Abstract
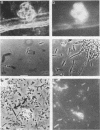
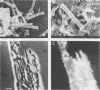
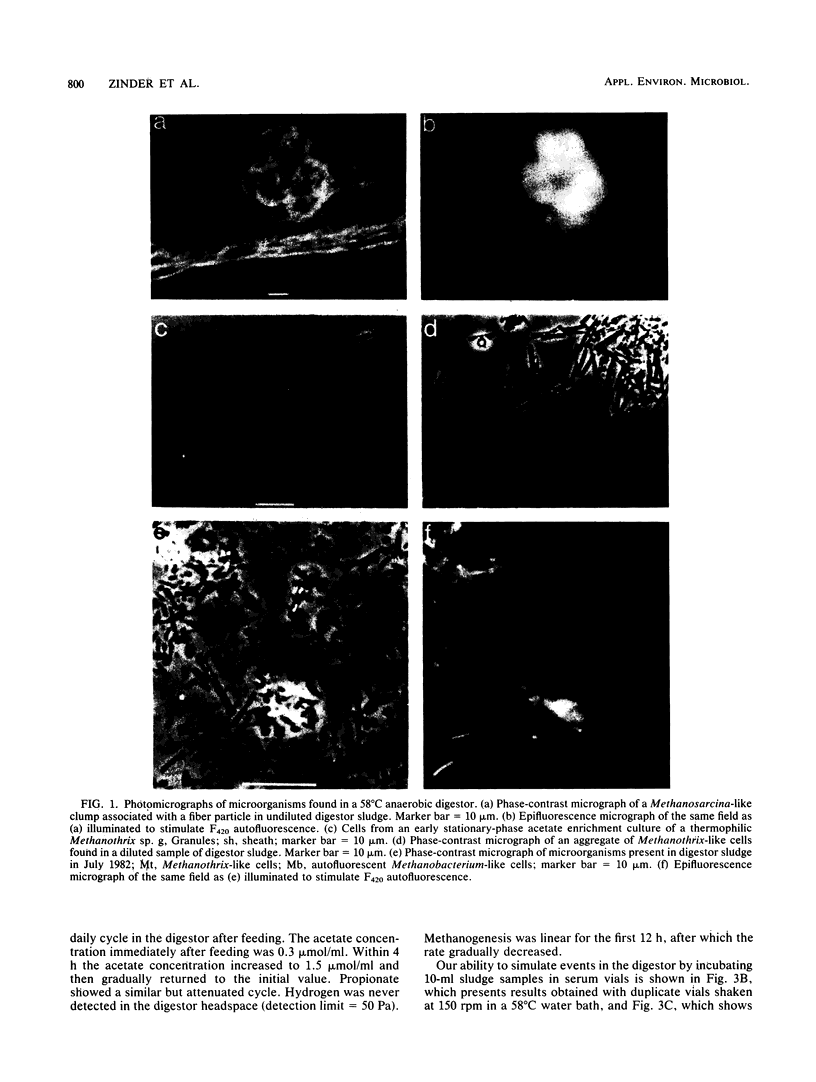
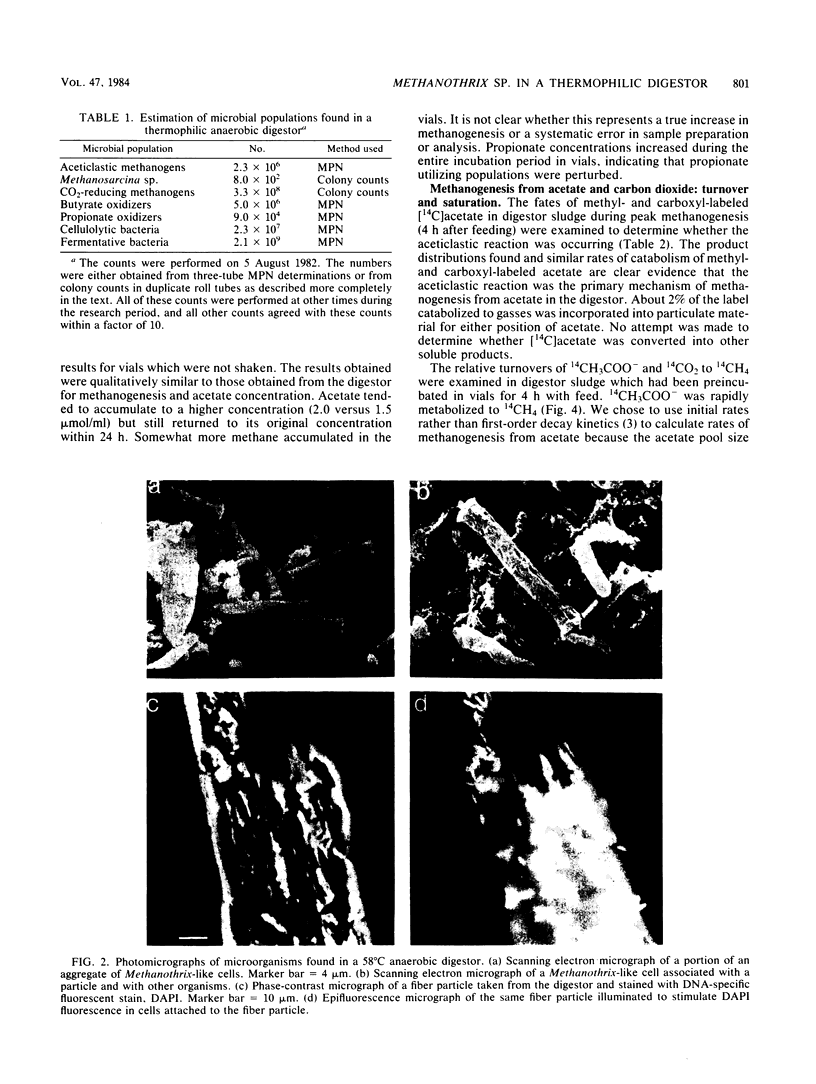
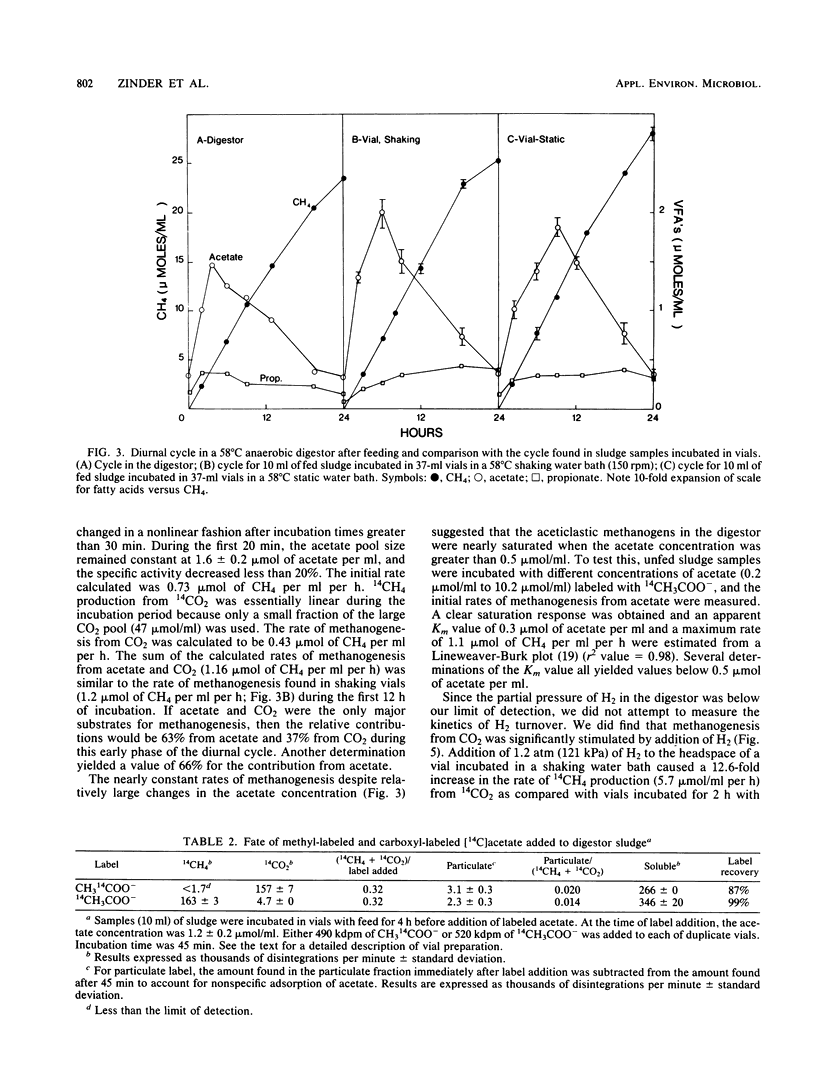
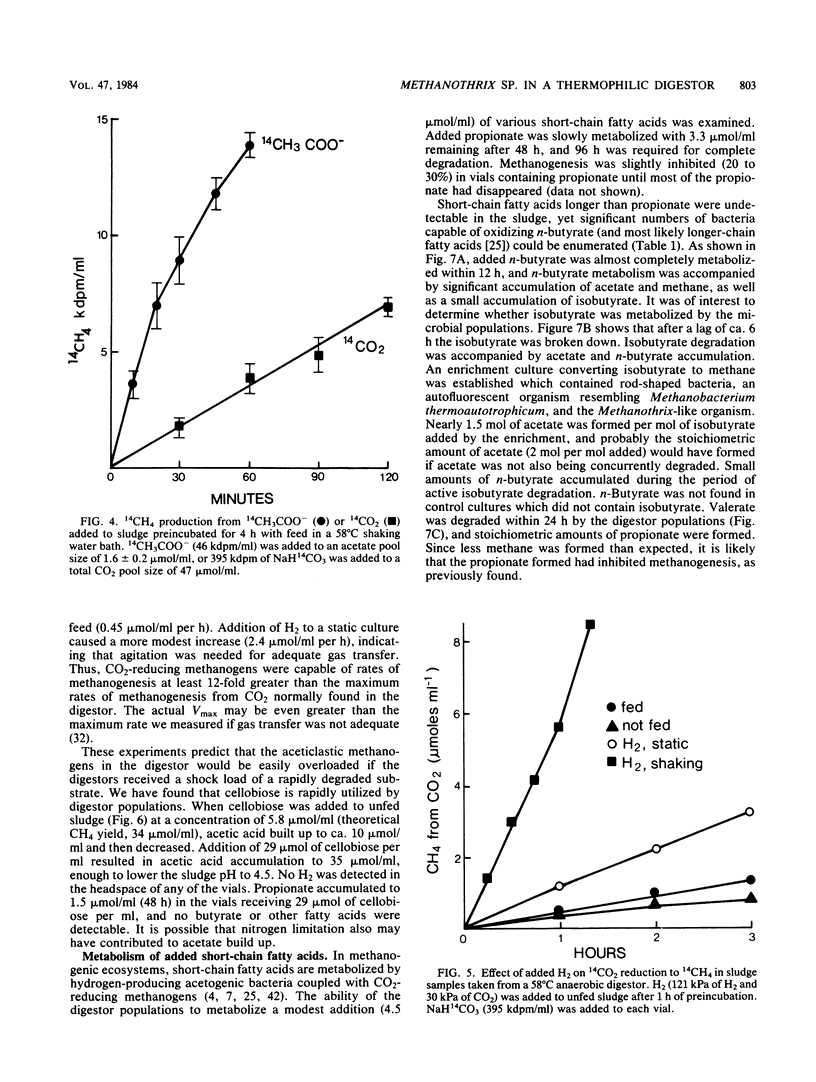
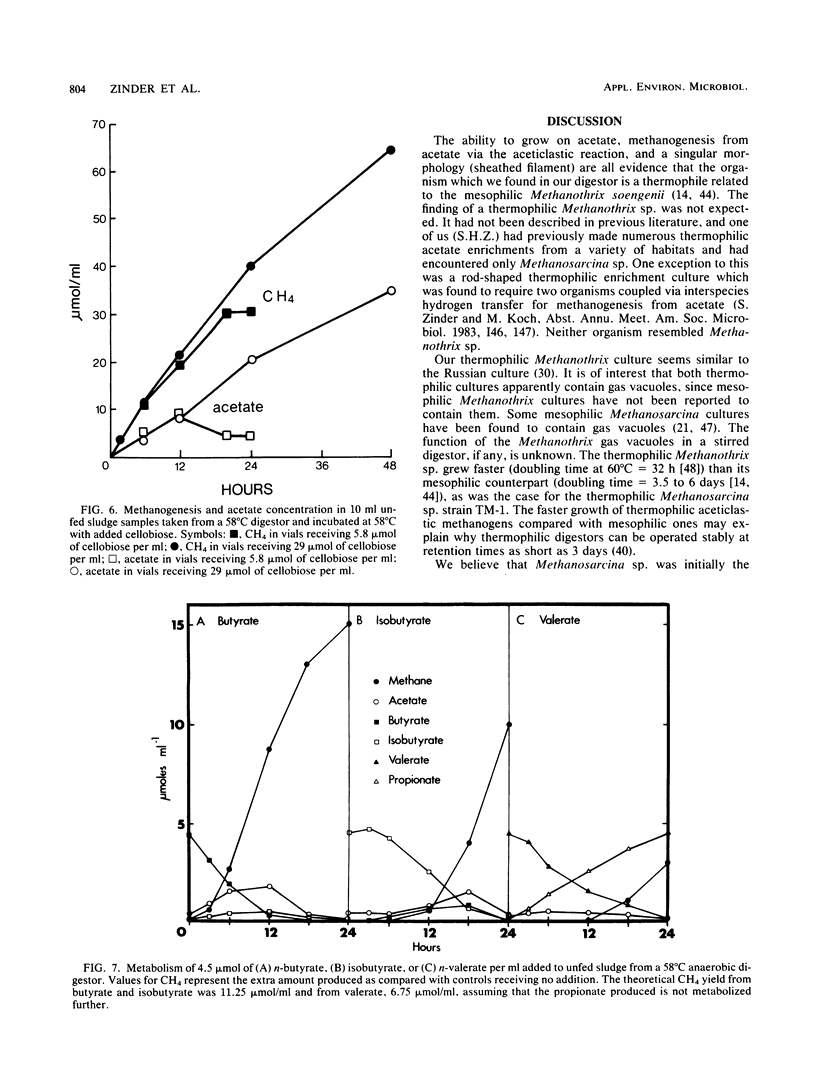
Aceticlastic methanogens and other microbial groups were enumerated in a 58°C laboratory-scale (3 liter) anaerobic digestor which was fed air-classified municipal refuse, a lignocellulosic waste (loading rate = 1.8 to 2.7 g of volatile solids per liter per day; retention time = 10 days). Two weeks after start-up, Methanosarcina sp. was present in high numbers (105 to 106 CFU/ml) and autofluorescent Methanosarcina-like clumps were abundant in sludge examined by using epifluorescence microscopy. After about 4 months of digestor operation, numbers of Methanosarcina sp. dropped 2 to 3 orders of magnitude and large numbers (most probable number = 106 to 107/ml) of a thermophilic aceticlastic methanogen morphologically resembing Methanothrix sp. were found. Methanothrix sp. had apparently displaced Methanosarcina sp. as the dominant aceticlastic methanogen in the digestor. During the period when Methanothrix sp. was apparently dominant, acetate concentrations varied between 0.3 and 1.5 μmol/ml during the daily feeding cycle, and acetate was the precursor of 63 to 66% of the methane produced during peak digestor methanogenesis. The apparent Km value obtained for methanogenesis from acetate, 0.3 μmol/ml, indicated that the aceticlastic methanogens were nearly saturated for substrate during most of the digestor cycle. CO2-reducing