Abstract
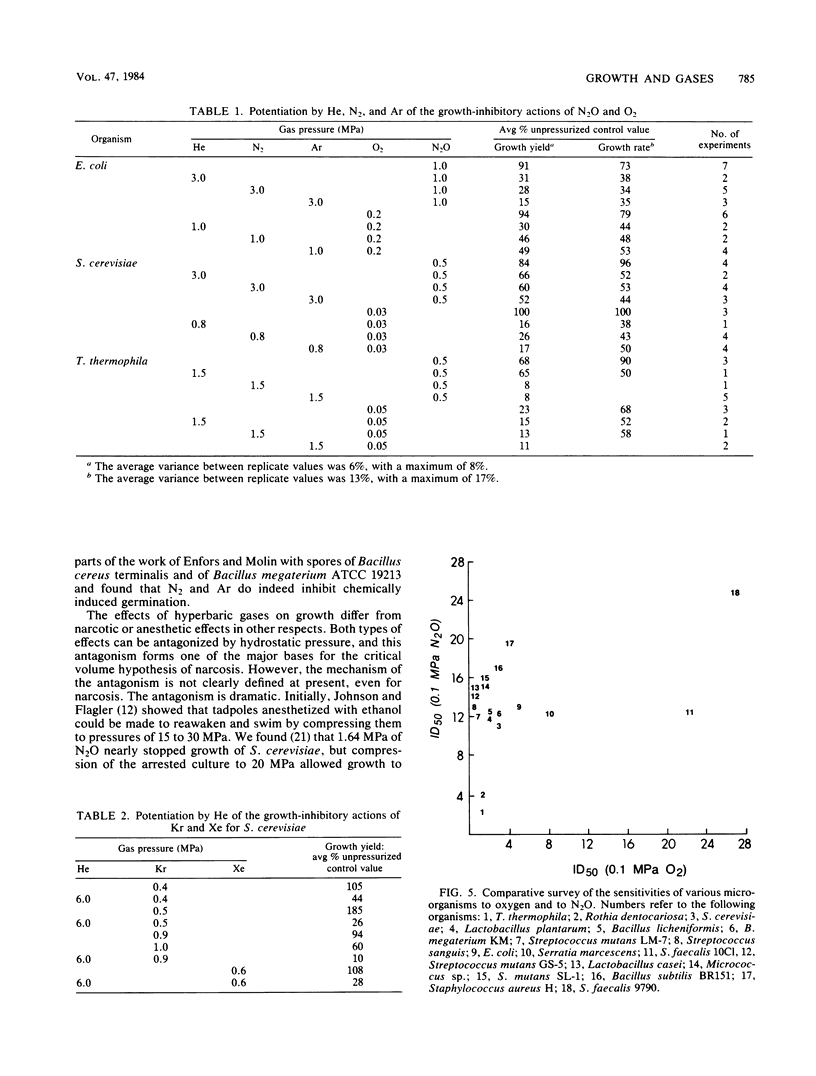
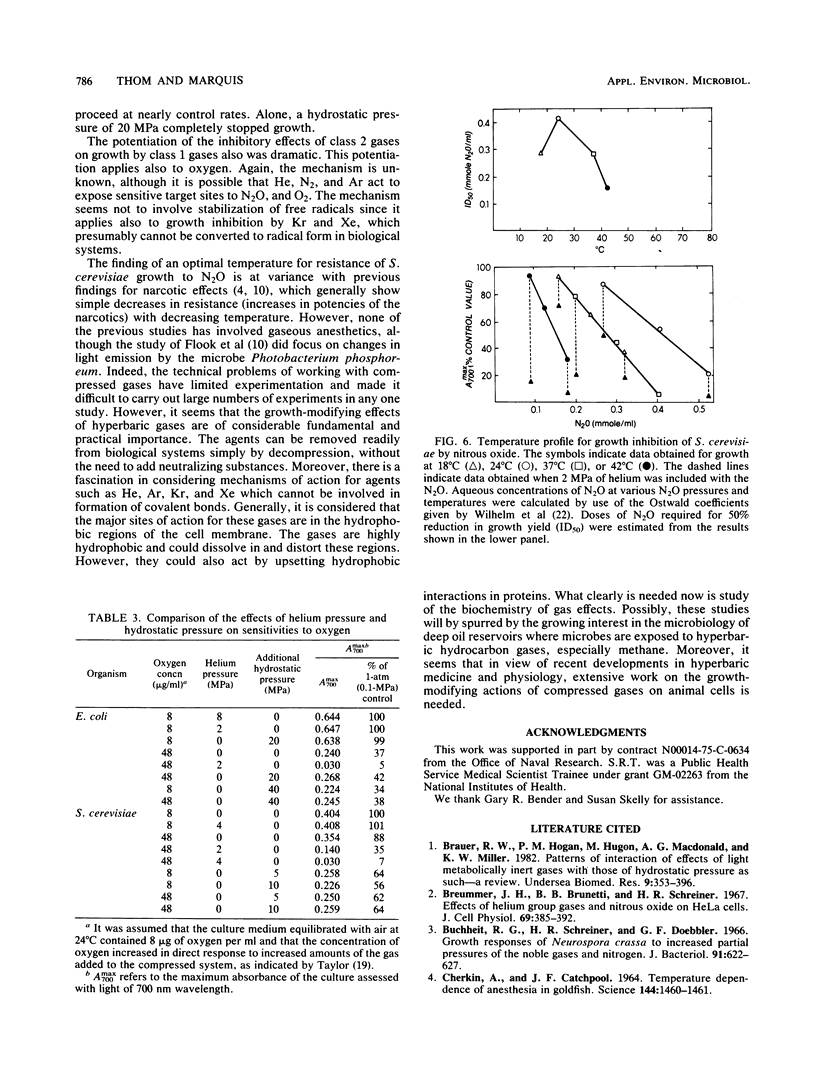
Studies of the growth-modifying actions for Escherichia coli, Saccharomyces cerevisiae, and Tetrahymena thermophila of helium, nitrogen, argon, krypton, xenon, and nitrous oxide led to the conclusion that there are two definable classes of gases. Class 1 gases, including He, N2, and Ar, are not growth inhibitors; in fact, they can reverse the growth inhibitory action of hydrostatic pressures. Class 2 gases, including Kr, Xe, and N2O, are potent growth inhibitors at low pressures. For example, at 24°C, 50% growth-inhibitory pressures of N2O were found to be ca. 1.7 MPa for E. coli, 1.0 MPa for S. cerevisiae, and 0.5 MPa for T. thermophila. Class 1 gases could act as potentiators for growth inhibition by N2O, O2, Kr, or Xe. Hydrostatic pressure alone is known to reverse N2O inhibition of growth, but we found that it did not greatly alter oxygen toxicity. Therefore, potentiation by class 1 gases appeared to be a gas effect rather than a pressure effect. The temperature profile for growth inhibition of S. cerevisiae by N2O revealed an optimal temperature for cell resistance of ca. 24°C, with lower resistance at higher and lower temperatures. Overall, it appeared that microbial growth modification by hyperbaric gases could not be related to their narcotic actions but reflected definably different physiological actions.
