Abstract
The neurosteroid 3α-hydroxysteroid-5α-pregnan-20-one (allopregnanolone) acts as a positive allosteric modulator of γ-aminobutyric acid at γ-aminobutyric acid type A receptors and hence is a powerful anxiolytic, anticonvulsant, and anesthetic agent. Allopregnanolone is synthesized from progesterone by reduction to 5α-dihydroprogesterone, mediated by 5α-reductase, and by reduction to allopregnanolone, mediated by 3α-hydroxysteroid dehydrogenase (3α-HSD). Previous reports suggested that some selective serotonin reuptake inhibitors (SSRIs) could alter concentrations of allopregnanolone in human cerebral spinal fluid and in rat brain sections. We determined whether SSRIs directly altered the activities of either 5α-reductase or 3α-HSD, using an in vitro system containing purified recombinant proteins. Although rats appear to express a single 3α-HSD isoform, the human brain contains several isoforms of this enzyme, including a new isoform we cloned from human fetal brains. Our results indicate that the SSRIs fluoxetine, sertraline, and paroxetine decrease the Km of the conversion of 5α-dihydroprogesterone to allopregnanolone by human 3α-HSD type III 10- to 30-fold. Only sertraline inhibited the reverse oxidative reaction. SSRIs also affected conversions of androgens to 3α- and 3α, 17β-reduced or -oxidized androgens mediated by 3α-HSD type IIBrain. Another antidepressant, imipramine, was without any effect on allopregnanolone or androstanediol production. The region-specific expression of 3α-HSD type IIBrain and 3α-HSD type III mRNAs suggest that SSRIs will affect neurosteroid production in a region-specific manner. Our results may thus help explain the rapid alleviation of the anxiety and dysphoria associated with late luteal phase dysphoria disorder and major unipolar depression by these SSRIs.
Keywords: 3α hydroxysteroid dehydrogenase, fluoxetine, allopregnanolone, dihydroprogesterone
Over the past decade, it has become clear that the brain synthesizes steroid hormones by using some of the same steroidogenic enzymes found in adrenals and gonads (reviewed in refs. 1 and 2). These compounds were given the name neurosteroids (3), and some of their functions have been elucidated (4). Neurosteroids that are derivatives of progesterone have been shown to act as allosteric modulators of the γ-aminobutyric acid type A (GABAA) receptor function (5, 6). They bind to a distinct site on these receptors and affect the frequency and duration of the channel opening. In this way, they modulate GABAergic transmission, and, as a result, neurosteroids may affect complex behaviors such as anxiety.
Changes in neurosteroid concentrations in the brain and in the plasma have been associated with the menstrual cycle in women (7, 8, 9). Changes in neurosteroid concentrations, but not in progesterone concentrations (10), also have been suggested to play a role in premenstrual syndrome (11). Firm conclusions cannot be drawn from these limited studies, however, as plasma concentrations of steroid may not reflect actual brain or cerebrospinal fluid levels of steroids.
Several recent studies have pointed to commonly used selective serotonin-reuptake inhibitors as potential modulators of neurosteroid synthesis in the brain. In the earliest study, investigators found that fluoxetine treatment could alleviate many symptoms of premenstrual dysphoria disorder, also called late luteal phase dysphoria disorder (12, 13). As this disorder correlates specifically with a specific phase of the menstrual cycle, it seemed logical that ovarian hormones, such as progesterone, might play a role in its etiology. Furthermore, because fluoxetine alleviated many symptoms of this disorder, investigators hypothesized that an additional effect of fluoxetine, besides inhibiting serotonin reuptake, might be to alter neurosteroid synthesis (14). In an elegant study, they showed that fluoxetine could indeed increase the abundance of the neurosteroid allopregnanolone, a derivative of progesterone, in the rat brain. The same investigators also recently showed that, in clinically depressed patients, neurosteroid concentrations in cerebrospinal fluid could be increased by treatment with fluoxetine or fluvoxamine (15). The potent GABAergic allopregnanolone is synthesized from progesterone by two sequential enzymatic reactions: In the first reaction, progesterone is converted to 5α-dihydroprogesterone (5α-pregnan-3, 20-dione or 5α-DHP) by the enzyme 5α-reductase. DHP then is converted to allopregnanolone, also known as 3α, 5α- tetrahydroprogesterone (5α-pregnan-3α, 20α-diol), by the enzyme 3α hydroxysteroid dehydrogenase (3α-HSD) (16). This enzymatic step is reversible and uses the cofactors NADP(H) or NAD(P), depending on the cellular localization of the enzyme, the particular isoform, and the substrate being used.
The results from the experiments by Uzunov et al. (14) suggest that selective serotonin reuptake inhibitors (SSRIs) increase the concentration of allopregnanolone only and do not substantially affect the brain concentrations of progesterone or DHP. Therefore, we wished to determine whether SSRIs would have any effect on 5α-reductase activity and whether they directly affect 3α-HSD activity, and the mechanism by which the alterations occur.
Materials and Methods
Materials.
