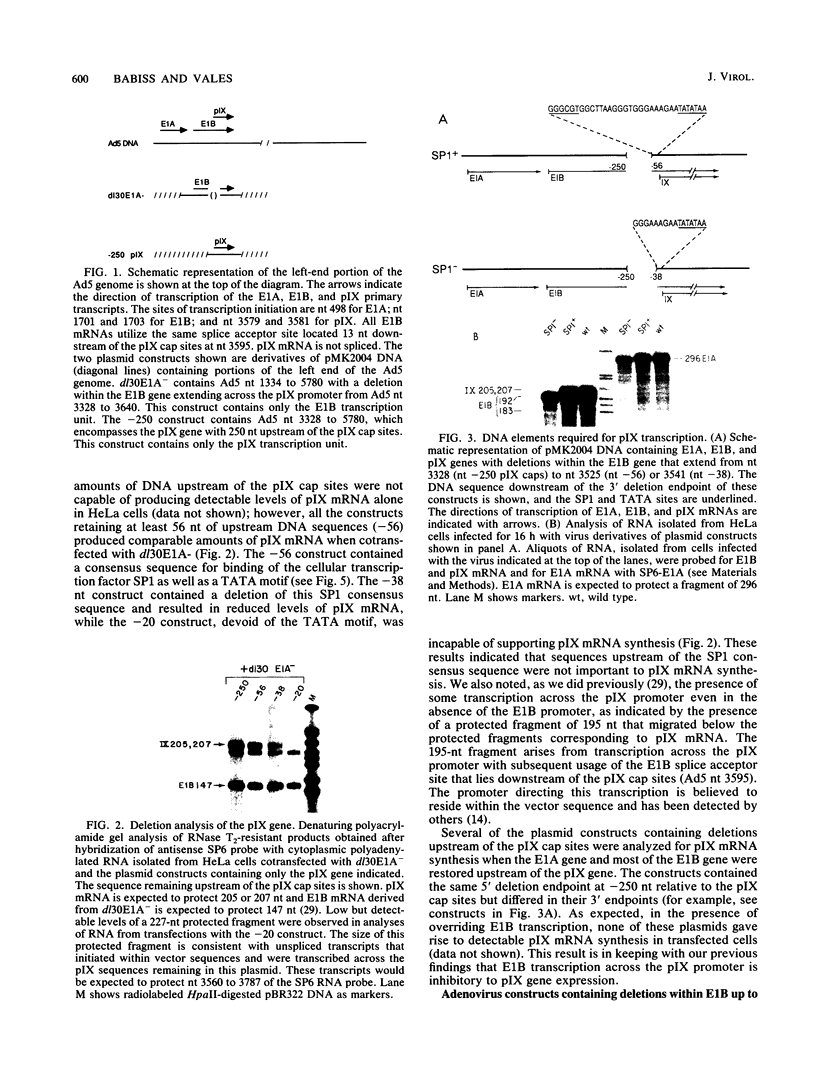
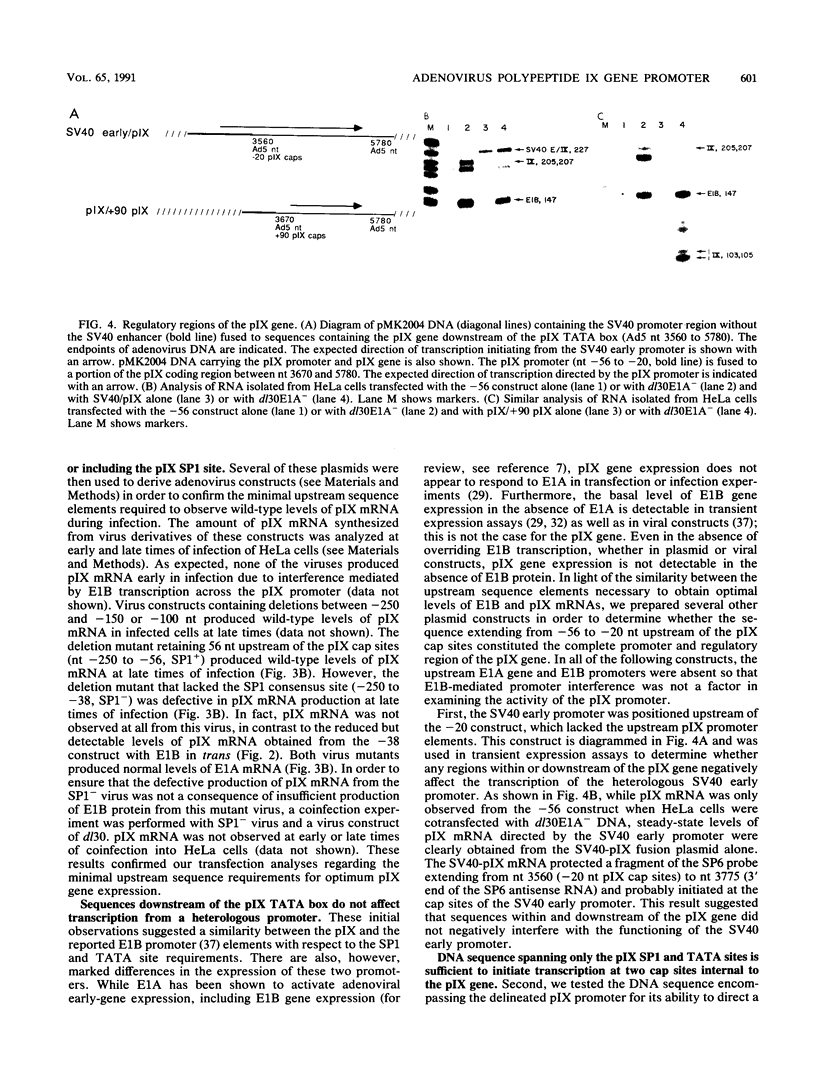
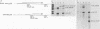Abstract
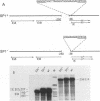
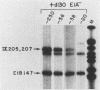
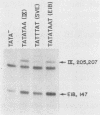
The promoter of the adenovirus polypeptide IX (pIX) gene consists of an SP1 binding site and a TATA box and is remarkably similar to the promoter of the E1B gene in which it is nested. Plasmid constructs containing the pIX gene with deletions in the SP1 or TATA sites were defective in pIX mRNA production in transient expression assays. These results were confirmed with analogous virus constructs. An oligonucleotide containing sequences within the pIX promoter region spanning the SP1 and TATA sites but not including the sequences downstream of the TATA box is sufficient to direct mRNA synthesis at +90 nucleotides within the pIX gene. While the simian virus 40 (SV40) early promoter is capable of directing pIX mRNA synthesis from the SV40 cap sites, substitution of the pIX TATA box with the SV40 TATA box results in barely detectable levels of pIX mRNA. These results will be discussed with respect to exchangeability of promoter elements and the possible role of the viral E1B 21-kDa protein in potentiating or stabilizing transcription factor TFIID binding to the pIX TATA element.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M. Promoter occlusion: transcription through a promoter may inhibit its activity. Cell. 1982 Jul;29(3):939–944. doi: 10.1016/0092-8674(82)90456-1. [DOI] [PubMed] [Google Scholar]
- Aleström P., Akusjärvi G., Perricaudet M., Mathews M. B., Klessig D. F., Pettersson U. The gene for polypeptide IX of adenovirus type 2 and its unspliced messenger RNA. Cell. 1980 Mar;19(3):671–681. doi: 10.1016/s0092-8674(80)80044-4. [DOI] [PubMed] [Google Scholar]
- Babiss L. E., Ginsberg H. S. Adenovirus type 5 early region 1b gene product is required for efficient shutoff of host protein synthesis. J Virol. 1984 Apr;50(1):202–212. doi: 10.1128/jvi.50.1.202-212.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman E., Paule M. R. Promoter occlusion during ribosomal RNA transcription. Cell. 1988 Sep 23;54(7):985–992. doi: 10.1016/0092-8674(88)90113-4. [DOI] [PubMed] [Google Scholar]
- Benoist C., Chambon P. In vivo sequence requirements of the SV40 early promotor region. Nature. 1981 Mar 26;290(5804):304–310. doi: 10.1038/290304a0. [DOI] [PubMed] [Google Scholar]
