Abstract
Neuropeptide Y (NPY) is an inhibitory neuromodulator expressed abundantly in the central nervous system that is suspected of being an endogenous antiepileptic agent that can control propagation of limbic seizures. Electrophysiological and pharmacological data suggest that these actions of NPY are mediated by G protein-coupled NPY Y2 and NPY Y5 receptors. To determine whether the NPY Y5 receptor (Y5R) is required for normal control of limbic seizures, we examined hippocampal function and responsiveness to kainic acid-induced seizures in Y5R-deficient (Y5R−/−) mice. We report that Y5R−/− mice do not exhibit spontaneous seizure-like activity; however, they are more sensitive to kainic acid-induced seizures. Electrophysiological examination of hippocampal slices from mutant mice revealed normal function, but the antiepileptic effects of exogenously applied NPY were absent. These data demonstrate that Y5R has an important role in mediating NPY’s inhibitory actions in the mouse hippocampus and suggest a role for Y5R in the control of limbic seizures.
Neuropeptide Y (NPY) is one of the most abundant neuropeptides in the mammalian peripheral and central nervous systems (1–3). It is expressed prominently in inhibitory GABA-ergic interneurons in the hippocampus (4, 5), where its expression increases in response to seizure activity (5–8). NPY selectively inhibits excitatory neurotransmission (9–11), and mice lacking NPY occasionally exhibit mild seizure-like activity, are more susceptible to seizures induced by the GABA antagonist, pentylenetetrazole (PTZ) (12), and are far more likely to die in response to kainic acid (KA)-induced seizures (13). Moreover, in rats, centrally administered NPY inhibits KA-induced seizures (14), PTZ-induced seizures (15), and electrically stimulated hippocampal seizures and their associated wet dog shakes (16). Consequently, NPY is considered to be an endogenous anticonvulsant.
Electrophysiological and pharmacological studies preceding the discovery of NPY Y5 receptor (Y5R) (17) have implicated Y2R in the modulation of NPY’s anticonvulsant actions in the rat hippocampus (18–20). These studies suggest that NPY suppresses excitatory transmission by activating Y2R expressed on presynaptic terminals of excitatory neurons and, consequently, inhibiting presynaptic glutamate release (18–20). More recent studies with rat hippocampal slices suggest the involvement of Y2R and, possibly, Y5R (21). The pharmacological profile of centrally administered NPY analogues capable of inhibiting KA-induced seizures in rats also suggests the involvement of Y5R (14). Taken together, these data implicate Y5R in the modulation of NPY’s anticonvulsant actions. To test this hypothesis, we used a genetic approach. Specifically, the ability of NPY and several of its analogues to block acute epileptiform activity in brain slice preparations from wild-type and Y5R-deficient (Y5R−/−) mice was evaluated. Additionally, the responsiveness of wild-type and Y5R−/− mice to peripherally and centrally administered KA was examined in freely behaving mice.
Materials and Methods
Peptides and Drugs.
Human PYY3–36, human NPY, human NPY13–36, and human pancreatic polypeptide were purchased from Bachem (Torrance, CA) and American Peptide (Sunnyvale, CA). KA and all other chemical reagents were from Sigma.
Animals.
NPY−/− mice on either a mixed C57BL/6J × 129/Sv or an inbred 129/Sv background were described previously (12, 13). Y5R−/− and wild-type littermate mice were generated by breeding mice heterozygous for the disrupted allele as described (22). Data were acquired from 14- to 20-week-old mice of the second generation on either the mixed or inbred background. Mice were housed individually and maintained under controlled temperature (23° ± 2°C) and lights (lights on 07:00 a.m. to 7:00 p.m.). Water and standard rodent chow [Teklad Rodent Diet (W) 8604; Harlan, Madison, WI] were available ad libitum. All animal procedures complied with National Institutes of Health guidelines and were approved by the University of Washington Animal Care Facility committee.
Intracerebroventricular (ICV) Cannulation and Injections.
