Abstract
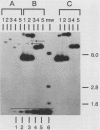
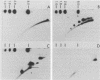
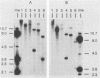
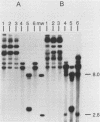
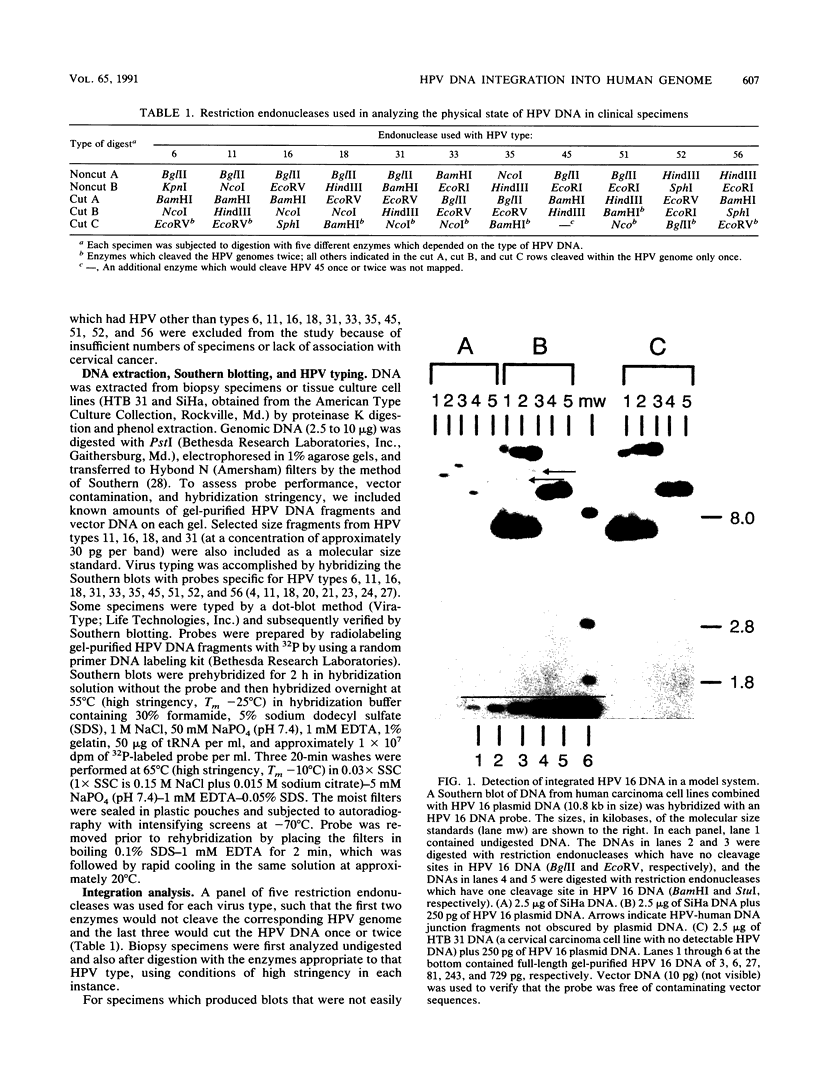
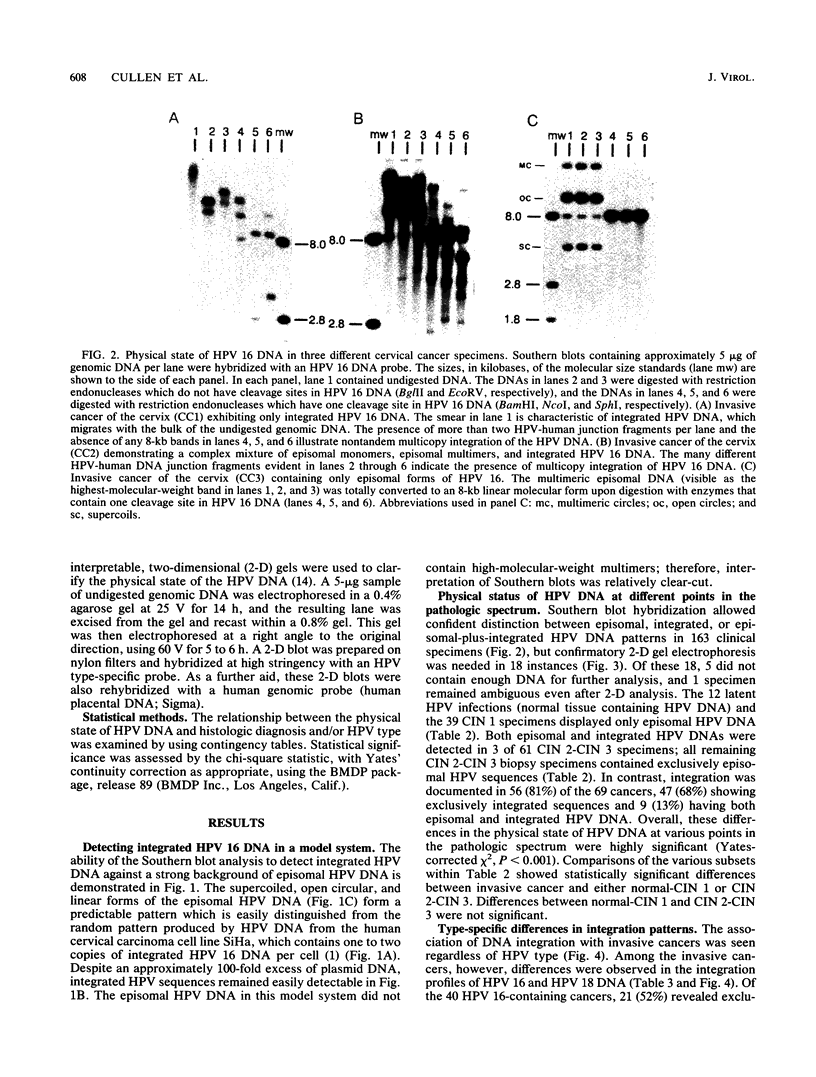
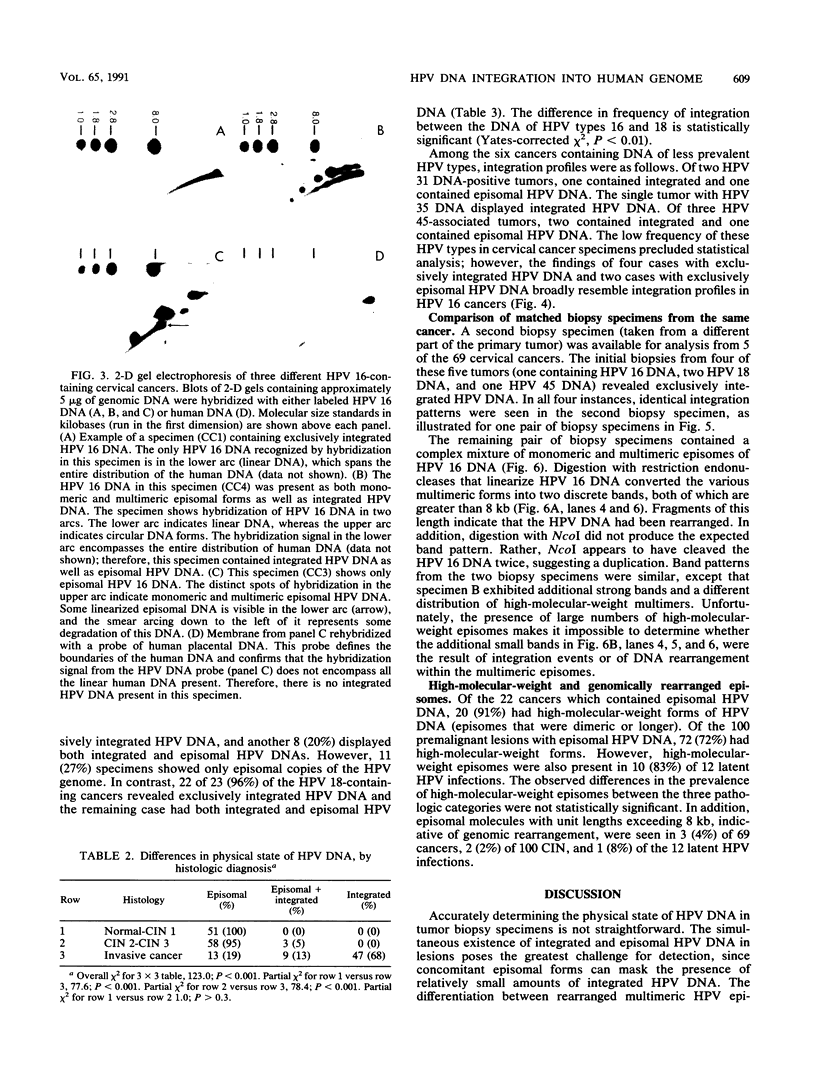
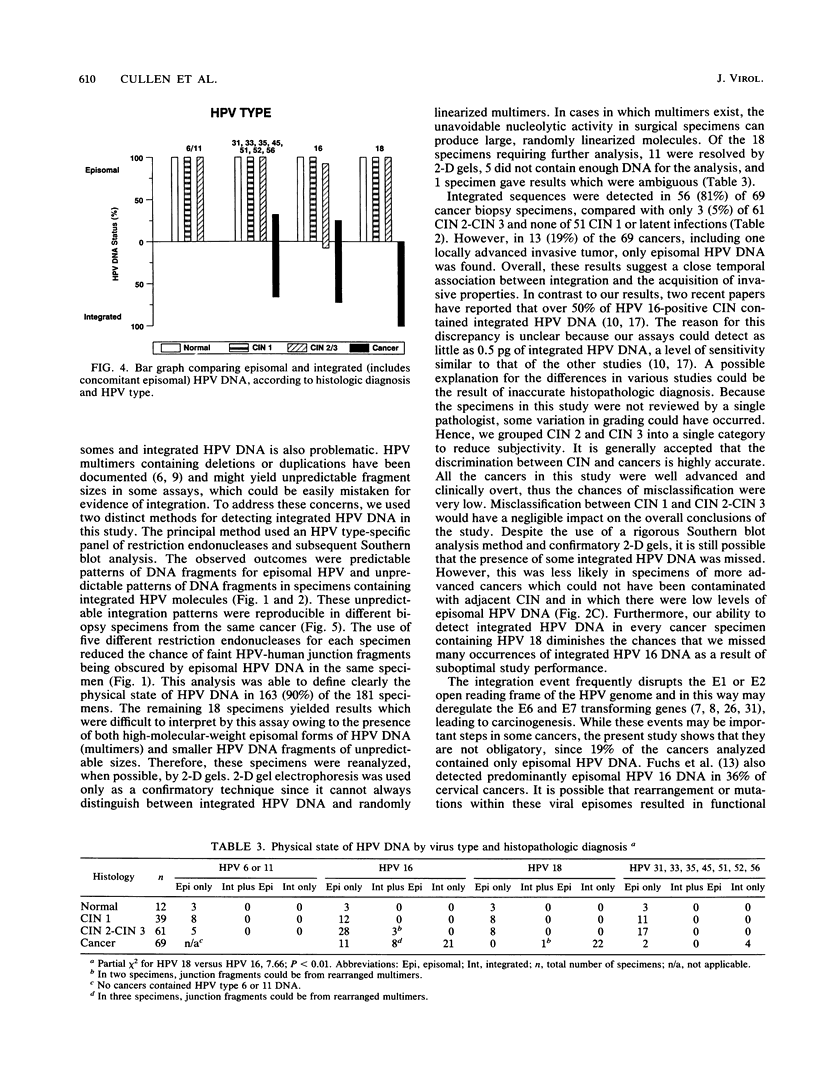
The integration of human papillomavirus (HPV) DNA into the human genome has been generally accepted as a characteristic of malignant lesions. To gain a better understanding of this phenomenon, genomic DNA from 181 cervical biopsy specimens was isolated and analyzed for HPV type and physical state of the HPV genome. These specimens represented the full spectrum of cervical disease, from condyloma to invasive carcinoma. Discrimination between integrated and episomal HPV DNA was accomplished by the detection of HPV-human DNA junction fragments on Southern blots. In most cases in which ambiguous Southern blot results were obtained, the specimens were reanalyzed by two-dimensional gel electrophoresis. Of the 100 biopsy specimens of cervical intraepithelial neoplasia analyzed, only 3 showed integrated HPV DNA, in contrast to 56 (81%) of 69 cervical carcinomas (P less than 0.001) showing integrated HPV DNA. Of the 40 carcinomas containing HPV 16 DNA, 29 (72%) had integrated HPV DNA, of which 8 (20%) also had episomal HPV DNA. In 11 (27%) cancers, only episomal HPV 16 DNA was detected. All 23 HPV 18-containing carcinomas had integrated HPV DNA, and 1 also had episomal HPV 18 DNA. The difference between HPV types 16 and 18 with respect to frequency of integration was statistically significant (P less than 0.01). The results of this study indicate that detectable integration of HPV DNA, regardless of type, occurs infrequently in cervical intraepithelial neoplasia. The absence of HPV 16 DNA integration in some carcinomas implies that integration is not always required for malignant progression. In contrast, the consistent integration of HPV 18 DNA in all cervical cancers examined may be related to its greater transforming efficiency in vitro and its reported clinical association with more aggressive cervical cancers.