Fluoxetine and paroxetine were obtained as Prozac (Eli Lilly) and Paxil (SmithKline Beecham) tablets and were dissolved in ethyl alcohol, and insoluble material was removed by centrifugation. Sertraline was obtained as Zoloft (Pfizer Diagnostics) tablets whereas imipramine was purchased from Sigma, and both were dissolved in water. 3H- and 14C steroid precursors were obtained from NEN-Amersham. Specific activities of each of the steroid precursors are 5α-dihydrotestosterone (DHT), 56.5 Ci/mmol; androstanediol, 41 Ci/mmol; DHP, 55.4 mCi/mmol; allopregnanolone, 65.0 Ci/mmol; and progesterone, 55.4 mCi/mmol. Blots containing human brain poly(A)+ RNA were obtained from CLONTECH.
Cloning 3α-HSD cDNAs and Expression in Bacteria.
Rat 3α-HSD from rat liver cDNA was cloned by using rat-specific primers that correspond to nucleotides 1–18 and nucleotides 948–966 (17). Human fetal brain 3α-HSD type II and type III cDNAs were cloned by using primers (5′, bases 1–18; 3′, bases 909–929) based on the sequences of the type II and III liver 3α-HSD (18, 19) cDNAs. These cDNAs were cloned into the prokaryotic expression vector pET (Novagen), and BL21(DE3) bacteria were transformed with these plasmids. Protein was induced in bacteria by 0.4 mM isopropyl β-d-thiogalactoside stimulation for 3 hours, and proteins were purified by preparation of bacterial inclusion bodies. Purity of the isolated proteins was assessed by SDS/PAGE, and protein concentration was determined by using a Pierce BCA Reagent Assay Kit (20).
Analysis of 3α-HSD Activities.
3α-HSD activity was determined by monitoring the conversion of radioactive dihydroprogesterone to allopregnanolone and also by monitoring the reverse reaction of allopregnanolone to 5α-DHP. For radiometric assays involving all substrates, bacterial extract (20 μl) was incubated with 40,000 cpm of radiolabeled steroid precursor and 10 nM–100 μM cold steroid precursor in 100 mM sodium phosphate buffer at pH 7.3 (with 2 mM NADPH) for the reductive reaction at 37°C for 20 min. Progesterone and DHP were 14C-labeled whereas all other compounds were 3H-labeled. Oxidative reactions were conducted with 2 mM NADP+ in 100 mM sodium phosphate at pH 8.9 (21). These conversions were assayed by thin layer chromatography, using chloroform/ethyl acetate (3:1) as a solvent system. Identification of each metabolite was based on reference standards run concomitantly on each plate. Rfs of the identified steroids were DHT, 0.35; DHP, 0.55; allopregnanolone, 0.39; progesterone, 0.49; 20α-dihydroprogesterone, 0.32; androstanediol, 0.22; androsterone, 0.34; and androstanedione, 0.48. No other bands were generated in these reactions. Bacterial extracts that were transformed with an unrelated plasmid, or that were not transformed, did not convert radioactive precursor. 3α-HSD activity also was assayed photometrically by monitoring the conversion of NADPH to NADP, by incubating the extracts with cold DHP for 2 min, and by monitoring conversion at 340 nm (17). The oxidative reactions using cold allopregnanolone also were assayed by monitoring conversion of NADP to NADPH at 340 nm. Reaction mixtures containing varying concentrations of substrate, as described above, were used, except that radioactive precursor was eliminated. Photometric assays were performed six times for each condition by using at least three different enzyme preparations whereas radiometric assays were performed in triplicate by using at least three different enzyme preparations.
Expression of 5α-Reductase Type I.
Rat 5α-reductase type I cDNA was provided by D. Russell (University of Texas Southwestern, Dallas) and was transfected into COS-1 cells by calcium phosphate precipitation. 5α-reductase activity was determined by incubating the cells, 72 hours after transfection, with 90,000 cpm 14C-progesterone for 1 hour and assaying production of 5α-DHP by thin layer chromatography, using 3:1 chloroform/ethyl acetate as a solvent system and steroidal standards.
Synthesis of 14C- 5α-Dihydroprogesterone.
14C-5α-dihydroprogesterone was synthesized from 14C-progesterone by using the transfected COS cell system described above. COS-1 cells transfected with 5α-reductase type I cDNA were incubated with 14C-progesterone for 12 hours, 72 hours after cell transfection. The major secreted steroidal product was 14C-5α-dihydroprogesterone, which was assayed and purified by thin layer chromatography.
Analysis of Human 3α-HSD mRNA Expression.
Human 3α-HSD mRNA was analyzed by Northern blots, using commercially available blots of human brain RNAs. These blots contained 2 μg of poly(A)+ mRNA/lane from different regions of normal adult human brains. Blots were probed with PCR-generated probes that corresponded to the least conserved regions of the 3′ coding regions of both the type IIBrain and III cDNAs. Hybridizing bands were quantitated by using a Molecular Dynamics PhosphorImager and imagequant computer software (Molecular Dynamics).
Analysis of 3α-HSD Activity in the Presence of SSRIs.
Determination of Km and Vmax of each enzyme was performed in the presence of 50 μM fluoxetine, paroxetine, sertraline, or imipramine as above. Dose-response curves were generated for each compound, and 50 μM was determined to be the concentration at which maximal effect was attained for the human type IIB and type III. Steady state levels of fluoxetine in human brain taken in a 50-mg daily dosing scheme are ≈10 μM (22). Purified enzyme was preincubated with one of the above three drugs for 25 min at 37°C before the addition of steroidal precursor and the appropriate cofactor. Radiometric assays were performed in triplicate whereas photometric assays were performed at least six times. Raw data from the above assays were analyzed by using the anemona (23) kinetics program.