The left lateral ventricle (0.6 mm posterior, 1.9 mm lateral, and 2.1 mm ventral to bregma) was cannulated as described (22). At least 7 days after surgery, ICV injections were performed in conscious mice without anesthesia. KA was dissolved in 5 mM Tris, pH 7.6, and the pH was adjusted to approximate neutrality with 1 M NaOH, as indicated by phenol red; the final concentration of KA was 0.75 μg/μl, and 1 μl was administered via a 10-μl Hamilton syringe in a syringe pump set to dispense 1 μl/min. Cannulae placement was verified at the end of the experiment by injection of 1 μl of a 1% cresyl violet solution, removal of the brain, and microscopic examination of coronal brain slices.
Scoring Seizure Severity.
Pharmacologically induced seizure severity was scored according to a modified version of the scoring system described by Racine (23). A score of 0 was assigned to mice that exhibited no signs of motor seizure activity; 1 for staring, mouth, or facial movements; 2 for head nodding or isolated twitches; 3 for unilateral/bilateral forelimb clonus; 4 for rearing; 5 for loss of posture, jumping, or status epilepticus (>30 min of seizure activity); and 6 for death. Motor seizure activity was defined as at least 15 consecutive sec of tonic clonic activity. Mice that survived the 2-hr observation period were assigned the maximum latency time of 120 min for death.
Hippocampal Slice Electrophysiology.
Acute hippocampal slices (450 μm) were prepared from male and female adult mice, as described (13). The resulting slices were transferred immediately to a holding chamber, where they remained submerged in oxygenated artificial cerebrospinal fluid (ACSF) consisting of 124 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 2 mM MgSO4, 26 mM NaHCO3, 2 mM CaCl2, and 10 mM dextrose (297 mosM). Slices were held at room temperature for ≥60 min before being transferred to a submersion-type recording chamber, where they were perfused with oxygenated recording medium at a rate of ≈2.5 ml/min (temperature = 32–34°C). To elicit epileptiform activity the perfusion medium was changed to either 0-Mg2+-ACSF (ACSF with MgSO4 omitted; 1.6 mM CaCl2 and 5 mM KCl) (23), picrotoxin-ACSF (ACSF with 200 μM picrotoxin/6 mM KCl), or 4-aminopyridine-ACSF (ACSF with 400 μM 4-AP/6 mM KCl). Extracellular recording electrodes (2 M NaCl, 2–10 MΩ) were used to record field potentials from the stratum pyramidal of CA1, CA3, or neocortex (somatosensory cortex, layers II–III or V–VI). For electrical stimulation of the tissue, a monopolar electrode (World Precision Instruments, Sarasota, FL) was placed on the surface of the slice in the stratum radiatum. Stimuli consisted of 150-μsec constant current pulses at 25–750 μA. Spontaneous field activity and responses to stimulation were analyzed and stored on-line by using axoscope software and an A/D board (Axon Instruments, Foster City, CA).
Statistical Analysis.
All values are reported as mean ± SEM. Data were analyzed either by the unpaired Student’s t test or one-way ANOVA followed by the least-significant-difference post hoc test when appropriate. P values <0.05 were reported as significant.
Results
In Vitro Electrophysiology.