- Bergsma D. J., Olive D. M., Hartzell S. W., Subramanian K. N. Territorial limits and functional anatomy of the simian virus 40 replication origin. Proc Natl Acad Sci U S A. 1982 Jan;79(2):381–385. doi: 10.1073/pnas.79.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J. Adenovirus promoters and E1A transactivation. Annu Rev Genet. 1986;20:45–79. doi: 10.1146/annurev.ge.20.120186.000401. [DOI] [PubMed] [Google Scholar]
- Brunet L. J., Babiss L. E., Young C. S., Mills D. R. Mutations in the adenovirus major late promoter: effects on viability and transcription during infection. Mol Cell Biol. 1987 Mar;7(3):1091–1100. doi: 10.1128/mcb.7.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnadurai G., Chinnadurai S., Green M. Enhanced infectivity of adenovirus type 2 DNA and a DNA-protein complex. J Virol. 1978 Apr;26(1):195–199. doi: 10.1128/jvi.26.1.195-199.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly S., Manley J. L. A CCAAT box sequence in the adenovirus major late promoter functions as part of an RNA polymerase II termination signal. Cell. 1989 May 19;57(4):561–571. doi: 10.1016/0092-8674(89)90126-8. [DOI] [PubMed] [Google Scholar]
- Corbin V., Maniatis T. Role of transcriptional interference in the Drosophila melanogaster Adh promoter switch. Nature. 1989 Jan 19;337(6204):279–282. doi: 10.1038/337279a0. [DOI] [PubMed] [Google Scholar]
- Crossland L. D., Raskas H. J. Identification of adenovirus genes that require template replication for expression. J Virol. 1983 Jun;46(3):737–748. doi: 10.1128/jvi.46.3.737-748.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R., Lomedico P. T., Ju G. Transcriptional interference in avian retroviruses--implications for the promoter insertion model of leukaemogenesis. Nature. 1984 Jan 19;307(5948):241–245. doi: 10.1038/307241a0. [DOI] [PubMed] [Google Scholar]
- Fuhrman S. A., Van Beveren C., Verma I. M. Identification of a RNA polymerase II initiation site in the long terminal repeat of Moloney murine leukemia viral DNA. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5411–5415. doi: 10.1073/pnas.78.9.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh-Choudhury G., Haj-Ahmad Y., Graham F. L. Protein IX, a minor component of the human adenovirus capsid, is essential for the packaging of full length genomes. EMBO J. 1987 Jun;6(6):1733–1739. doi: 10.1002/j.1460-2075.1987.tb02425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn S., Buratowski S., Sharp P. A., Guarente L. Yeast TATA-binding protein TFIID binds to TATA elements with both consensus and nonconsensus DNA sequences. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5718–5722. doi: 10.1073/pnas.86.15.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson S. L., Ryan K., Sollner-Webb B. The promoter-proximal rDNA terminator augments initiation by preventing disruption of the stable transcription complex caused by polymerase read-in. Genes Dev. 1989 Feb;3(2):212–223. doi: 10.1101/gad.3.2.212. [DOI] [PubMed] [Google Scholar]
- Herrmann C. H., Mathews M. B. The adenovirus E1B 19-kilodalton protein stimulates gene expression by increasing DNA levels. Mol Cell Biol. 1989 Dec;9(12):5412–5423. doi: 10.1128/mcb.9.12.5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. C., Rigby P. W., Ziff E. B. Trans-acting protein factors and the regulation of eukaryotic transcription: lessons from studies on DNA tumor viruses. Genes Dev. 1988 Mar;2(3):267–281. doi: 10.1101/gad.2.3.267. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Matsui T. Novel regulation of transcription initiation of the peptide IX gene of adenovirus 2. Mol Cell Biol. 1989 Oct;9(10):4265–4271. doi: 10.1128/mcb.9.10.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McStay B., Reeder R. H. An RNA polymerase I termination site can stimulate the adjacent ribosomal gene promoter by two distinct mechanisms in Xenopus laevis. Genes Dev. 1990 Jul;4(7):1240–1251. doi: 10.1101/gad.4.7.1240. [DOI] [PubMed] [Google Scholar]
- Mermelstein F. H., Flores O., Reinberg D. Initiation of transcription by RNA polymerase II. Biochim Biophys Acta. 1989 Sep 21;1009(1):1–10. doi: 10.1016/0167-4781(89)90071-7. [DOI] [PubMed] [Google Scholar]
- Montell C., Fisher E. F., Caruthers M. H., Berk A. J. Control of adenovirus E1B mRNA synthesis by a shift in the activities of RNA splice sites. Mol Cell Biol. 1984 May;4(5):966–972. doi: 10.1128/mcb.4.5.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. Definition and mapping of adenovirus 2 nuclear transcription. Methods Enzymol. 1980;65(1):768–785. doi: 10.1016/s0076-6879(80)65072-1. [DOI] [PubMed] [Google Scholar]
- Pilder S., Logan J., Shenk T. Deletion of the gene encoding the adenovirus 5 early region 1b 21,000-molecular-weight polypeptide leads to degradation of viral and host cell DNA. J Virol. 1984 Nov;52(2):664–671. doi: 10.1128/jvi.52.2.664-671.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. C., Fisch T. M., Benecke B. J., Nevins J. R., Heintz N. Definition of multiple, functionally distinct TATA elements, one of which is a target in the hsp70 promoter for E1A regulation. Cell. 1988 Mar 11;52(5):723–729. doi: 10.1016/0092-8674(88)90410-2. [DOI] [PubMed] [Google Scholar]