Analysis of 5α-Reductase Activity in the Presence of SSRIs.
COS-1 cells transfected with 5α-reductase type I cDNA were incubated with 14C-progesterone in the presence or absence of fluoxetine for 1 hour, 72 hours after cell transfection. Steroid product secreted into the media was collected, was extracted with isooctane:ethyl acetate (1:1, vol:vol), and was assayed by thin layer chromatography. The major secreted product was 14C-5α dihydroprogesterone. Steroid product was quantitated after exposure on a phosphor screen and was analyzed by using a Molecular Dynamics PhosphorImager and the imagequant software program.
Results
Effect of Fluoxetine on Rat 5α-Reductase Activities.
Rat 5α-reductase cDNA was transfected into COS-1 cells to determine whether fluoxetine had an effect on the conversion of progesterone to 5α-dihydroprogesterone. This conversion then was assayed by thin layer chromatography. Analysis of this data showed that there was no alteration in the production of DHP with the addition of fluoxetine (Fig. 1).
Figure 1.
Effect of fluoxetine on 5α-reductase activity. Rat type I 5α-reductase was expressed in COS-1 cells after transfection. Cells were incubated with 14C-progesterone for 1 hour at 37°C, 72 hours after transfection in the presence (+) or absence (−) of 50 μM fluoxetine. Steroid product was extracted and analyzed by thin layer chromatography. Conversion of progesterone (PROG) to DHP was determined by phosphoimager analysis of the thin layer chromatography and was determined to be 43.5 and 42.5% in the absence and the presence of fluoxetine, respectively.
Effect of Fluoxetine on Rat 3α-HSD Activities.
Rat 3α-HSD cDNA was cloned and was expressed in bacteria. The reductive activity of this enzyme was determined by monitoring the conversion of radioactive dihydroprogesterone to allopregnanolone, and its oxidative activity by monitoring the reverse reaction of allopregnanolone to dihydroprogesterone. Enzymatic activity was determined at various doses of substrate. Kms and Vmaxs were determined from the data analyzed by Lineweaver-Burk plots and were confirmed by analysis using the anemona kinetics program.
The data derived from the Lineweaver-Burk plot and other similar plots are shown in Table 1. Table 1 represents the data from the reaction DHP to allopregnanolone. The Km for rat 3α-HSD was ≈59 nM whereas the Vmax was ≈200 nmol/mg protein/min. These values are consistent with the Km and Vmax previously reported by other investigators (24). When fluoxetine was added to the reaction, the Km of the enzyme decreased dramatically to only 0.6 nM: that is, a 100-fold decrement in the Km. This indicates that fluoxetine has dramatically increased the affinity of the enzyme for the substrate DHP. The Vmax for the enzyme decreased 2-fold in the presence of fluoxetine.
Table 1.
Summary of rat 3α-HSD activity
| DHP ⇒ allopregnanolone | |||
|---|---|---|---|
| Km, nM | Vmax, nmol/mg/min | Enzyme efficiency | |
| DHP alone | 59.4 ± 0.08 | 222.4 ± 3.3 | 3.7 |
| (+)fluoxetine | 0.6 ± 0.01 | 103 ± 0.02 | 171.2 |
| (+)paroxetine | 1.6 ± 0.01 | 103 ± 0.14 | 64.3 |
| (+)imipramine | 60 ± 0.02 | 202.7 ± 3.1 | 3.4 |
| Allopregnanolone ⇒ DHP | |||
|---|---|---|---|
| Km, μM | Vmax, nmol/mg/min | Enzyme efficiency | |
| Allo alone | 10.3 ± 0.01 | 29.9 ± 0.01 | 0.003 |
| (+)fluoxetine | 10.6 ± 0.01 | 16.5 ± 0.01 | 0.002 |
| (+)paroxetine | 8.6 ± 0.3 | 11.1 ± 2.7 | 0.001 |
| (+)imipramine | 10.1 ± 0.09 | 11.1 ± 1.0 | 0.001 |
Mean ± SE.
By contrast, when allopregnanolone was used as substrate for the enzyme, the Km was 10.3 μM. This suggests that this enzyme favors the reductive pathway production of allopregnanolone over the oxidative production of DHP because the Km for the reductive pathway is 200× less than for the oxidative pathway. When fluoxetine was added to the reaction, there was no change in the Km of the enzyme.
The efficiency of the enzyme, the ratio of Vmax to Km, then was calculated. The enzymatic efficiency of rat 3α-HSD, in the conversion from DHP to allopregnanolone, was 3.7 and was 0.003 in the conversion of allopregnanolone to DHP. The enzyme efficiency of the reductive reaction increased ≈46-fold in the presence of fluoxetine. Fluoxetine did not alter the oxidative reaction. Thus, fluoxetine dramatically enhances the efficiency of the enzyme, but only in the conversion of DHP to allopregnanolone.
Effect of Other SSRIs on Rat 3α-HSD Activities.