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbosa M. S., Schlegel R. The E6 and E7 genes of HPV-18 are sufficient for inducing two-stage in vitro transformation of human keratinocytes. Oncogene. 1989 Dec;4(12):1529–1532. [PubMed] [Google Scholar]
- Barnes W., Delgado G., Kurman R. J., Petrilli E. S., Smith D. M., Ahmed S., Lorincz A. T., Temple G. F., Jenson A. B., Lancaster W. D. Possible prognostic significance of human papillomavirus type in cervical cancer. Gynecol Oncol. 1988 Mar;29(3):267–273. doi: 10.1016/0090-8258(88)90225-9. [DOI] [PubMed] [Google Scholar]
- Beaudenon S., Kremsdorf D., Croissant O., Jablonska S., Wain-Hobson S., Orth G. A novel type of human papillomavirus associated with genital neoplasias. Nature. 1986 May 15;321(6067):246–249. doi: 10.1038/321246a0. [DOI] [PubMed] [Google Scholar]
- Beaudenon S., Kremsdorf D., Obalek S., Jablonska S., Pehau-Arnaudet G., Croissant O., Orth G. Plurality of genital human papillomaviruses: characterization of two new types with distinct biological properties. Virology. 1987 Dec;161(2):374–384. doi: 10.1016/0042-6822(87)90130-9. [DOI] [PubMed] [Google Scholar]
- Choo K. B., Cheung W. F., Liew L. N., Lee H. H., Han S. H. Presence of catenated human papillomavirus type 16 episomes in a cervical carcinoma cell line. J Virol. 1989 Feb;63(2):782–789. doi: 10.1128/jvi.63.2.782-789.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo K. B., Lee H. H., Pan C. C., Wu S. M., Liew L. N., Cheung W. F., Han S. H. Sequence duplication and internal deletion in the integrated human papillomavirus type 16 genome cloned from a cervical carcinoma. J Virol. 1988 May;62(5):1659–1666. doi: 10.1128/jvi.62.5.1659-1666.