methanogens were capable of methanogenesis at rates more than 12 times greater than those usually found in the digestor. Added propionate (4.5 μmol/ml) was metabolized slowly by the digestor populations and slightly inhibited methanogenesis. Added n-butyrate, isobutyrate, or n-valerate (4.5 μmol/ml each) were broken down within 24 h. Isobutyrate was oxidized to acetate, a novel reaction possibly involving isomerization to n-butyrate. The rapid growth rate and versatile metabolism of Methanosarcina sp. make it a likely organism to be involved in start-up, whereas the low Km value of Methanothrix sp. for acetate may cause it to be favored in stable digestors operated with long retention times.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone D. R., Bryant M. P. Propionate-Degrading Bacterium, Syntrophobacter wolinii sp. nov. gen. nov., from Methanogenic Ecosystems. Appl Environ Microbiol. 1980 Sep;40(3):626–632. doi: 10.1128/aem.40.3.626-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone D. R. Terminal reactions in the anaerobic digestion of animal waste. Appl Environ Microbiol. 1982 Jan;43(1):57–64. doi: 10.1128/aem.43.1.57-64.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doddema H. J., Vogels G. D. Improved identification of methanogenic bacteria by fluorescence microscopy. Appl Environ Microbiol. 1978 Nov;36(5):752–754. doi: 10.1128/aem.36.5.752-754.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson T. J., Mah R. A. Isolation and characterization of an h(2)-oxidizing thermophilic methanogen. Appl Environ Microbiol. 1983 Jan;45(1):265–274. doi: 10.1128/aem.45.1.265-274.