Other selective serotonin reuptake inhibitors, as well as another antidepressant with serotonergic properties, were tested to determine whether they would similarly affect 3α-HSD activity. Our results demonstrate that the SSRI paroxetine also decreased the Km of the enzyme when DHP was used as substrate (from 59 nM to 1.6 nM) and slightly decreased the Km when allopregnanolone was used as substrate (from 10.3 μM to 8.6 μM) (Table 1). The tricyclic imipramine was ineffective in altering the Km or Vmax of either reaction. The enzymatic efficiency of the reductive reaction increased 15-fold in the presence of paroxetine (from 4.4 to 64.3), although it was not changed with imipramine in either direction.
Cloning of Human 3α-HSD cDNAs.
We determined whether fluoxetine could similarly affect the enzymatic activity of the human 3α-HSD. Unlike rodents, which appear to have only a single 3α-HSD isoform, human beings have multiple 3α-HSD isoforms. It was not previously known whether any brain-specific isoforms existed. Therefore, human brain 3α-HSD cDNAs were cloned by using fetal brain RNA. Full-length cDNAs were expressed in bacteria, and their activities were determined. The effects of fluoxetine on these activities then were determined, as had been done for rat 3α-HSD.
Two different human brain 3α-HSD cDNAs were cloned, a type II and a type III. The type III enzyme was identical to a type III enzyme isolated from human prostate (19). We cloned a novel type II 3α-HSD cDNA, which we have designated as IIBrain (Fig. 2). This cDNA also was cloned from adult human brain RNA, indicating that this RNA is expressed in both the fetus and the adult. This new type IIBrain enzyme is 89.8% identical to the type III at the nucleotide level and 87.9% identical at the amino acid level. Furthermore, this novel type II was shown to be 99.7 and 99.3% identical at the nucleotide and amino acid levels, respectively, to the type II isolated from prostate, differing by only two amino acids, at amino acids 38 and 89 (18). Human type IIBrain is 99.5 and 98.7% identical to the human type II isoform from liver, differing by four amino acids at amino acid positions 38, 75, 89, and 175 (21). It also has 85 and 83.9% identity (nucleotide and amino acid, respectively) to the type I isoform, which is liver-specific (21). The type III isoform is in turn 85.7% identical at the amino acid level to the type II-prostate specific form and 97.8% identical to human 20α-hydroxysteroid dehydrogenase. This suggested that type IIBrain might have a substrate specificity intermediate between type II from prostate and type III.
Figure 2.
(A) Nucleotide sequence and the predicted amino acid sequence of the human brain 3α-HSD clone. The ORF contains 969 nucleotides and encodes a protein of 323 amino acids. (B) Comparison of the amino acid sequences of mammalian 3α-HSDs. Only amino acids differing from the human brain type IIBrain 3α(20α, 17β)-HSD sequence are shown. Human type 1 is also chlordecone reductase and DD4 (21, 33); human type 3 is also bile acid binding protein (31); human 20αHSD is also DD1 (34, 35); x, no amino acid (rat 3αHSD is one amino acid shorter than the human forms).
Enzymatic Activities of Human 3α-HSDs.
Human type IIBrain and type III not only differ in sequence but also differ dramatically in their activities. Human 3α-HSD type III and type IIBrain were expressed in bacteria. The Km and Vmax for the human 3α-HSD type III were determined. The Km for the conversion of DHP to allopregnanolone was 7.2 nM, and the Vmax was ≈126 nmol/mg/min (Table 2). Fluoxetine decreased the Km to 0.63nM but did not substantially alter the Vmax. The Km for the conversion of allopregnanolone to DHP was 43 μM, and the Vmax was 7.1 nmol/mg/min. Fluoxetine decreased the Km slightly but increased the Vmax 3-fold. Calculation of the enzymatic efficiency for the conversion of DHP to allopregnanolone showed that fluoxetine increased the efficiency 15-fold whereas the effect on the conversion from allopregnanolone to DHP was 4-fold (Table 2). In contrast to the effect seen with the purified rat 3α-HSD, paroxetine appeared to have a greater effect on enzyme kinetics, as it decreased the Km of the conversion of DHP to allopregnanolone from 7.2 to 0.26 nM, resulting in a 18-fold increase in enzyme efficiency. Paroxetine had a slightly lesser effect on the oxidative reaction, increasing the enzyme efficiency only 3-fold. Sertraline also decreased the Km of the conversion of DHP to allopregnanolone from 7.2 to 0.69 nM, a 10-fold increase in enzyme efficiency. Unlike fluoxetine and paroxetine, sertraline increased the Km of the conversion of allopregnanolone to DHP from 43 to 130.4 μM, a 2.5-fold reduction in oxidative enzyme efficiency. Imipramine had no cumulative effect on the enzyme, either in the oxidative or reductive reaction.
Table 2.
Summary of human type III activity
| DHP ⇒ allopregnanolone | |||
|---|---|---|---|
| Km, nM | Vmax, nmol/mg/min | Enzyme efficiency | |
| DHP alone | 7.2 ± 0.01 | 126 ± 0.44 | 17.5 |
| (+)fluoxetine | 0.63 ± 0.01 | 169.8 ± 0.56 | 269.5 |
| (+)paroxetine | 0.26 ± 0.01 | 82.13 ± 0.69 | 316.5 |
| (+)sertraline | 0.69 ± 0.03 | 120.1 ± 0.57 | 174.1 |
| (+)imipramine | 9.93 ± 0.04 | 114.2 ± 3.53 | 11.5 |
| Allopregnanolone ⇒ DHP | |||
|---|---|---|---|
| Km, μM | Vmax, nmol/mg/min | Enzyme efficiency | |
| Allo alone | 43.0 ± 0.02 | 7.1 ± 0.01 | 0.0002 |
| (+)fluoxetine | 30.9 ± 0.03 | 27.0 ± 0.37 | 0.0008 |
| (+)paroxetine | 15.0 ± 0.01 | 8.9 ± 0.01 | 0.0006 |
| (+)sertraline | 130.4 ± 0.16 | 10.8 ± 0.43 | 0.00008 |
| (+)imipramine | 71.4 ± 0.22 | 39.0 ± 3.33 | 0.0005 |
Mean ± SE.
