Abstract
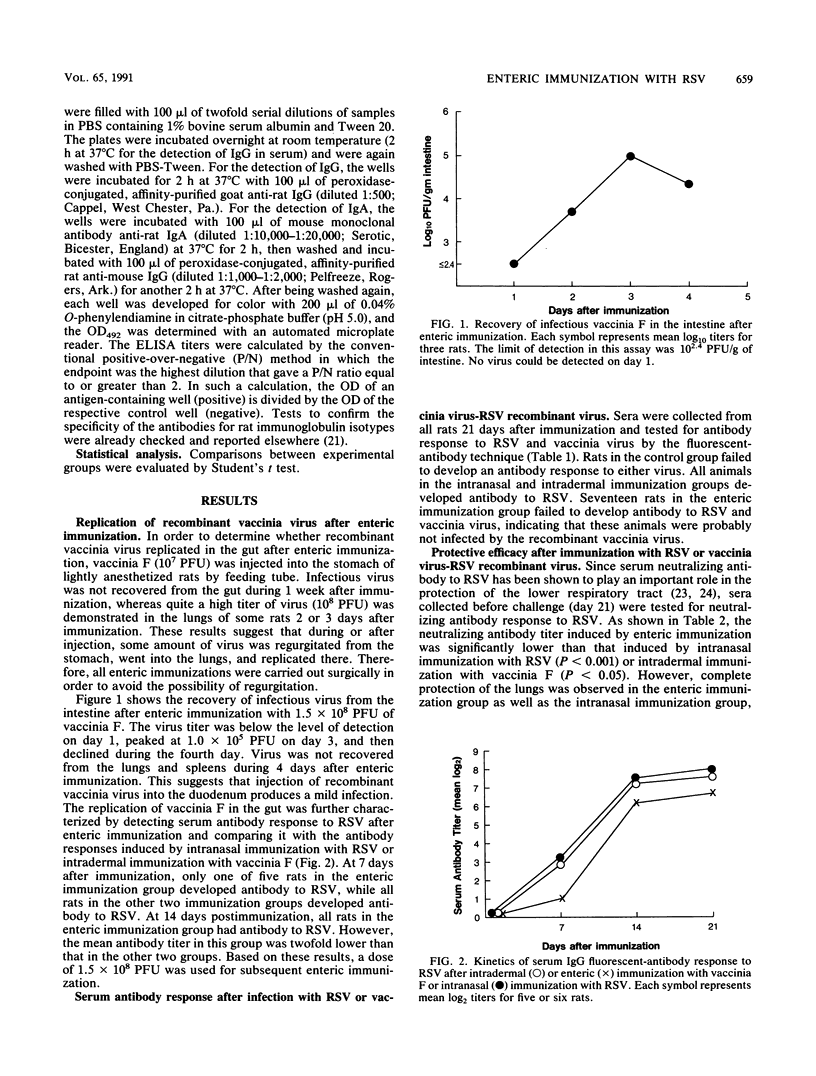
Cotton rats were immunized via intranasal, intradermal, or enteric routes with respiratory syncytial virus (RSV) or a live recombinant vaccinia virus expressing the RSV F glycoprotein (vaccinia F). The animals were tested for the appearance of RSV-specific antibody responses in the serum, bronchoalveolar lavage, and nasal wash after immunization and for virus replication 4 days after intranasal challenge with RSV. RSV antibody response in the serum and respiratory tract was demonstrated in all immunization groups and was significantly increased after intranasal challenge with RSV. Immunoglobulin A (IgA) antibody response in bronchoalveolar lavage fluid after intranasal or enteric immunization was two- to threefold higher than that after intradermal immunization. Nasal-wash IgA antibody response was not significantly different among three immunization groups, although mean antibody titer was the highest in intranasal immunization group. Complete resistance to replication of RSV challenge was observed in the lungs of cotton rats immunized by the intranasal or enteric routes, whereas a low level of replication was detected in the lungs of rats immunized intradermally. Enteric or intradermal immunization conferred partial protection to the upper respiratory tract, but complete protection of the upper respiratory tract was observed in the intranasal immunization group. These observations suggest that while enteric immunization is quite effective in inducing antibody responses in the respiratory tract, the magnitude of antiviral immunity induced in the respiratory tract after intranasal immunization may be superior to that observed after enteric immunization.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belshe R. B., Van Voris L. P., Mufson M. A. Parenteral administration of live respiratory syncytial virus vaccine: results of a field trial. J Infect Dis. 1982 Mar;145(3):311–319. doi: 10.1093/infdis/145.3.311. [DOI] [PubMed] [Google Scholar]
- Bergmann K. C., Waldman R. H., Tischner H., Pohl W. D. Antibody in tears, saliva and nasal secretions following oral immunization of humans with inactivated influenza virus vaccine. Int Arch Allergy Appl Immunol. 1986;80(1):107–109. doi: 10.1159/000234034. [DOI] [PubMed] [Google Scholar]
- Buynak E. B., Weibel R. E., McLean A. A., Hilleman M. R. Live respiratory syncytial virus vaccine administered parenterally. Proc Soc Exp Biol Med. 1978 Apr;157(4):636–642. doi: 10.3181/00379727-157-40112. [DOI] [PubMed] [Google Scholar]
- Coates H. V., Alling D. W., Chanock R. M. An antigenic analysis of respiratory syncytial virus isolates by a plaque reduction neutralization test. Am J Epidemiol. 1966 Mar;83(2):299–313. doi: 10.1093/oxfordjournals.aje.a120586. [DOI] [PubMed] [Google Scholar]
- Czerkinsky C., Prince S. J., Michalek S. M., Jackson S., Russell M. W., Moldoveanu Z., McGhee J. R., Mestecky J. IgA antibody-producing cells in peripheral blood after antigen ingestion: evidence for a common mucosal immune system in humans. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2449–2453. doi: 10.1073/pnas.84.8.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elango N., Prince G. A., Murphy B. R., Venkatesan S., Chanock R. M., Moss B. Resistance to human respiratory syncytial virus (RSV) infection induced by immunization of cotton rats with a recombinant vaccinia virus expressing the RSV G glycoprotein. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1906–1910. doi: 10.1073/pnas.83.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. W., Arrobio J. O., Brandt C. D., Wright P., Hodes D., Chanock R. M., Parrott R. H. Safety and antigenicity of temperature sensitive (TS) mutant respiratory syncytial virus (RSV) in infants and children. Pediatrics. 1973 Jul;52(1):56–63. [PubMed] [Google Scholar]