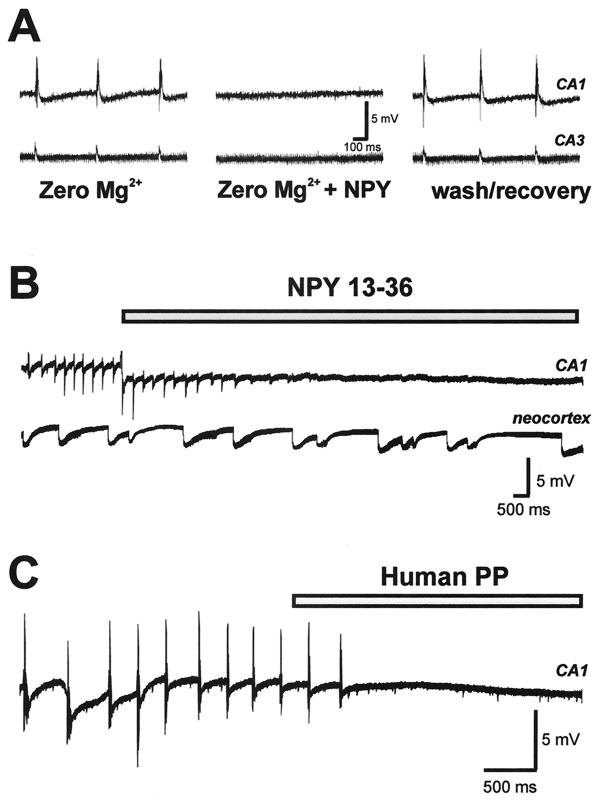
To determine whether NPY exerts antiepileptic actions in the mouse hippocampus, we investigated the effect of exogenous peptide application on seizure activity in vitro. Extracellular field responses were monitored simultaneously in the CA1 and CA3 pyramidal cell regions of hippocampal slices from wild-type mice (n = 16 slices from 7 animals). Spontaneous epileptiform discharges (10- to 75-ms duration; 2- to 8-mV amplitude) were observed after bath perfusion with 0-Mg2+-ACSF (0-Mg-ACSF) (24). After the development of a stable level of spontaneous discharge (0.1–0.5 Hz; 40–180 min), slices then were perfused with 0-Mg-ACSF containing 1 μM human NPY. NPY abolished spontaneous epileptiform discharge activity in all slices tested (n = 6; Fig. 1A); this effect was reversible upon drug washout (baseline = 0.16 ± 0.03 Hz; NPY = 0.01 ± 0.01 Hz; wash = 0.13 ± 0.03 Hz). In contrast, exogenous NPY application did not reduce spontaneous epileptiform discharge activity observed in neocortex (n = 4; data not shown). Similar results were observed in the picrotoxin (n = 2) and 4-aminopyridine (n = 2) models of in vitro seizure activity (data not shown).
Figure 1.
NPY blockade of spontaneous epileptiform burst discharge in hippocampal slices from wild-type mice. Epileptiform activity was induced by slice perfusion with bathing medium containing no magnesium. (A) Field recordings were obtained simultaneously from CA1 (Upper) and CA3 (Lower) pyramidal cell layers. After ≈45 min of perfusion with 0-Mg-ACSF, spontaneous burst discharges occurred in CA1 and CA3. This discharge pattern was abolished after a 10-min perfusion with 0-Mg-ACSF containing 1 μM human NPY. Recovery of burst activity was seen within 15 min after return to 0-Mg-ACSF. (B) Spontaneous epileptiform burst discharges occurring in CA1 were abolished by perfusion with 0-Mg-ACSF containing 1 μM human NPY13–36; burst-discharge activity occurring in neocortex (layer II–III) was unchanged. (C) Spontaneous epileptiform burst discharges occurring in CA1 were abolished by perfusion with 0-Mg-ACSF containing 1 μM human pancreatic polypeptide.
To determine which NPY receptor subtypes might be involved in mediating the antiepileptic actions of NPY in the mouse hippocampus, several commonly used peptide agonists were tested. Exogenous application of 1 μM NPY13–36, a peptide agonist with preference for Y2R (25), mimicked the effect of NPY (n = 8; Fig. 1B). Dose-response studies indicated that NPY13–36 reversibly blocked hippocampal seizure activity in an all-or-none manner at concentrations between 0.25 and 1 μM (n = 3). Exogenous application of 1 μM human pancreatic polypeptide (hPP), a peptide with preference for Y5R (17), also mimicked the effect of human NPY (n = 4; Fig. 1C; baseline = 0.37 ± 0.08 Hz; hPP = 0.02 ± 0.01 Hz). However, exogenous application of 1 μM [Leu31Pro34]NPY, a peptide agonist with preference for Y1R (26), had no effect on burst-discharge activity (n = 3; baseline = 0.46 ± 0.20 Hz; [Leu31Pro34]NPY = 0.43 ± 0.20 Hz).