The effects of fluoxetine, paroxetine, and imipramine on the enzymatic activity of human 3α-HSD type IIBrain also were determined. Unlike human type III, human type IIBrain did not appreciably convert DHP to allopregnanolone or allopregnanolone to DHP. However, the human type IIBrain had considerable 20α-HSD activity and converted progesterone to 20α-dihydroprogesterone (4-pregnen-20α-ol-3, 5-dione). In addition, human type IIBrain possesses 17β-HSD activity and converts androstanediol to androsterone (Fig. 3A). Fluoxetine affected the Km of the 20α-HSD function of the type II enzyme (Table 3). Fluoxetine, but not paroxetine or imipramine, increased the Km of this reaction from 142 to 238 μM, resulting in a slightly less efficient 20α-HSD activity. Thus, fluoxetine slightly inhibits the side reaction: progesterone to 20α-dihydroprogesterone.
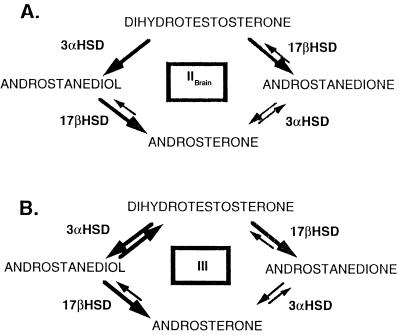
Figure 3.
Schematic representation of the activities of the human type III and type IIBrain 3αHSDs using androgens as substrates. (A) Type IIBrain. (B) Type III. Activities definitively determined by using DHT and androstanediol are shown by thick arrows. Reactions denoted by thin arrows may be catalyzed by the enzymes but were not tested.
Table 3.
Summary of type IIBrain activity with progesterone
| Km, μM | Vmax, nmol/mg/min | Enzyme efficiency | |
|---|---|---|---|
| Prog alone | 142.1 ± 0.16 | 20.1 ± 1.8 | 0.00014 |
| (+)fluoxetine | 238.0 ± 0.02 | 30.0 ± 0.29 | 0.00013 |
| (+)paroxetine | 121.0 ± 0.27 | 18.58 ± 4.4 | 0.00015 |
| (+)imipramine | 149.9 ± 0.39 | 20.85 ± 1.5 | 0.00014 |
Mean ± SE.
Although the type IIBrain isoform did not use progestins as substrates, it did use androgens as substrates. It did not convert DHP to allopregnanolone but converted androgens such as 5α-dihydrotestosterone (5α-androstan-17β-ol-3-one) to androstanediol (5α-androstan-3α, 17β-diol). By comparison, the rat 3α-HSD is a pure 3α-HSD and has no additional activities. The type IIBrain enzyme did not appreciably oxidize androstanediol to DHT; instead, androstanediol was converted to androsterone (5α-androstan-3α-ol-17-one), through the 17β HSD activity of this 3α-HSD. The type III also has 17β-HSD activity as it converts DHT to androstanedione (5α-androstan-3α, 17β-dione) and androstanediol to androsterone (Fig. 3B). The 3α activity of type IIBrain was tested to ascertain whether the SSRIs affected the conversion of androgens in a manner similar to the way SSRIs affected the conversion of progestins by human type III (see above).
Fluoxetine and paroxetine affected the reduction of DHT to androstanediol in a similar manner to the way the conversion of DHP to allopregnanolone was affected by the type III enzyme. However, the conversion of DHT to androstanediol required micromolar concentrations of DHT (Km 2.37 μM) whereas the conversion of DHP to allopregnanolone by the type III enzyme or rat 3αHSD required only nanomolar concentrations of substrate. Both fluoxetine and paroxetine decreased the Km of the enzyme (47-fold and 6-fold, respectively) and also increased the Vmax (3.6-fold and 11-fold) (Table 4). The enzymatic efficiency of the conversion of DHT to androstanediol increased 163-fold when the enzyme was incubated with fluoxetine and 63-fold with paroxetine but did not change substantially with imipramine. These results suggest that both fluoxetine and paroxetine enhance the 3α activity of 3αHSD type IIBrain when androgens are used as a substrate. The 17β-hydroxysteroid dehydrogenase activity of the 3αHSD type IIBrain also was affected by paroxetine. The conversion of androstanediol to androsterone is altered in the presence of paroxetine, with both a 2-fold increase in Km and a 5-fold increase in Vmax. Paroxetine decreases the Km slightly and increases the Vmax 5-fold. Imipramine also appeared to have an effect on the conversion of androstanediol to androsterone, the mechanism for which is unknown.
Table 4.