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar H. F., Wuhrmann K. Kinetic parameters and relative turnovers of some important catabolic reactions in digesting sludge. Appl Environ Microbiol. 1978 Jul;36(1):1–7. doi: 10.1128/aem.36.1.1-7.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M., Dolfing J., Wuhrmann K., Zehnder A. J. Pathways of propionate degradation by enriched methanogenic cultures. Appl Environ Microbiol. 1983 Apr;45(4):1411–1414. doi: 10.1128/aem.45.4.1411-1414.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie R. I., Bryant M. P. Metabolic Activity of Fatty Acid-Oxidizing Bacteria and the Contribution of Acetate, Propionate, Butyrate, and CO(2) to Methanogenesis in Cattle Waste at 40 and 60 degrees C. Appl Environ Microbiol. 1981 Jun;41(6):1363–1373. doi: 10.1128/aem.41.6.1363-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah R. A., Smith M. R., Baresi L. Studies on an acetate-fermenting strain of Methanosarcina. Appl Environ Microbiol. 1978 Jun;35(6):1174–1184. doi: 10.1128/aem.35.6.1174-1184.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah R. A., Ward D. M., Baresi L., Glass T. L. Biogenesis of methane. Annu Rev Microbiol. 1977;31:309–341. doi: 10.1146/annurev.mi.31.100177.001521. [DOI] [PubMed] [Google Scholar]
- Matin A., Veldkamp H. Physiological basis of the selective advantage of a Spirillum sp. in a carbon-limited environment. J Gen Microbiol. 1978 Apr;105(2):187–197. doi: 10.1099/00221287-105-2-187. [DOI] [PubMed] [Google Scholar]