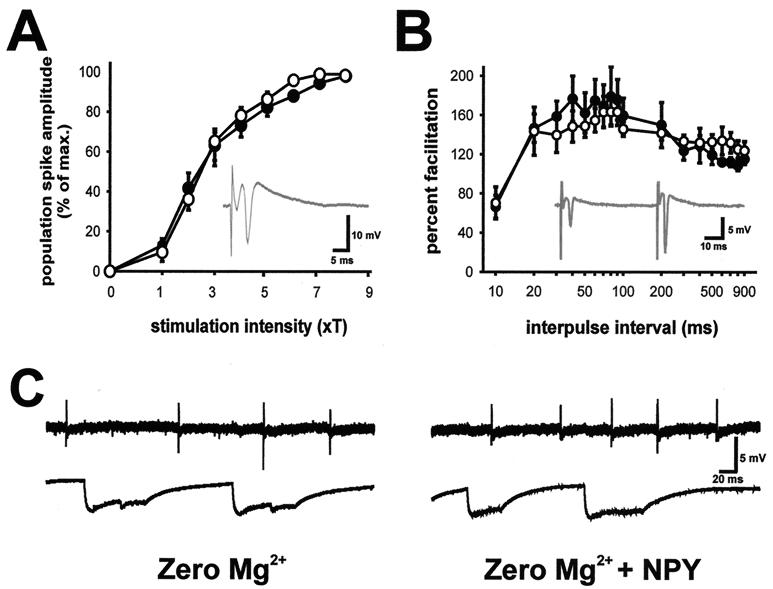
To investigate the potential role of Y5R in modulation of seizure activity, electrophysiology studies in hippocampal slices from Y5R−/− mice were performed. Initial studies, using conventional stimulation paradigms (27, 28), demonstrated that synaptic function was unaltered in the hippocampus of Y5R−/− mice. Input–output curves after single-pulse stimulation in stratum radiatum were constructed for slices from Y5R−/− (n = 9 slices from 4 mice) and wild-type littermate (n = 8 slices from 4 mice) mice; no differences in Schaffer collateral-CA1 synapse function were observed (Fig. 2A). Similarly, a paired-pulse stimulation paradigm failed to reveal differences in synaptic function at this synapse (Fig. 2B). Bath perfusion with 0-Mg-ACSF elicited spontaneous epileptiform discharge activity in slices from Y5R−/− mice. Exogenous application of human NPY (0.5–4 μM) had no effect on seizure activity observed in either hippocampus or neocortex (n = 9; baseline = 0.10 ± 0.02 Hz; NPY = 0.10 ± 0.01 Hz). A representative extracellular field recording during an NPY challenge in a slice from a wild-type mouse is shown in Fig. 2C. Exogenous application of 1 μM human NPY13–36 also had no effect (n = 3; baseline = 0.14 ± 0.03 Hz; NPY13–36 = 0.13 ± 0.01 Hz). Subsequent application of 1 μM tetrodotoxin, a Na+ channel antagonist, abolished epileptiform activity, in both hippocampus and neocortex, of all slices tested (n = 5), indicating that bursting can be abolished in slices from Y5R−/− mice.
Figure 2.
Synaptic function and epileptiform-discharge activity in hippocampal slices from Y5R−/− mice. (A) Input–output curves of population spike responses recorded in the CA1 pyramidal cell region (see Inset) to stimulation of the Schaffer collaterals. Threshold for stimulation was defined for each slice as the minimum current required to elicit a detectable population spike; the x axis shows stimulus intensity in terms of threshold multiples. Responses were normalized with respect to maximum population spike amplitude to allow averaging of responses from wild-type (○) and Y5R−/− (●) mice. (B) Plot of paired-pulse facilitation (amplitude of population spike response to second stimulus divided by amplitude of the response to first stimulus) in the CA1 pyramidal cell region (see Inset) for hippocampal slices from wild-type and Y5R−/− mice. Stimulation intensity was set at four times the threshold. (C) Spontaneous epileptiform burst-discharge activity in a hippocampal slice from a Y5R−/− mouse during perfusion with 0-Mg-ACSF (Left) and 20 min after perfusion with 0-Mg-ACSF containing 1 μM human NPY (Right). Field recordings were obtained from hippocampus (CA1 pyramidal cell layer, Upper) and neocortex (layer II–III, Lower).
Comparison of Handling-Induced Seizures in NPY−/− and Y5R−/− Mice.