Summary of IIBrain activity with androgens
| DHT ⇒ androstanediol | |||
|---|---|---|---|
| Km, μM | Vmax, nmol/mg/min | Enzyme efficiency | |
| DHT alone | 2.37 ± 0.01 | 1.8 ± 0.02 | 0.0008 |
| (+)fluoxetine | 0.05 ± 0.07 | 6.5 ± 0.02 | 0.13 |
| (+)paroxetine | 0.39 ± 0.01 | 20.0 ± 0.39 | 0.05 |
| (+)imipramine | 17.8 ± 1.8 | 40.0 ± 0.90 | 0.002 |
| Androstanediol ⇒ androsterone | |||
|---|---|---|---|
| Km, μM | Vmax, nmol/mg/min | Enzyme efficiency | |
| Adiol alone | 1 ± 0.03 | 4.4 ± 0.07 | 0.004 |
| (+)fluoxetine | 7.2 ± 0.05 | 29.1 ± 0.93 | 0.004 |
| (+)paroxetine | 0.46 ± 0.07 | 22.2 ± 0.15 | 0.05 |
| (+)imipramine | 0.21 ± 0.03 | 8.63 ± 0.24 | 0.04 |
Mean ± SE.
Expression of 3α-HSD mRNAs in Human Brain.
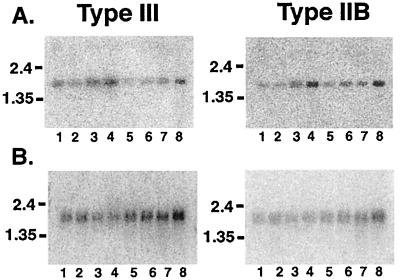
Because there are multiple human 3α-HSDs with dramatically different activities, we determined where these mRNAs were expressed in human brain. Northern blots containing 2 μg of human brain poly(A)+RNA from different regions of adult human brain (unknown) were probed with human type IIBrain- and type III-specific cDNA probes. Our data demonstrate that there was region-specific expression of these mRNAs. Type III mRNA was mainly expressed in cerebellum, medulla, spinal cord, and putamen whereas type IIBrain mRNA was mainly expressed medulla, spinal cord, frontotemporal lobes, and putamen (Fig. 4A). Type III mRNA also was expressed in many of the subcortical nuclei of the brain, including amygdala, caudate, and thalamus, as well as in hippocampus, substantia nigra, and subthalamic nuclei (Fig. 4B). Type IIBrain mRNA appeared to be predominately expressed in thalamus, subthalamic nuclei, and amygdala but was present in lesser degrees in the hippocampus, substantia nigra, and caudate (Fig. 4B).
Figure 4.
Regional distribution of type IIBrain and type III in adult human brain. Northern blots containing 2 μg poly-A+ RNA per lane from human brain were hybridized with a PCR-generated probe corresponding to the less conserved 3′ ends of type IIBrain and type III. (A) Lanes: 1, cerebellum; 2, cortex; 3, medulla; 4, spinal cord; 5, occipital lobe; 6, frontal lobe; 7, temporal lobe; 8, putamen. (B) Lanes: 1, amygdala; 2, caudate nucleus; 3, corpus callosum; 4, hippocampus; 5, whole brain; 6, substantia nigra; 7, subthalamic nucleus; 8, thalamus.
Discussion
Our data show that human beings have multiple forms of 3α-HSD in the brain, with different and distinct enzymatic activities. These experiments directly demonstrate a novel molecular mechanism for specific SSRI action. Fluoxetine, paroxetine, and sertraline increase allopregnanolone production through increased efficiency of conversion of DHP to allopregnanolone. Fluoxetine also may have some effect through the inhibition of a competing pathway (progesterone to 20α-dihydroprogesterone). These experiments show that the actions of fluoxetine, paroxetine, sertraline, and perhaps other SSRIs are 3α-HSD-isoform specific, as paroxetine has a greater effect on the human type III enzyme than the human type II (or rat 3α-HSD) whereas only fluoxetine inhibits the 20α-HSD activity of the type II enzyme. Both fluoxetine and paroxetine also affected the conversion of DHT to androstanediol whereas fluoxetine further affected conversion of androstanediol to androsterone. Both androstanediol and androsterone, like allopregnanolone, may be neuroactive (25, 26, 27). Because the two 3α-HSD isoforms are differentially expressed in specific regions of the human brain, SSRIs may alter neurosteroid production differentially in particular brain regions and thus provide a mechanism for modulation of specific behaviors.
The 3α-, 20α-, and 17β-hydroxysteroid dehydrogenases (HSDs) are part of the aldo-ketoreductase protein superfamily. These proteins are monomeric and are generally 34–39 kDa in size. They share a common (α/β)8-barrel three-dimensional fold and possess a highly conserved nicotinamide-cofactor-binding pocket (28). The aldo-ketoreductases maintain the general barrel scaffold for cofactor and substrate binding and provide for substrate specificity through modification of protein loops near the active site. The newly discovered type IIBrain isoform contains the conserved catalytic tetrad Asp 50, Tyr 55, Lys 84, and His 117 (numbering based on rat 3αHSD sequence) that are common to the other HSDs but lacks a Tyr-X-X-X-Lys motif that is found in the short-chain dehydrogenase/reductase superfamily. The human type IIBrain isoform differs from the prostate type II isoform at amino acids 38 and 89. The first position (amino acid 89) has been shown by site-directed mutagenesis¶ to be important for conferring both 3α- and 20α-HSD activity on the protein. The prostate isoform was not noted to have 20α-HSD activity (18). Type IIBrain also differs from the type II liver isoform at these two positions as well as amino acids 75 and 175. Positions 75, 85, and 175 are not part of the catalytic tetrad but instead appear to be in the loops on the C-terminal side of the barrel that are thought to be responsible for determining the stereospecificity of the HSDs (28). All three type II isoforms differ substantially from the type III enzyme, with the majority of those amino acid changes occurring in the 3′ end of the protein, or the region that would be crucial for discrimination among substrates.