Full text
PDF
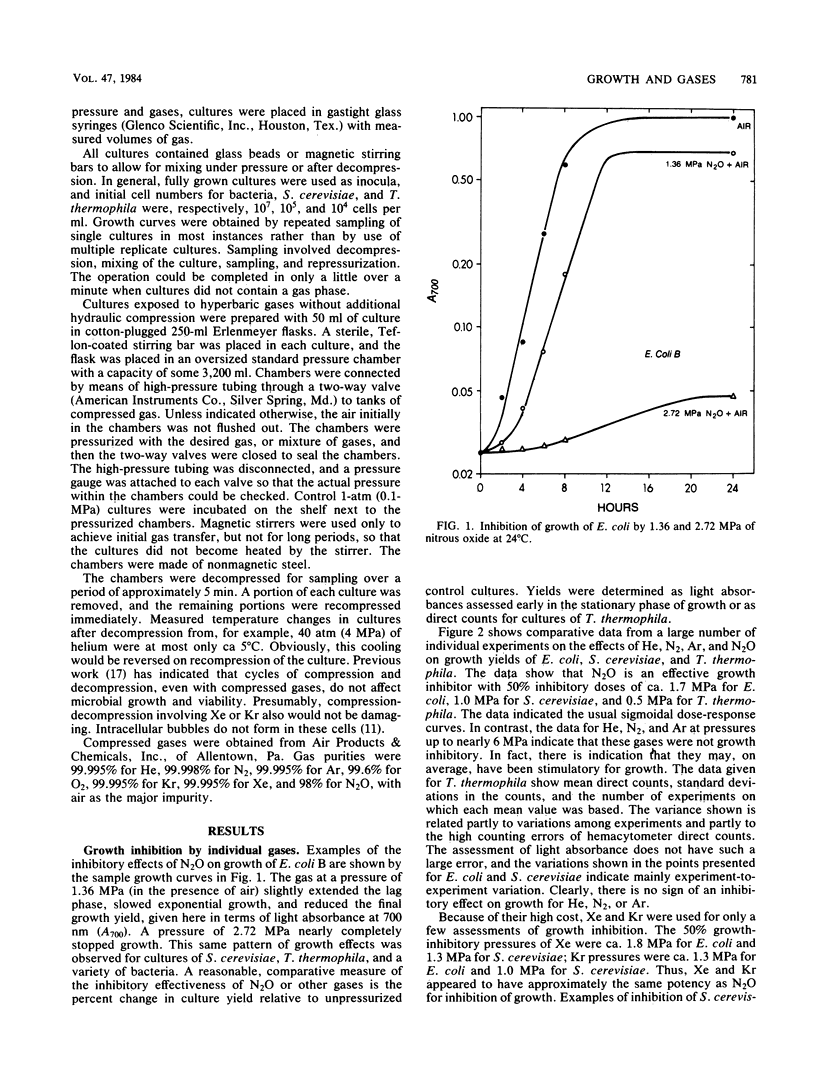
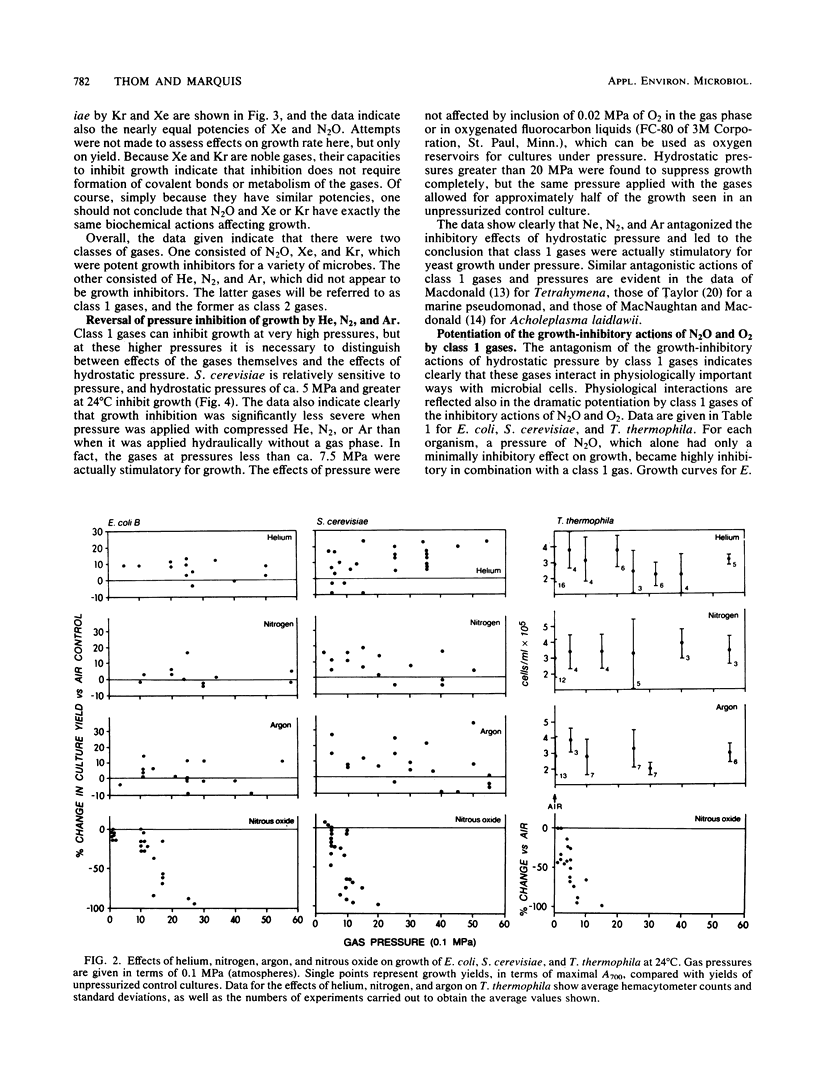
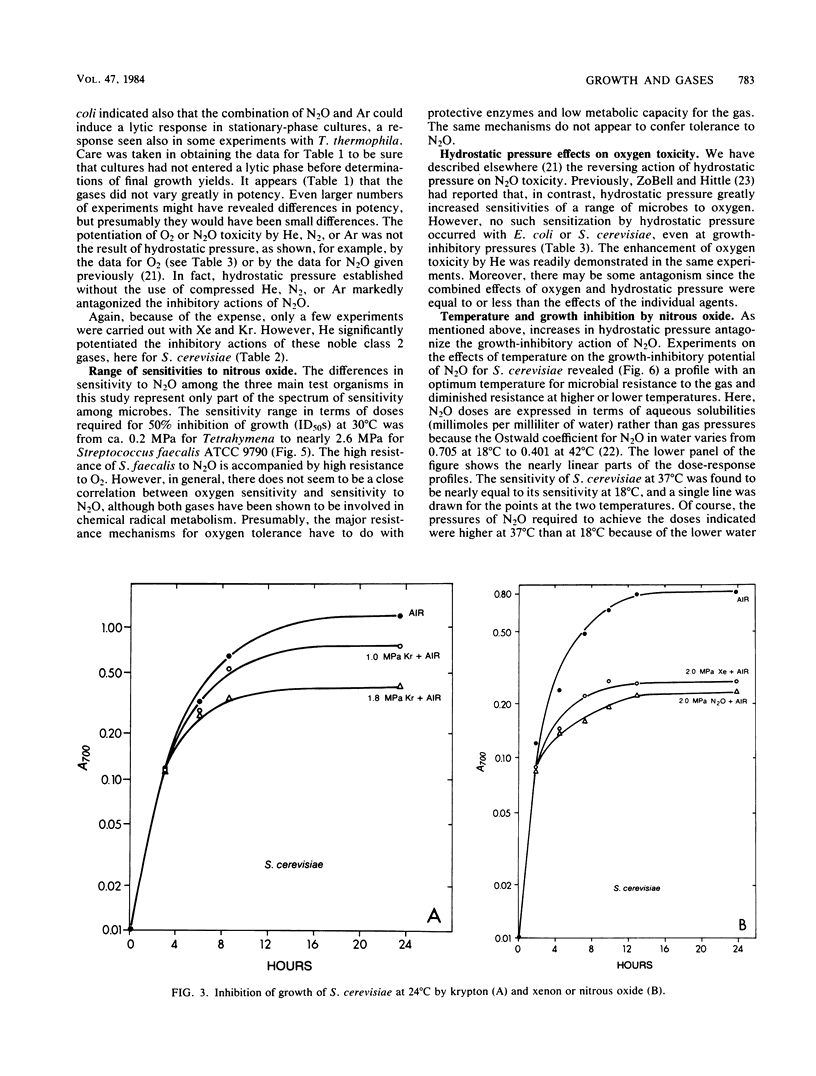
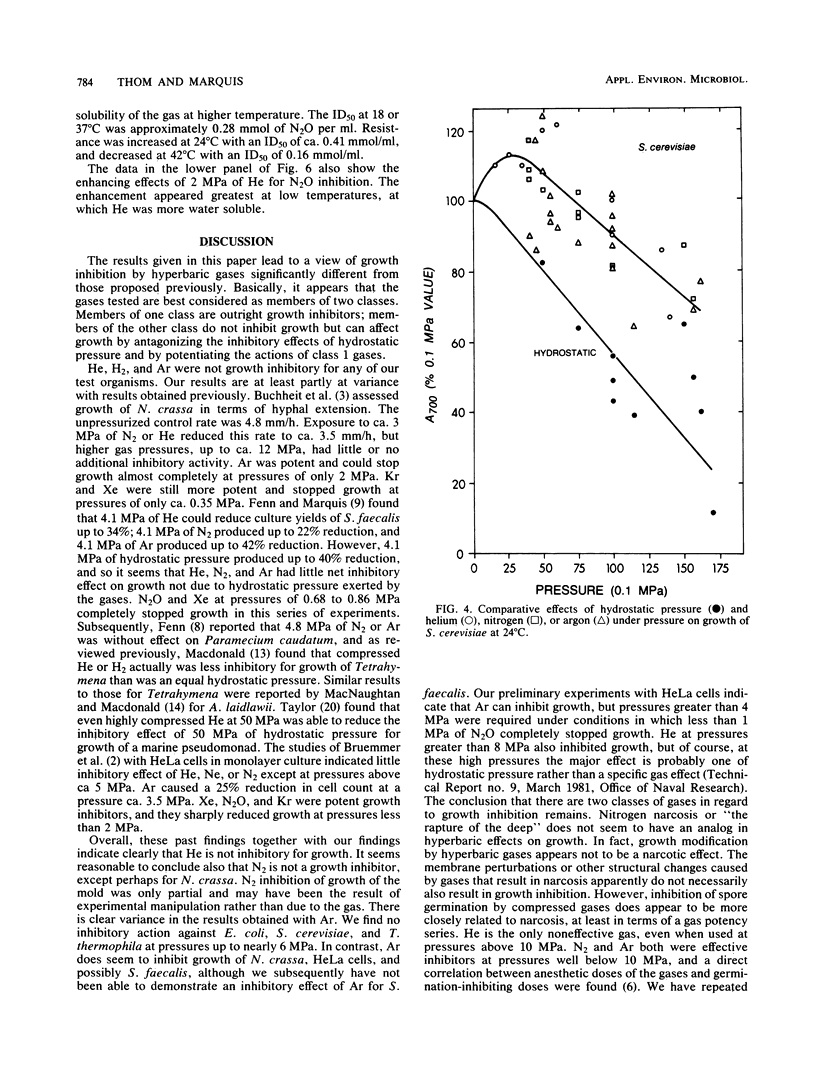

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brauer R. W., Hogan P. M., Hugon M., Macdonald A. G., Miller K. W. Patterns of interaction of effects of light metabolically inert gases with those of hydrostatic pressure as such--a review. Undersea Biomed Res. 1982 Dec;9(4):353–396. [PubMed] [Google Scholar]
- Bruemmer J. H., Brunetti B. B., Schreiner H. R. Effects of helium group gases and nitrous oxide on HeLa cells. J Cell Physiol. 1967 Jun;69(3):385–392. doi: 10.1002/jcp.1040690315. [DOI] [PubMed] [Google Scholar]
- Buchheit R. G., Schreiner H. R., Doebbler G. F. Growth responses of Neurospora crassa to increased partial pressures of the noble gases and nitrogen. J Bacteriol. 1966 Feb;91(2):622–627. doi: 10.1128/jb.91.2.622-627.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHERKIN A., CATCHPOOL J. F. TEMPERATURE DEPENDENCE OF ANESTHESIA IN GOLDFISH. Science. 1964 Jun 19;144(3625):1460–1462. doi: 10.1126/science.144.3625.1460. [DOI] [PubMed] [Google Scholar]