Visual observations of male and female Y5R−/− mice of various ages on either a mixed C57BL/6J × 129/Sv genetic background or an inbred 129/Sv background for 30 min revealed no spontaneous, seizure-like behaviors when housed under standard vivarium conditions, and they responded normally to stress induced by handling, tail suspension, or placement on the cage top. This is in contrast to NPY-deficient mice, which exhibit seizure activity in response to mild forms of stress, including handling, tail suspension (D.J.M., unpublished observation), and placement of mice on cage tops (12). This seizure phenotype is more severe on an inbred 129/Sv background. Adult NPY−/− mutant mice occasionally display motor seizures characterized by tonic clonic activity and loss of posture in response to mild stressors (D.J.M., unpublished observation).
Response of Y5R−/− Mice to Peripherally Administered KA.
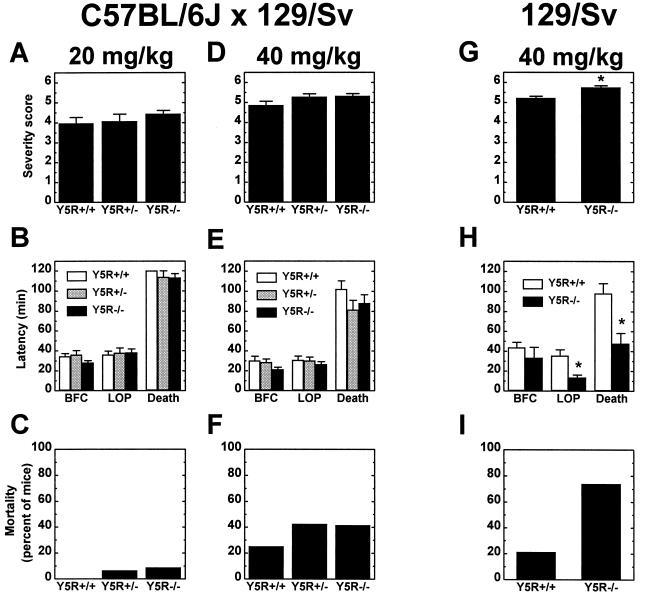
To determine whether the Y5R is involved in the modulation of limbic seizure activity, we evaluated the response of wild-type and Y5R−/− mice to peripherally administered KA. KA is an analogue of glutamate capable of inducing robust limbic motor seizures (29). On a mixed C57BL/6J × 129/Sv genetic background, mice of all genotypes exhibited similar behavioral responses to both low (20 mg/kg; Fig. 3 A–C) and high (40 mg/kg; Fig. 3 D–F) doses of KA. At both doses the latency times to bilateral forelimb clonus (BFC), loss of posture (LOP), and death were similar in control and mutant mice (Fig. 3 B and E). Although not significant, Y5R−/− mice on a mixed genetic background displayed a trend toward greater seizure severity (Fig. 3 A and D) and were more likely to die than wild-type littermates in response to KA (Fig. 3 C and F). Heterozygous mutant (Y5R+/−) mice also exhibited greater seizure severity and increased death when challenged with KA (Fig. 3 C, D, and F). In response to the higher dose of KA, twice as many mutant mice died as compared with wild-type littermates (Fig. 3F). These results and the observation that genetic background influences the seizure susceptibility of NPY-deficient mice prompted us to explore the response of inbred 129/Sv Y5R−/− mice to peripherally administered KA.
Figure 3.
Response of wild-type and mutant mice to peripherally administered KA. Female wild-type (Y5R+/+), heterozygous (Y5R+/−), and homozygous Y5R−/− littermate mice on a mixed C57BL/6J × 129/Sv (A–F) genetic background or female wild-type and Y5R−/− mice on an inbred 129/Sv (G–I) genetic background were injected (i.p.) with either 20 (A–C) or 40 (D–I) mg/kg KA and observed for 2 hr after injection. (A, D, and G) Motor seizure severity was rated on a scale from 0 to 6 as described in Materials and Methods (n = 16–35 per genotype; *, P < 0.001). (B, E, and H) Latency to loss of posture (LOP), bilateral forelimb clonus (BFC), and death in response to KA was measured in minutes for control and mutant mice (n = 9–35 per genotype; *, P < 0.004). (C, F, and I) Plot of the percent mortality of control and mutant mice after KA administration (n = 16–35 per genotype).