- Mountfort D. O., Asher R. A. Changes in proportions of acetate and carbon dioxide used as methane precursors during the anaerobic digestion of bovine waste. Appl Environ Microbiol. 1978 Apr;35(4):648–654. doi: 10.1128/aem.35.4.648-654.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. R., Zeikus J. G. Rapid method for the radioisotopic analysis of gaseous end products of anaerobic metabolism. Appl Microbiol. 1974 Aug;28(2):258–261. doi: 10.1128/am.28.2.258-261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T. K., Weimer T. K., Zeikus J. G. Cellulolytic and physiological properties of Clostridium thermocellum. Arch Microbiol. 1977 Jul 26;114(1):1–7. doi: 10.1007/BF00429622. [DOI] [PubMed] [Google Scholar]
- Paul J. H. Use of hoechst dyes 33258 and 33342 for enumeration of attached and planktonic bacteria. Appl Environ Microbiol. 1982 Apr;43(4):939–944. doi: 10.1128/aem.43.4.939-944.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. A., Tiedje J. M. Kinetics of hydrogen consumption by rumen fluid, anaerobic digestor sludge, and sediment. Appl Environ Microbiol. 1982 Dec;44(6):1374–1384. doi: 10.1128/aem.44.6.1374-1384.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R., Mah R. A. Growth and methanogenesis by Methanosarcina strain 227 on acetate and methanol. Appl Environ Microbiol. 1978 Dec;36(6):870–879. doi: 10.1128/aem.36.6.870-879.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. H., Mah R. A. Kinetics of acetate metabolism during sludge digestion. Appl Microbiol. 1966 May;14(3):368–371. doi: 10.1128/am.14.3.368-371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varel V. H., Isaacson H. R., Bryant M. P. Thermophilic methane production from cattle waste. Appl Environ Microbiol. 1977 Feb;33(2):298–307. doi: 10.1128/aem.33.2.298-307.