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo K. B., Pan C. C., Han S. H. Integration of human papillomavirus type 16 into cellular DNA of cervical carcinoma: preferential deletion of the E2 gene and invariable retention of the long control region and the E6/E7 open reading frames. Virology. 1987 Nov;161(1):259–261. doi: 10.1016/0042-6822(87)90195-4. [DOI] [PubMed] [Google Scholar]
- Di Luca D., Caselli E., Monini P., Rotola A., Savioli A., Cassai E. Episomal HPV 16 DNA isolated from a cervical carcinoma presents a partial duplication of the early region. Virus Res. 1989 Sep;14(1):49–55. doi: 10.1016/0168-1702(89)90068-3. [DOI] [PubMed] [Google Scholar]
- Di Luca D., Pilotti S., Stefanon B., Rotola A., Monini P., Tognon M., De Palo G., Rilke F., Cassai E. Human papillomavirus type 16 DNA in genital tumours: a pathological and molecular analysis. J Gen Virol. 1986 Mar;67(Pt 3):583–589. doi: 10.1099/0022-1317-67-3-583. [DOI] [PubMed] [Google Scholar]
- Dürst M., Gissmann L., Ikenberg H., zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürst M., Kleinheinz A., Hotz M., Gissmann L. The physical state of human papillomavirus type 16 DNA in benign and malignant genital tumours. J Gen Virol. 1985 Jul;66(Pt 7):1515–1522. doi: 10.1099/0022-1317-66-7-1515. [DOI] [PubMed] [Google Scholar]
- Fuchs P. G., Girardi F., Pfister H. Human papillomavirus 16 DNA in cervical cancers and in lymph nodes of cervical cancer patients: a diagnostic marker for early metastases? Int J Cancer. 1989 Jan 15;43(1):41–44. doi: 10.1002/ijc.2910430110. [DOI] [PubMed] [Google Scholar]
- Johnson P. H., Grossman L. I. Electrophoresis of DNA in agarose gels. Optimizing separations of conformational isomers of double- and single-stranded DNAs. Biochemistry. 1977 Sep 20;16(19):4217–4225. doi: 10.1021/bi00638a014. [DOI] [PubMed] [Google Scholar]
- Kurman R. J., Schiffman M. H., Lancaster W. D., Reid R., Jenson A. B., Temple G. F., Lorincz A. T. Analysis of individual human papillomavirus types in cervical neoplasia: a possible role for type 18 in rapid progression. Am J Obstet Gynecol. 1988 Aug;159(2):293–296. doi: 10.1016/s0002-9378(88)80070-x. [DOI] [PubMed] [Google Scholar]