We evaluated the response of wild-type and Y5R−/− mice on the 129/Sv background to a 40-mg/kg dose of KA (Fig. 3 G–I). Y5R−/− mice exhibited motor seizures of significantly greater severity than did wild-type mice (Fig. 3G), and the latency times to loss of posture and death were significantly shorter for Y5R−/− mice (Fig. 3H). Approximately 75% of the Y5R−/− mice died compared with 20% of controls (Fig. 3I).
Response of Y5R−/− Mice to Centrally Administered KA.
We also evaluated the response of Y5R−/− mice on the mixed genetic background to KA administered ICV, because this paradigm tends to elicit more reproducible behavioral effects. Permanent indwelling cannulae were implanted into the left lateral ventricles of male Y5R−/− and wild-type littermate mice on a mixed genetic background. A dose of KA (0.75 μg) capable of reproducibly generating level 5 motor seizures (i.e., loss of posture and status epilepticus) in wild-type mice was chosen. The Y5R−/− mice were hypersensitive to the effects of centrally administered KA. They exhibited significantly shorter latency times to motor seizure activity (Y5R−/− = 2.33 ± 0.65 min vs. wild type = 15.2 ± 2.6 min; P < 0.03; n = 8–9 for both groups) and all other behavioral indices. This dose of KA killed 50% of the wild-type mice and all of the Y5R−/− mice.
Discussion
Both in vitro and in vivo studies have substantiated the role of NPY as an endogenous anticonvulsant; however, the NPY receptor subtype(s) mediating these effects has not been clearly defined. Electrophysiological and pharmacological studies with rats have suggested the involvement of Y2R (18–20) and probably Y5R (21), but the lack of validated NPY receptor subtype-specific antagonists has hampered a definitive conclusion. Consequently, we have studied mice lacking Y5R to determine its role in modulation of NPY’s anticonvulsant activities.
Exogenously applied NPY inhibited epileptiform discharges elicited by perfusing hippocampal slices from wild-type mice with 0-Mg-ACSF. Interestingly, NPY inhibited discharges only in the hippocampus and did not affect spontaneous epileptiform activity in the mouse somatosensory cortex. This lack of an effect on cortical activity is inconsistent with NPY’s abilities to inhibit epileptiform activity in rat cortical slices (21). These studies suggest that the antiepileptic actions of NPY in the rat frontal cortex (but perhaps not other regions of the cortex) may be mediated predominantly by Y1R (21). Alternatively, this disparity may be due to species differences in the expression patterns of the various NPY receptor subtypes expressed in the central nervous system. Y1R is present at high levels in the rat cortex (3, 30, 31), but only moderately expressed in the mouse cortex (32). Y1R predominates in the rat cortex, but Y2R and Y5R also have been detected (3, 30, 31, 33), but neither Y2R nor Y5R have been detected in the mouse cortex (32). In contrast, Y1R, Y2R, and Y5R are all expressed in the hippocampal formation of both the rat (17, 30, 31, 33) and mouse (32).
Consistent with the rat hippocampal slice data (18–21), electrophysiological and pharmacological studies with several NPY analogues with receptor subtype selectivity suggest the involvement of at least Y2R and/or Y5R in the modulation of NPY’s anticonvulsant actions in the mouse hippocampus. Similar experiments with hippocampal slices from Y5R−/− mice have validated the involvement of Y5R. Interestingly, neither NPY nor the Y2R-preferential agonist, NPY13–36, had an effect on spontaneous epileptiform discharges in hippocampal slices from Y5R−/− mice, demonstrating an absolute requirement for Y5R in mediating NPY’s anticonvulsant actions in mouse hippocampal slices. These studies also suggest that the anticonvulsant actions of NPY13–36 in mouse hippocampal slices may be mediated by Y5R.
Electrophysiological evaluation of hippocampal function in Y5R−/− mice suggests that Y5R is not required for hippocampal function under normal conditions. Neither standard input-output nor paired-pulse paradigms revealed any deficits at the Schaffer collateral to CA1 synapse, a synapse shown to be modulated by NPY (13), in hippocampal slices from Y5R−/− mice. These in vitro results are consistent with the normal behavior exhibited by Y5R−/− mice. Because both NPY−/− (13) and Y5R−/− mice are more likely to die than wild-type littermate mice in response to KA-induced seizures, endogenous NPY apparently modulates limbic seizure activity, at least in part, through activation of Y5R. In contrast to NPY−/− mice (12), Y5R−/− mice do not exhibit seizure-like activity in response to handling, suggesting that other NPY receptor subtypes modulate limbic activity in vivo in addition to Y5R.
