Abstract
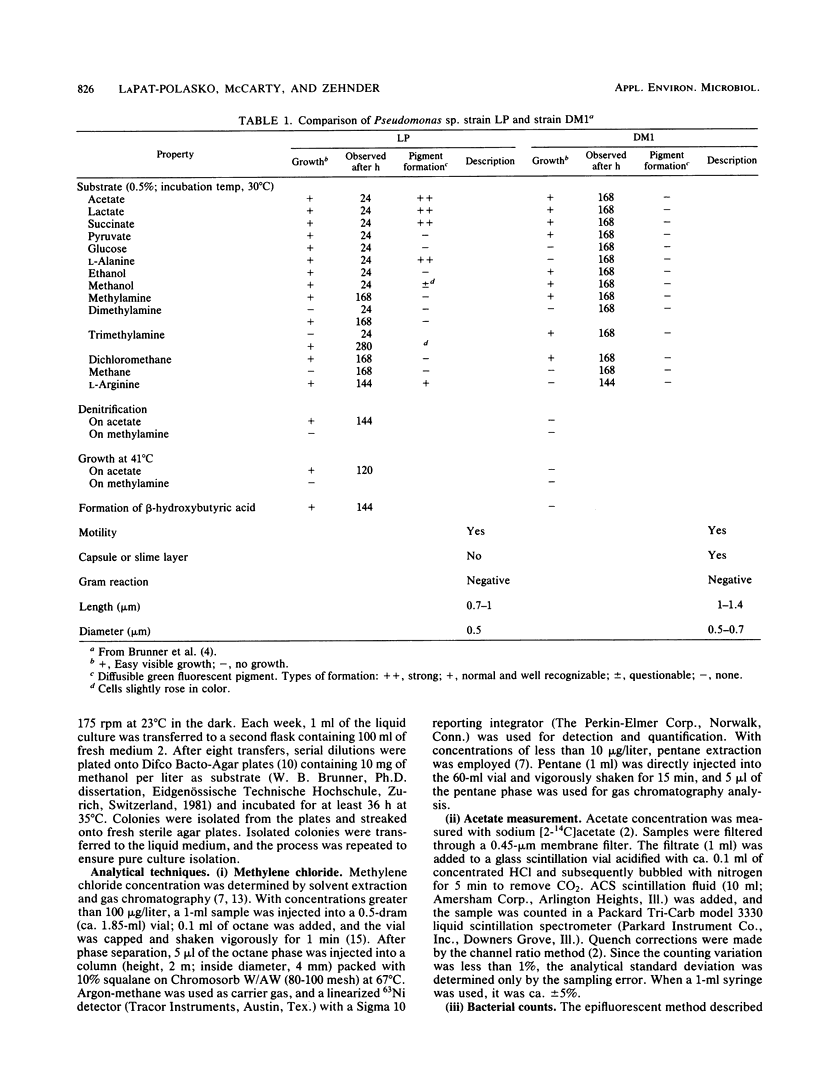
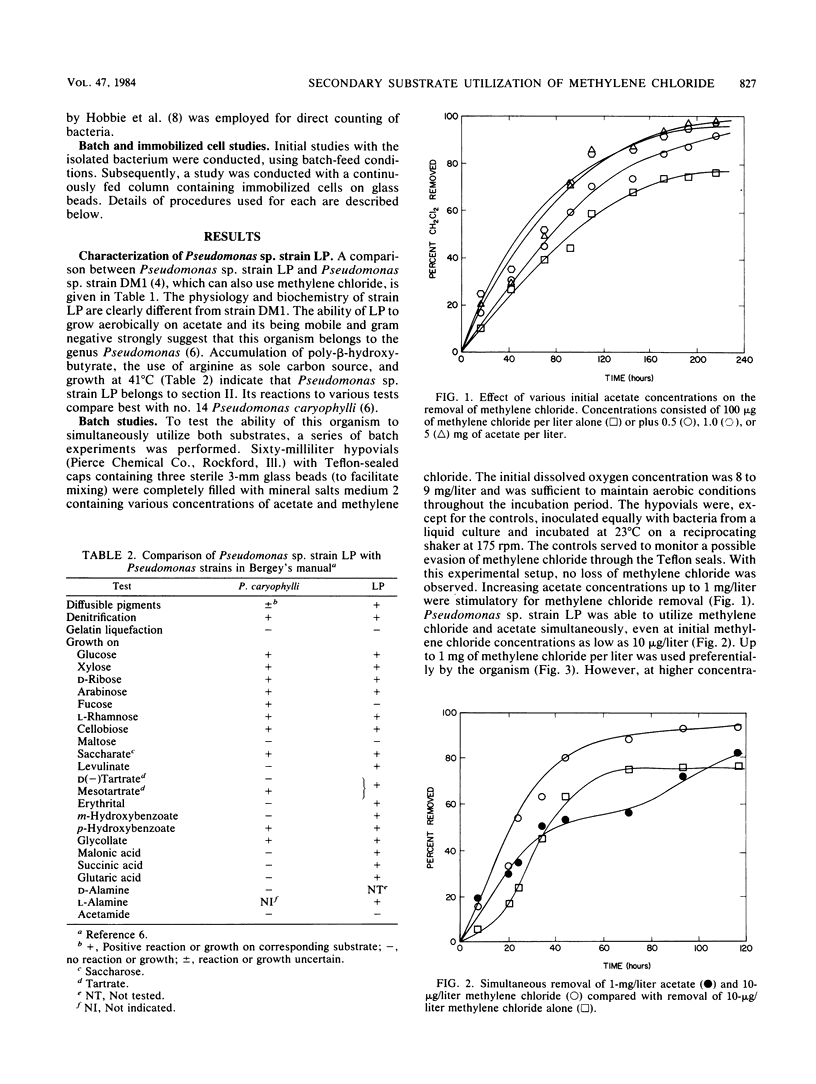
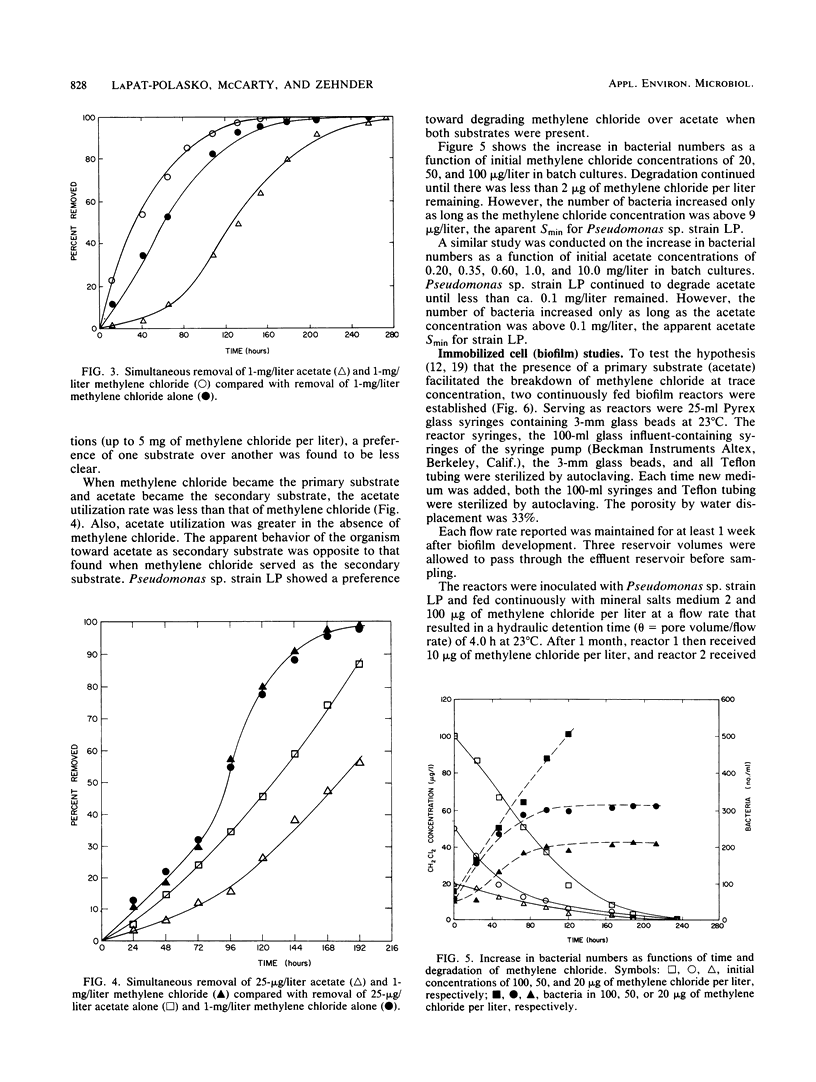
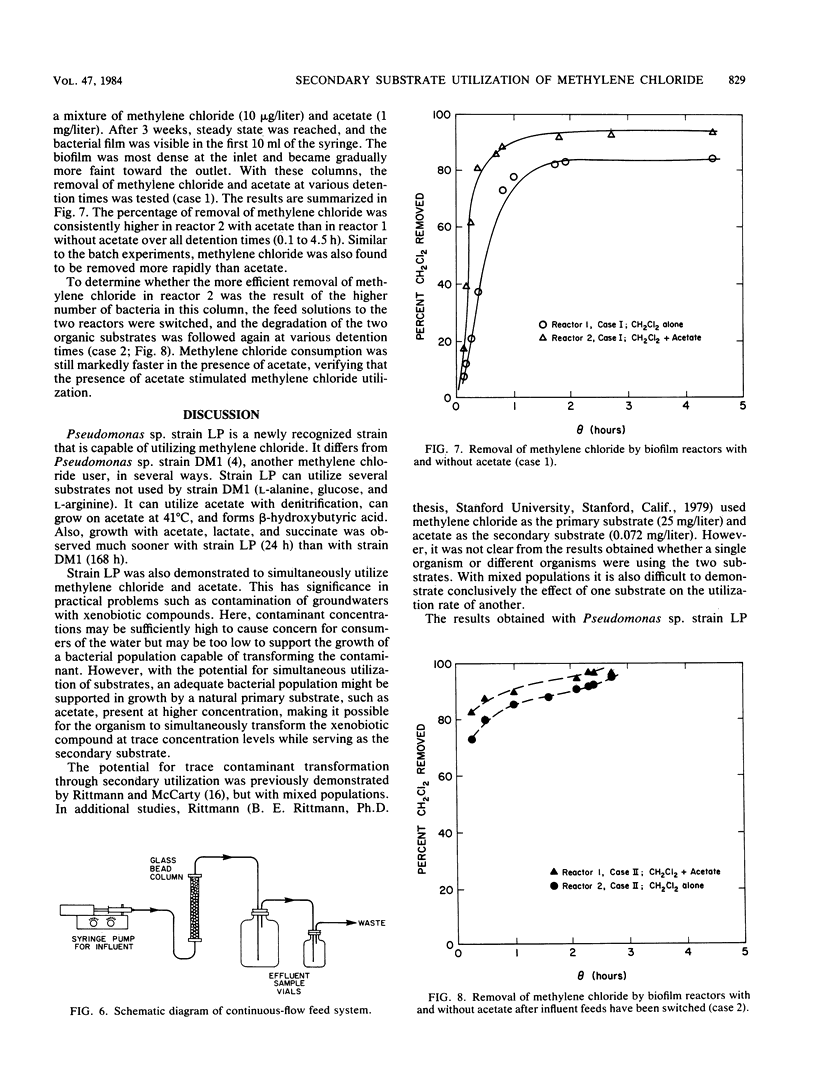
Secondary substrate utilization of methylene chloride was analyzed by using Pseudomonas sp. strain LP. Both batch and continuously fed reactors demonstrated that this strain was capable of simultaneously consuming two substrates at different concentrations: the primary substrate at the higher concentration (milligrams per liter) and the secondary substrate at the lower concentration (micrograms per liter). The rate of methylene chloride utilization at trace concentrations was greater in the presence of the primary substrate, acetate, than without it. However, when the substrate roles were changed, the acetate secondary substrate utilization rate was less when methylene chloride was present. Thus, substrate interactions are important in the kinetics of secondary substrate