The specific mechanism by which the SSRIs alter the enzyme kinetics of the three 3α- HSDs tested here is currently unknown. There are, however, several possible mechanisms. The human type I 3α-HSD isoform has been shown to be activated by sulfobromophthalein, an agent that is used for testing liver function (29). It is thought that this compound activates the enzyme by binding to both the enzyme and its binary complex and inducing a conformational change in the active site of the enzyme. In this instance and in other cases of activation of aldo-ketoreductase proteins (30, 31), the stimulatory anions are thought to interact with Lys-262 and weaken the binding between the protein and the 2′-phosphate group of NADPH, leading to the rapid release of product, and the alterations in Km. It is possible that the fluoride groups of both paroxetine and fluoxetine function in a similar manner. Alternatively, paroxetine and fluoxetine may facilitate proton donation or removal by Tyr-55 by altering the pKb or pKa of this residue. Mutational analysis of the amino acid residues of the catalytic tetrad indicates that Tyr-55 is the major contributor to enzyme rate enhancement, as it functions as the general acid/base in catalysis (32). In addition, the mechanism by which sertraline acts may be different from that of paroxetine and fluoxetine, as we show that sertraline both augments the forward reductive reaction and inhibits the reverse, oxidative, reaction.
The preferential use of androgens by the type IIBrain isoform suggests potential new roles for androgens in the brain. The role of androgens in behaviors other than those that are sex-related has not been extensively explored. Androsterone and androstanediol, like the 3α, 5α reduced products of progesterone metabolism, might act as positive allosteric modulators of the GABAA receptor (24, 25, 26) and may, like the progestins, affect GABA-associated behaviors. The discovery of this human brain isoform of 3αHSD and its dramatic response to the SSRIs suggests that androgens could play a role in affective disorders such as unipolar depression. In addition, the presence of an androgen-specific 3αHSD may be important for the conversion of active steroid hormone into inactive metabolites at the androgen receptor.
We demonstrate here a mechanism by which certain SSRIs may act in brain—that is, by increasing neurosteroid production in the human brain and thereby potentially modulating GABA- associated behaviors. This work suggests that dysregulation of neurosteroidogenesis in humans could represent an important etiology of certain affective disorders, such as late luteal phase dysphoria disorder in women or unipolar depression in women or men. Our ability to understand this novel, additional action of SSRIs on modulation of neurosteroidogenic enzymatic activity may now enable us to design specific compounds that differentially affect these enzymes, and therefore provide more efficacious treatment of mood disorders.
Acknowledgments
We thank Ms. Casey Brown for excellent technical assistance. This work was supported by National Institutes of Health Grants HD27970 (to S.H.M.) and NS01979 (to L.D.G.) and by a grant from the National Alliance for Research on Schizophrenia and Depression (to S.H.M.).
Abbreviations
- 3α-HSD
3α hydroxysteroid dehydrogenase
- DHP
5α-dihydroprogesterone
- DHT
5α-dihydrotestosterone
- GABAA
γ-aminobutyric acid type A
- SSRI
selective serotonin reuptake inhibitor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AF149416).
Dufort, I., Robert, A. & Luv-The, V., Eighth Adrenal Cortex Conference, June 13–16, 1998, Orford, QC, Canada.