Interestingly, differences in genetic background influence the sensitivity of Y5R−/− mice, but not wild-type mice, to KA-induced seizures. Y5R−/− mice maintained on an inbred 129/Sv background exhibit a stronger phenotype in response to peripherally administered KA than do mutant mice on a mixed 129/Sv × C57BL/6J background, whereas wild-type mice on either background exhibit similar responses. These data imply that some gene in the C57BL/6J genetic background influences the antiepileptic actions of NPY mediated by Y5R. The results are consistent with the observation that NPY deficiency results in more severe and more frequent handling-induced seizures on an inbred 129/Sv background.
The present studies have demonstrated the requirement for Y5R in the mediation of the antiepileptic activity of NPY in the mouse hippocampus and support a role for Y5R modulation of limbic seizures in vivo. However, Y5R does not appear to be required for normal hippocampal function or the control of normal excitatory signaling. These data suggest that Y5R-selective agonists may provide a novel approach for the treatment of epilepsy.
Acknowledgments
We thank Drs. William Colmers and David Woldbye for their constructive suggestions regarding this manuscript.
Abbreviations
- NPY
neuropeptide Y
- Y1R
Y2R, and Y5R, neuropeptide Y1, Y2, and Y5 receptors, respectively
- Y5R−/− mice
Y5R-deficient mice
- KA
kainic acid
- ICV
intracerebroventricular
- ACSF
artificial cerebrospinal fluid
References
- 1.Allen Y S, Adrian T E, Allen J M, Tatemoto K, Crow T J, Bloom S R, Polak J M. Science. 1983;221:877–879. doi: 10.1126/science.6136091. [DOI] [PubMed] [Google Scholar]
- 2.Chronwall B M, DiMaggio D A, Massari V J, Pickel V M, Ruggiero D A, O’Donohue T L. Neuroscience. 1985;15:1159–1181. doi: 10.1016/0306-4522(85)90260-x. [DOI] [PubMed] [Google Scholar]
- 3.Dumont Y, Martel J C, Fournier A, St.-Pierre S, Quirion R. Prog Neurobiol. 1992;38:125–167. doi: 10.1016/0301-0082(92)90038-g. [DOI] [PubMed] [Google Scholar]
- 4.Morris B J. J Comp Neurol. 1989;290:358–368. doi: 10.1002/cne.902900305. [DOI] [PubMed] [Google Scholar]
- 5.Gruber B, Greber S, Rupp E, Sperk G. Hippocampus. 1994;4:474–482. doi: 10.1002/hipo.450040409. [DOI] [PubMed] [Google Scholar]
- 6.Sloviter R S. In: The Hippocampus-New Vistas. Chan-Palay V, Kohler C, editors. New York: Liss; 1989. pp. 443–461. [Google Scholar]
- 7.Kragh J, Tønder N, Finsen B R, Zimmer J, Bolwig T G. Exp Brain Res. 1994;98:305–313. doi: 10.1007/BF00228418. [DOI] [PubMed] [Google Scholar]
- 8.Tønder N, Kragh J, Finsen B R, Bolwig T G, Zimmer J. Epilepsia. 1994;35:1299–1308. doi: 10.1111/j.1528-1157.1994.tb01802.x. [DOI] [PubMed] [Google Scholar]
- 9.Colmers W F, Lukowiak K, Pittman Q L. J Physiol (London) 1987;383:285–299. doi: 10.1113/jphysiol.1987.sp016409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haas H L, Hermann A, Greene R W, Chan-Palay V. J Comp Neurol. 1987;257:208–215. doi: 10.1002/cne.902570207. [DOI] [PubMed] [Google Scholar]
- 11.Klapstein G J, Colmers W F. Hippocampus. 1993;3:103–112. doi: 10.1002/hipo.450030111. [DOI] [PubMed] [Google Scholar]