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnder A. J., Huser B. A., Brock T. D., Wuhrmann K. Characterization of an acetate-decarboxylating, non-hydrogen-oxidizing methane bacterium. Arch Microbiol. 1980 Jan;124(1):1–11. doi: 10.1007/BF00407022. [DOI] [PubMed] [Google Scholar]
- Zehnder A. J., Huser B., Brock T. D. Measuring radioactive methane with the liquid scintillation counter. Appl Environ Microbiol. 1979 May;37(5):897–899. doi: 10.1128/aem.37.5.897-899.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G. The biology of methanogenic bacteria. Bacteriol Rev. 1977 Jun;41(2):514–541. doi: 10.1128/br.41.2.514-541.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Wolfe R. S. Methanobacterium thermoautotrophicus sp. n., an anaerobic, autotrophic, extreme thermophile. J Bacteriol. 1972 Feb;109(2):707–715. doi: 10.1128/jb.109.2.707-713.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhilina T. N., Zavarzin G. A. Sravnitel'naia tsitologiia metanosartsin i opisanie Methanosarcina vacuolata n. sp. Mikrobiologiia. 1979 Mar-Apr;48(2):279–285. [PubMed] [Google Scholar]
- Zinder S. H., Anguish T., Cardwell S. C. Effects of Temperature on Methanogenesis in a Thermophilic (58 degrees C) Anaerobic Digestor. Appl Environ Microbiol. 1984 Apr;47(4):808–813. doi: 10.1128/aem.47.4.808-813.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinder S. H., Mah R. A. Isolation and Characterization of a Thermophilic Strain of Methanosarcina Unable to Use H(2)-CO(2) for Methanogenesis. Appl Environ Microbiol. 1979 Nov;38(5):996–1008. doi: 10.1128/aem.38.5.996-1008.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]