utilization. Pseudomonas sp. strain LP showed a preference toward degrading methylene chloride over acetate, whether it was the primary or secondary substrate, providing it was below an inhibitory concentration of ca. 10 mg/liter.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. Biodegradation of chemicals of environmental concern. Science. 1981 Jan 9;211(4478):132–138. doi: 10.1126/science.7444456. [DOI] [PubMed] [Google Scholar]
- Boethling R. S., Alexander M. Effect of concentration of organic chemicals on their biodegradation by natural microbial communities. Appl Environ Microbiol. 1979 Jun;37(6):1211–1216. doi: 10.1128/aem.37.6.1211-1216.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner W., Staub D., Leisinger T. Bacterial degradation of dichloromethane. Appl Environ Microbiol. 1980 Nov;40(5):950–958. doi: 10.1128/aem.40.5.950-958.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongen W. M., Alink G. M., Koeman J. H. Mutagenic effect of dichloromethane on Salmonella typhimurium. Mutat Res. 1978 Jan;56(3):245–248. doi: 10.1016/0027-5107(78)90191-4. [DOI] [PubMed] [Google Scholar]
- Kaper J. B., Mills A. L., Colwell R. R. Evaluation of the accuracy and precision of enumerating aerobic heterotrophs in water samples by the spread plate method. Appl Environ Microbiol. 1978 Apr;35(4):756–761. doi: 10.1128/aem.35.4.756-761.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittmann B. E., McCarty P. L. Utilization of dichloromethane by suspended and fixed-film bacteria. Appl Environ Microbiol. 1980 Jun;39(6):1225–1226. doi: 10.1128/aem.39.6.1225-1226.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart R. D., Fisher T. N., Hosko M. J., Peterson J. E., Baretta E. D., Dodd H. C. Carboxyhemoglobin elevation after exposure to dichloromethane. Science. 1972 Apr 21;176(4032):295–296. doi: 10.1126/science.176.4032.295. [DOI] [PubMed] [Google Scholar]