- Kim H. W., Canchola J. G., Brandt C. D., Pyles G., Chanock R. M., Jensen K., Parrott R. H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969 Apr;89(4):422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- Kumagai T., Wong D. T., Ogra P. L. Development of cell-mediated cytotoxic activity in the respiratory tract after experimental infection with respiratory syncytial virus. Clin Exp Immunol. 1985 Aug;61(2):351–359. [PMC free article] [PubMed] [Google Scholar]
- Liew F. Y., Russell S. M., Appleyard G., Brand C. M., Beale J. Cross-protection in mice infected with influenza A virus by the respiratory route is correlated with local IgA antibody rather than serum antibody or cytotoxic T cell reactivity. Eur J Immunol. 1984 Apr;14(4):350–356. doi: 10.1002/eji.1830140414. [DOI] [PubMed] [Google Scholar]
- Mazanec M. B., Nedrud J. G., Lamm M. E. Immunoglobulin A monoclonal antibodies protect against Sendai virus. J Virol. 1987 Aug;61(8):2624–2626. doi: 10.1128/jvi.61.8.2624-2626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott M. R., Bienenstock J. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979 May;122(5):1892–1898. [PubMed] [Google Scholar]
- McIntosh K., Masters H. B., Orr I., Chao R. K., Barkin R. M. The immunologic response to infection with respiratory syncytial virus in infants. J Infect Dis. 1978 Jul;138(1):24–32. doi: 10.1093/infdis/138.1.24. [DOI] [PubMed] [Google Scholar]
- McIntosh K., McQuillin J., Gardner P. S. Cell-free and cell-bound antibody in nasal secretions from infants with respiratory syncytial virus infection. Infect Immun. 1979 Feb;23(2):276–281. doi: 10.1128/iai.23.2.276-281.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J., 5th, Van Kirk J. E., Wright P. F., Chanock R. M. Experimental respiratory syncytial virus infection of adults. Possible mechanisms of resistance to infection and illness. J Immunol. 1971 Jul;107(1):123–130. [PubMed] [Google Scholar]
- Murphy B. R., Collins P. L., Lawrence L., Zubak J., Chanock R. M., Prince G. A. Immunosuppression of the antibody response to respiratory syncytial virus (RSV) by pre-existing serum antibodies: partial prevention by topical infection of the respiratory tract with vaccinia virus-RSV recombinants. J Gen Virol. 1989 Aug;70(Pt 8):2185–2190. doi: 10.1099/0022-1317-70-8-2185. [DOI] [PubMed] [Google Scholar]
- Nedrud J. G., Liang X. P., Hague N., Lamm M. E. Combined oral/nasal immunization protects mice from Sendai virus infection. J Immunol. 1987 Nov 15;139(10):3484–3492. [PubMed] [Google Scholar]
- Ogra P. L., Karzon D. T. Distribution of poliovirus antibody in serum, nasopharynx and alimentary tract following segmental immunization of lower alimentary tract with poliovaccine. J Immunol. 1969 Jun;102(6):1423–1430. [PubMed] [Google Scholar]
- Okamoto Y., Freihorst J., Ogra P. L. Maternal determinants of neonatal immune response to ovalbumin: effect of breast feeding on development of anti-ovalbumin antibody in the neonate. Int Arch Allergy Appl Immunol. 1989;89(1):83–89. doi: 10.1159/000234928. [DOI] [PubMed] [Google Scholar]