- Subramanian T., Kuppuswamy M., Gysbers J., Mak S., Chinnadurai G. 19-kDa tumor antigen coded by early region E1b of adenovirus 2 is required for efficient synthesis and for protection of viral DNA. J Biol Chem. 1984 Oct 10;259(19):11777–11783. [PubMed] [Google Scholar]
- Vales L. D., Darnell J. E., Jr Promoter occlusion prevents transcription of adenovirus polypeptide IX mRNA until after DNA replication. Genes Dev. 1989 Jan;3(1):49–59. doi: 10.1101/gad.3.1.49. [DOI] [PubMed] [Google Scholar]
- Volkert F. C., Young C. S. The genetic analysis of recombination using adenovirus overlapping terminal DNA fragments. Virology. 1983 Feb;125(1):175–193. doi: 10.1016/0042-6822(83)90072-7. [DOI] [PubMed] [Google Scholar]
- Wefald F. C., Devlin B. H., Williams R. S. Functional heterogeneity of mammalian TATA-box sequences revealed by interaction with a cell-specific enhancer. Nature. 1990 Mar 15;344(6263):260–262. doi: 10.1038/344260a0. [DOI] [PubMed] [Google Scholar]
- White E., Grodzicker T., Stillman B. W. Mutations in the gene encoding the adenovirus early region 1B 19,000-molecular-weight tumor antigen cause the degradation of chromosomal DNA. J Virol. 1984 Nov;52(2):410–419. doi: 10.1128/jvi.52.2.410-419.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. C., Fraser N. W., Darnell J. E., Jr Mapping of RNA initiation sites by high doses of uv irradiation: evidence for three independent promoters within the left 11% of the Ad-2 genome. Virology. 1979 Apr 15;94(1):175–184. doi: 10.1016/0042-6822(79)90447-1. [DOI] [PubMed] [Google Scholar]
- Wu L., Berk A. Constraints on spacing between transcription factor binding sites in a simple adenovirus promoter. Genes Dev. 1988 Apr;2(4):403–411. doi: 10.1101/gad.2.4.403. [DOI] [PubMed] [Google Scholar]
- Wu L., Rosser D. S., Schmidt M. C., Berk A. A TATA box implicated in E1A transcriptional activation of a simple adenovirus 2 promoter. Nature. 1987 Apr 2;326(6112):512–515. doi: 10.1038/326512a0. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Venkatesh L., Kuppuswamy M., Chinnadurai G. Adenovirus transforming 19-kD T antigen has an enhancer-dependent trans-activation function and relieves enhancer repression mediated by viral and cellular genes. Genes Dev. 1987 Sep;1(7):645–658. doi: 10.1101/gad.1.7.645. [DOI] [PubMed] [Google Scholar]