References
- 1.Compagnone, N. A. & Mellon, S. H. (1999) Front. Neuroendocrinol., in press. [DOI] [PubMed]
- 2.Mensah-Nyagan A G, Do-Rego J-L, Luu-The V, Pelletier G, Vaudry H. Pharmacol Rev. 1999;51:63–81. [PubMed] [Google Scholar]
- 3.Baulieu E E. Biol Cell. 1991;71:3–10. doi: 10.1016/0248-4900(91)90045-o. [DOI] [PubMed] [Google Scholar]
- 4.Mellon S H. J Clin Endocrinol Metab. 1994;78:1003–1008. doi: 10.1210/jcem.78.5.8175951. [DOI] [PubMed] [Google Scholar]
- 5.Harrison N L, Simmonds M A. Brain Res. 1984;323:287–292. doi: 10.1016/0006-8993(84)90299-3. [DOI] [PubMed] [Google Scholar]
- 6.Majewska M D, Harrison N L, Schwartz R D, Barker J L, Paul S M. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 7.Bixo M, Andersson A, Winblad B, Purdy R H, Backstrom T. Brain Res. 1997;764:173–178. doi: 10.1016/s0006-8993(97)00455-1. [DOI] [PubMed] [Google Scholar]
- 8.Korneyev A, Guidotti A, Costa E. J Neurochem. 1994;61:2041–2047. doi: 10.1111/j.1471-4159.1993.tb07440.x. [DOI] [PubMed] [Google Scholar]
- 9.Milewich L, Gomez-Sanchez C, Crowley G, Porter J C, Madden J D, MacDonald P C. J Clin Endocrinol Metab. 1977;45:617–622. doi: 10.1210/jcem-45-4-617. [DOI] [PubMed] [Google Scholar]
- 10.Lewis L L. Nurs Res. 1995;44:111–116. [PubMed] [Google Scholar]
- 11.Wang M, Seippel L, Purdy R H, Backstrom T. J Clin Endocrinol Metab. 1996;81:1076–1082. doi: 10.1210/jcem.81.3.8772579. [DOI] [PubMed] [Google Scholar]
- 12.Steiner M, Steinberg S, Stewart D, Carter D, Berger C, Reid R, Grover D, Streiner D. N Engl J Med. 1995;332:1529–1534. doi: 10.1056/NEJM199506083322301. [DOI] [PubMed] [Google Scholar]
- 13.Su T-P, Schmidt P J, Danaceau M A, Tobin M B, Rosenstein D L, Murphy D L, Rubinow D R. Neuropsychopharmacology. 1997;16:346–356. doi: 10.1016/S0893-133X(96)00245-X. [DOI] [PubMed] [Google Scholar]
- 14.Uzunov D P, Cooper T B, Costa E, Guidotti A. Proc Natl Acad Sci USA. 1996;93:12599–12604. doi: 10.1073/pnas.93.22.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uzunova V, Sheline Y, Davis M, Rasmusson A, Uzunov P, Costa A, Guidotti A. Proc Natl Acad Sci USA. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson J A, Karavolas H J. Endocrinology. 1973;93:430–435. doi: 10.1210/endo-93-2-430. [DOI] [PubMed] [Google Scholar]
- 17.Cheng K-C, White P C, Qin K-N. Mol Endocrinol. 1991;5:823–828. doi: 10.1210/mend-5-6-823. [DOI] [PubMed] [Google Scholar]
- 18.Lin H-K, Jez J M, Schlegel B P, Peehl D M, Pachter J A, Penning T M. Mol Endocrinol. 1997;11:1971–1984. doi: 10.1210/mend.11.13.0026. [DOI] [PubMed] [Google Scholar]
- 19.Dufort I, Soucy P, Labrie F, Luu-The V. Biochem Biophys Res Commun. 1996;228:474–479. doi: 10.1006/bbrc.1996.1684. [DOI] [PubMed] [Google Scholar]
- 20.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 21.Khanna M, Qin K-N, Wang R, Cheng K-C. J Biol Chem. 1995;270:20162–20168. doi: 10.1074/jbc.270.34.20162. [DOI] [PubMed] [Google Scholar]
- 22.Altamura A C, Moro A R, Percudani M. Clin Pharmacokinet. 1994;26:201–214. doi: 10.2165/00003088-199426030-00004. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez A, Ruiz M T. Bioinformatics. 1998;14:27–28. doi: 10.1093/bioinformatics/14.2.227. [DOI] [PubMed] [Google Scholar]
- 24.Karavolas H J, Hodges D R. In: Neurosteroids and Brain Function. Costa E, Paul S M, editors. New York: Thieme; 1991. pp. 135–145. [Google Scholar]
- 25.Bitran D, Hilvers R J, Frye C A, Erskine M S. Life Sci. 1996;58:573–583. doi: 10.1016/0024-3205(95)02326-7. [DOI] [PubMed] [Google Scholar]
- 26.Kellogg C K, Olson V G, Pleger G L. Brain Res Dev Brain Res. 1998;108:131–137. doi: 10.1016/s0165-3806(98)00042-x. [DOI] [PubMed] [Google Scholar]
- 27.Wilson M A, Biscardi R. Life Sci. 1997;60:1679–1691. doi: 10.1016/s0024-3205(97)00110-0. [DOI] [PubMed] [Google Scholar]
- 28.Jez J M, Bennett M J, Schlegel B P, Lewis M, Penning T M. Biochem J. 1997;326:625–636. doi: 10.1042/bj3260625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuura K, Tamada Y, Isawa H, Miwa G, Deyashiki Y, Hara A. Biochem J. 1997;322:89–93. doi: 10.1042/bj3220089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrison D H, Bohren K M, Ringe D, Petsko G A, Gabbay K H. Biochemistry. 1994;33:2011–2020. doi: 10.1021/bi00174a006. [DOI] [PubMed] [Google Scholar]
- 31.Bohren K M, Page J L, Shanklar R, Henry S P, Gabbay K H. J Biol Chem. 1991;266:24031–24037. [PubMed] [Google Scholar]
- 32.Schlegel B P, Jez J M, Penning T M. Biochemistry. 1998;37:3538–3548. doi: 10.1021/bi9723055. [DOI] [PubMed] [Google Scholar]
- 33.Deyashiki Y, Ogasawara A, Nakayama T, Nakashani M, Miyabe Y, Sato K, Hara A. Biochem J. 1994;299:545–552. doi: 10.1042/bj2990545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoltz A, Hammond L, Lou H, Takikawa H, Ronk M, Shively J E. J Biol Chem. 1993;268:10448–10457. [PubMed] [Google Scholar]
- 35.Hara A, Matsuura K, Tamada Y, Sato K, Miyabe Y, Deyashiki Y, Ishida N. Biochem J. 1996;313:373–376. doi: 10.1042/bj3130373. [DOI] [PMC free article] [PubMed] [Google Scholar]