- 12.Erickson J C, Clegg K E, Palmiter R D. Nature (London) 1996;381:415–418. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- 13.Baraban S C, Hollopeter G, Erickson J C, Schwartzkroin P A, Palmiter R D. J Neurosci. 1997;17:8927–8936. doi: 10.1523/JNEUROSCI.17-23-08927.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woldbye D P D, Larsen P J, Mikkelsen J D, Klemp K, Madsen T M, Bolwig T G. Nat Med. 1997;3:761–764. doi: 10.1038/nm0797-761. [DOI] [PubMed] [Google Scholar]
- 15.Woldbye D P D. Regul Pept. 1998;75–76:279–282. doi: 10.1016/s0167-0115(98)00079-2. [DOI] [PubMed] [Google Scholar]
- 16.Woldbye D P D, Madsen T M, Larsen P J, Mikkelsen J D, Bolwig T G. Brain Res. 1996;737:162–168. doi: 10.1016/0006-8993(96)00730-5. [DOI] [PubMed] [Google Scholar]
- 17.Gerald C, et al. Nature (London) 1996;382:168–171. doi: 10.1038/382168a0. [DOI] [PubMed] [Google Scholar]
- 18.Colmers W F, Lukowiak K, Pittman Q L. J Neurosci. 1988;8:3827–3837. doi: 10.1523/JNEUROSCI.08-10-03827.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colmers W F, Klapstein G J, Fournier A, St.-Pierre S, Treherne K A. Br J Pharmacol. 1991;102:41–44. doi: 10.1111/j.1476-5381.1991.tb12129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bleakman D, Harrison N L, Colmers W F, Miller R J. Br J Pharmacol. 1992;107:334–340. doi: 10.1111/j.1476-5381.1992.tb12747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bijak M. Neurosci Lett. 1999;268:115–118. doi: 10.1016/s0304-3940(99)00381-x. [DOI] [PubMed] [Google Scholar]
- 22.Marsh D J, Hollopeter G, Kafer K E, Palmiter R D. Nat Med. 1998;4:718–721. doi: 10.1038/nm0698-718. [DOI] [PubMed] [Google Scholar]
- 23.Racine R J. Electroenceph Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 24.Mody I, Lambert J D, Heinemann U. J Neurophysiol. 1987;57:869–888. doi: 10.1152/jn.1987.57.3.869. [DOI] [PubMed] [Google Scholar]
- 25.Hedlund P, Bjelke B, Aguirre J A, Fuxe K. Acta Physiol Scand. 1991;141:279–280. doi: 10.1111/j.1748-1716.1991.tb09078.x. [DOI] [PubMed] [Google Scholar]
- 26.Fuhlendorff J, Gether U, Aakerlund L, Langeland-Johansen N, Thogersen H, Melberg S G, Olsen U B, Thastrup O, Schwartz T W. Proc Nat Acad Sci USA. 1990;87:182–186. doi: 10.1073/pnas.87.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gribkoff V K, Ashe J H. Brain Res Bull. 1985;15:273–278. doi: 10.1016/0361-9230(85)90150-9. [DOI] [PubMed] [Google Scholar]
- 28.Low W C, BeMent S L, Whitehorn D. Exp Neurol. 1983;80:9–22. doi: 10.1016/0014-4886(83)90002-x. [DOI] [PubMed] [Google Scholar]
- 29.Sperk G. Prog Neurobiol. 1994;42:1–32. doi: 10.1016/0301-0082(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 30.Larsen P J, Sheikh S P, Jakobsen C R, Schwartz T W, Mikkelsen J D. Eur J Neurosci. 1993;5:1622–1637. doi: 10.1111/j.1460-9568.1993.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 31.Dumont Y, Fournier A, Quirion R. J Neurosci. 1998;18:5565–5574. doi: 10.1523/JNEUROSCI.18-15-05565.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naveilhan P, Neveu I, Arenas E, Ernfors P. Neuroscience. 1998;87:289–302. doi: 10.1016/s0306-4522(98)00141-9. [DOI] [PubMed] [Google Scholar]
- 33.Dumont Y, Fournier A, St.-Pierre S, Quirion R. Synapse. 1996;22:139–158. doi: 10.1002/(SICI)1098-2396(199602)22:2<139::AID-SYN7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]