- CULLEN S. C., GROSS E. G. The anesthetic properties of xenon in animals and human beings, with additional observations on krypton. Science. 1951 May 18;113(2942):580–582. doi: 10.1126/science.113.2942.580. [DOI] [PubMed] [Google Scholar]
- Enfors S. O., Molin G. The influence of high concentrations of carbon dioxide on the germination of bacterial spores. J Appl Bacteriol. 1978 Oct;45(2):279–285. doi: 10.1111/j.1365-2672.1978.tb04223.x. [DOI] [PubMed] [Google Scholar]
- Fenn W. O., Marquis R. E. Growth of Streptococcus faecalis under high hydrostatic pressure and high partial pressures of inert gases. J Gen Physiol. 1968 Nov;52(5):810–824. doi: 10.1085/jgp.52.5.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flook V., Adey G. D., Dundas C. R., White D. C. Effect of temperature on potency of anesthetic agents. J Appl Physiol. 1974 Oct;37(4):552–555. doi: 10.1152/jappl.1974.37.4.552. [DOI] [PubMed] [Google Scholar]
- Hemmingsen E. A., Hemmingsen B. B. Lack of intracellular bubble formation in microorganisms at very high gas supersaturations. J Appl Physiol Respir Environ Exerc Physiol. 1979 Dec;47(6):1270–1277. doi: 10.1152/jappl.1979.47.6.1270. [DOI] [PubMed] [Google Scholar]