- Olmsted R. A., Elango N., Prince G. A., Murphy B. R., Johnson P. R., Moss B., Chanock R. M., Collins P. L. Expression of the F glycoprotein of respiratory syncytial virus by a recombinant vaccinia virus: comparison of the individual contributions of the F and G glycoproteins to host immunity. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7462–7466. doi: 10.1073/pnas.83.19.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince G. A., Horswood R. L., Camargo E., Koenig D., Chanock R. M. Mechanisms of immunity to respiratory syncytial virus in cotton rats. Infect Immun. 1983 Oct;42(1):81–87. doi: 10.1128/iai.42.1.81-87.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince G. A., Horswood R. L., Chanock R. M. Quantitative aspects of passive immunity to respiratory syncytial virus infection in infant cotton rats. J Virol. 1985 Sep;55(3):517–520. doi: 10.1128/jvi.55.3.517-520.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. W., Mestecky J. Induction of the mucosal immune response. Rev Infect Dis. 1988 Jul-Aug;10 (Suppl 2):S440–S446. doi: 10.1093/cid/10.supplement_2.s440. [DOI] [PubMed] [Google Scholar]
- Smith C. B., Purcell R. H., Bellanti J. A., Chanock R. M. Protective effect of antibody to parainfluenza type 1 virus. N Engl J Med. 1966 Nov 24;275(21):1145–1152. doi: 10.1056/NEJM196611242752101. [DOI] [PubMed] [Google Scholar]
- Smith T. J., Buescher E. L., Top F. H., Jr, Altemeier W. A., McCown J. M. Experimental respiratory infection with type 4 adenovirus vaccine in volunteers: clinical and immunological responses. J Infect Dis. 1970 Oct;122(4):239–248. doi: 10.1093/infdis/122.4.239. [DOI] [PubMed] [Google Scholar]
- Stott E. J., Taylor G., Ball L. A., Anderson K., Young K. K., King A. M., Wertz G. W. Immune and histopathological responses in animals vaccinated with recombinant vaccinia viruses that express individual genes of human respiratory syncytial virus. J Virol. 1987 Dec;61(12):3855–3861. doi: 10.1128/jvi.61.12.3855-3861.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G., Stott E. J., Bew M., Fernie B. F., Cote P. J., Collins A. P., Hughes M., Jebbett J. Monoclonal antibodies protect against respiratory syncytial virus infection in mice. Immunology. 1984 May;52(1):137–142. [PMC free article] [PubMed] [Google Scholar]
- Ueba O. Respiratory syncytial virus. I. Concentration and purification of the infectious virus. Acta Med Okayama. 1978 Aug;32(4):265–272. [PubMed] [Google Scholar]
- Walsh E. E., Hall C. B., Briselli M., Brandriss M. W., Schlesinger J. J. Immunization with glycoprotein subunits of respiratory syncytial virus to protect cotton rats against viral infection. J Infect Dis. 1987 Jun;155(6):1198–1204. doi: 10.1093/infdis/155.6.1198. [DOI] [PubMed] [Google Scholar]
- Walsh E. E., Schlesinger J. J., Brandriss M. W. Protection from respiratory syncytial virus infection in cotton rats by passive transfer of monoclonal antibodies. Infect Immun. 1984 Feb;43(2):756–758. doi: 10.1128/iai.43.2.756-758.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz-Carrington P., Roux M. E., McWilliams M., PHILLIPS-Quagliata J. M., Lamm M. E. Organ and isotype distribution of plasma cells producing specific antibody after oral immunization: evidence for a generalized secretory immune system. J Immunol. 1979 Oct;123(4):1705–1708. [PubMed] [Google Scholar]
- Wright P. F., Shinozaki T., Fleet W., Sell S. H., Thompson J., Karzon D. T. Evaluation of a live, attenuated respiratory syncytial virus vaccine in infants. J Pediatr. 1976 Jun;88(6):931–936. doi: 10.1016/s0022-3476(76)81044-x. [DOI] [PubMed] [Google Scholar]