- Lehn H., Krieg P., Sauer G. Papillomavirus genomes in human cervical tumors: analysis of their transcriptional activity. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5540–5544. doi: 10.1073/pnas.82.16.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehn H., Villa L. L., Marziona F., Hilgarth M., Hillemans H. G., Sauer G. Physical state and biological activity of human papillomavirus genomes in precancerous lesions of the female genital tract. J Gen Virol. 1988 Jan;69(Pt 1):187–196. doi: 10.1099/0022-1317-69-1-187. [DOI] [PubMed] [Google Scholar]
- Lorincz A. T., Quinn A. P., Lancaster W. D., Temple G. F. A new type of papillomavirus associated with cancer of the uterine cervix. Virology. 1987 Jul;159(1):187–190. doi: 10.1016/0042-6822(87)90366-7. [DOI] [PubMed] [Google Scholar]
- Lorincz A. T., Temple G. F., Kurman R. J., Jenson A. B., Lancaster W. D. Oncogenic association of specific human papillomavirus types with cervical neoplasia. J Natl Cancer Inst. 1987 Oct;79(4):671–677. [PubMed] [Google Scholar]
- Lörincz A. T., Quinn A. P., Goldsborough M. D., McAllister P., Temple G. F. Human papillomavirus type 56: a new virus detected in cervical cancers. J Gen Virol. 1989 Nov;70(Pt 11):3099–3104. doi: 10.1099/0022-1317-70-11-3099. [DOI] [PubMed] [Google Scholar]
- Lörincz A. T., Quinn A. P., Goldsborough M. D., Schmidt B. J., Temple G. F. Cloning and partial DNA sequencing of two new human papillomavirus types associated with condylomas and low-grade cervical neoplasia. J Virol. 1989 Jun;63(6):2829–2834. doi: 10.1128/jvi.63.6.2829-2834.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukura T., Koi S., Sugase M. Both episomal and integrated forms of human papillomavirus type 16 are involved in invasive cervical cancers. Virology. 1989 Sep;172(1):63–72. doi: 10.1016/0042-6822(89)90107-4. [DOI] [PubMed] [Google Scholar]