- JOHNSON F. H., FLAGLER E. A. Activity of narcotized amphibian larvae under hydrostatic pressure. J Cell Physiol. 1951 Feb;37(1):15–25. doi: 10.1002/jcp.1030370103. [DOI] [PubMed] [Google Scholar]
- MacDonald A. G. The effect of helium and of hydrogen at high pressure on the cell division of Tetrahymena pyriformis W. J Cell Physiol. 1975 Jun;85(3):511–528. doi: 10.1002/jcp.1040850303. [DOI] [PubMed] [Google Scholar]
- MacNaughtan W., Macdonald A. G. Effects of pressure and pressure antagonists on the growth and membrane-bound ATP-ase of Acholeplasma laidlawii B. Comp Biochem Physiol A Comp Physiol. 1982;72(2):405–414. doi: 10.1016/0300-9629(82)90238-9. [DOI] [PubMed] [Google Scholar]
- Marquis R. E. High-pressure microbial physiology. Adv Microb Physiol. 1976;14(11):159–241. doi: 10.1016/s0065-2911(08)60228-3. [DOI] [PubMed] [Google Scholar]
- Marquis R. E., Porterfield N., Matsumura P. Acid-base titration of streptococci and the physical states of intracellular ions. J Bacteriol. 1973 May;114(2):491–498. doi: 10.1128/jb.114.2.491-498.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis R. E., Thom S. R., Crookshank C. A. Interactions of helium, oxygen, and nitrous oxide affecting bacterial growth. Undersea Biomed Res. 1978 Jun;5(2):189–198. [PubMed] [Google Scholar]
- Schreiner H. R. General biological effects of the helium-xenon series of elements. Fed Proc. 1968 May-Jun;27(3):872–878. [PubMed] [Google Scholar]
- Taylor C. D. Solubility of oxygen in a seawater medium in equilibrium with a high-pressure oxy-helium atmosphere. Undersea Biomed Res. 1979 Jun;6(2):147–154. [PubMed] [Google Scholar]
- Taylor C. D. The effect of pressure upon the solubility of oxygen in water. Implications of the deviation from the ideal gas law upon measurements of fluorescence quenching. Arch Biochem Biophys. 1978 Nov;191(1):375–384. doi: 10.1016/0003-9861(78)90101-7. [DOI] [PubMed] [Google Scholar]
- ZoBell C. E., Hittle L. L. Some effects of hyperbaric oxygenation on bacteria at increased hydrostatic pressures. Can J Microbiol. 1967 Oct;13(10):1311–1319. doi: 10.1139/m67-177. [DOI] [PubMed] [Google Scholar]