- Naghashfar Z. S., Rosenshein N. B., Lorincz A. T., Buscema J., Shah K. V. Characterization of human papillomavirus type 45, a new type 18-related virus of the genital tract. J Gen Virol. 1987 Dec;68(Pt 12):3073–3079. doi: 10.1099/0022-1317-68-12-3073. [DOI] [PubMed] [Google Scholar]
- Nuovo G. J., Crum C. P., De Villiers E. M., Levine R. U., Silverstein S. J. Isolation of a novel human papillomavirus (type 51) from a cervical condyloma. J Virol. 1988 Apr;62(4):1452–1455. doi: 10.1128/jvi.62.4.1452-1455.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid R., Greenberg M., Jenson A. B., Husain M., Willett J., Daoud Y., Temple G., Stanhope C. R., Sherman A. I., Phibbs G. D. Sexually transmitted papillomaviral infections. I. The anatomic distribution and pathologic grade of neoplastic lesions associated with different viral types. Am J Obstet Gynecol. 1987 Jan;156(1):212–222. doi: 10.1016/0002-9378(87)90241-9. [DOI] [PubMed] [Google Scholar]
- Shimoda K., Lorincz A. T., Temple G. F., Lancaster W. D. Human papillomavirus type 52: a new virus associated with cervical neoplasia. J Gen Virol. 1988 Nov;69(Pt 11):2925–2928. doi: 10.1099/0022-1317-69-11-2925. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Walker J., Bloss J. D., Liao S. Y., Berman M., Bergen S., Wilczynski S. P. Human papillomavirus genotype as a prognostic indicator in carcinoma of the uterine cervix. Obstet Gynecol. 1989 Nov;74(5):781–785. [PubMed] [Google Scholar]
- Yee C., Krishnan-Hewlett I., Baker C. C., Schlegel R., Howley P. M. Presence and expression of human papillomavirus sequences in human cervical carcinoma cell lines. Am J Pathol. 1985 Jun;119(3):361–366. [PMC free article] [PubMed] [Google Scholar]
- el Awady M. K., Kaplan J. B., O'Brien S. J., Burk R. D. Molecular analysis of integrated human papillomavirus 16 sequences in the cervical cancer cell line SiHa. Virology. 1987 Aug;159(2):389–398. doi: 10.1016/0042-6822(87)90478-8. [DOI] [PubMed] [Google Scholar]