Abstract
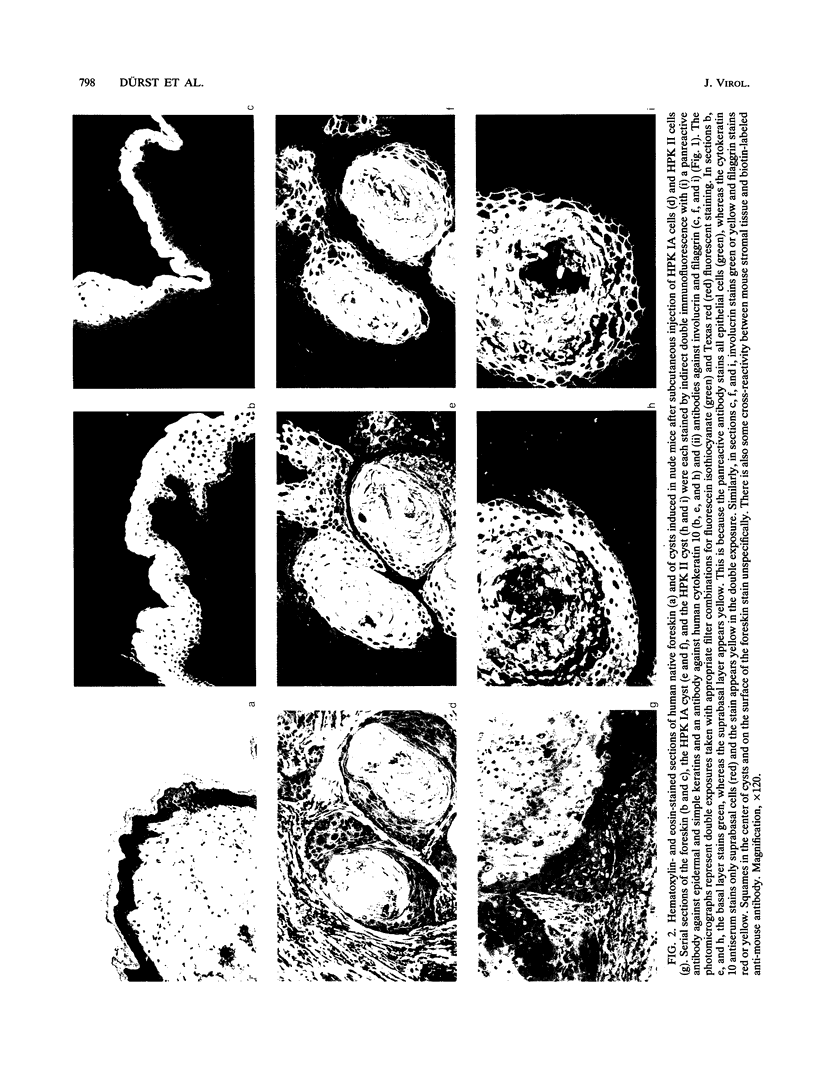
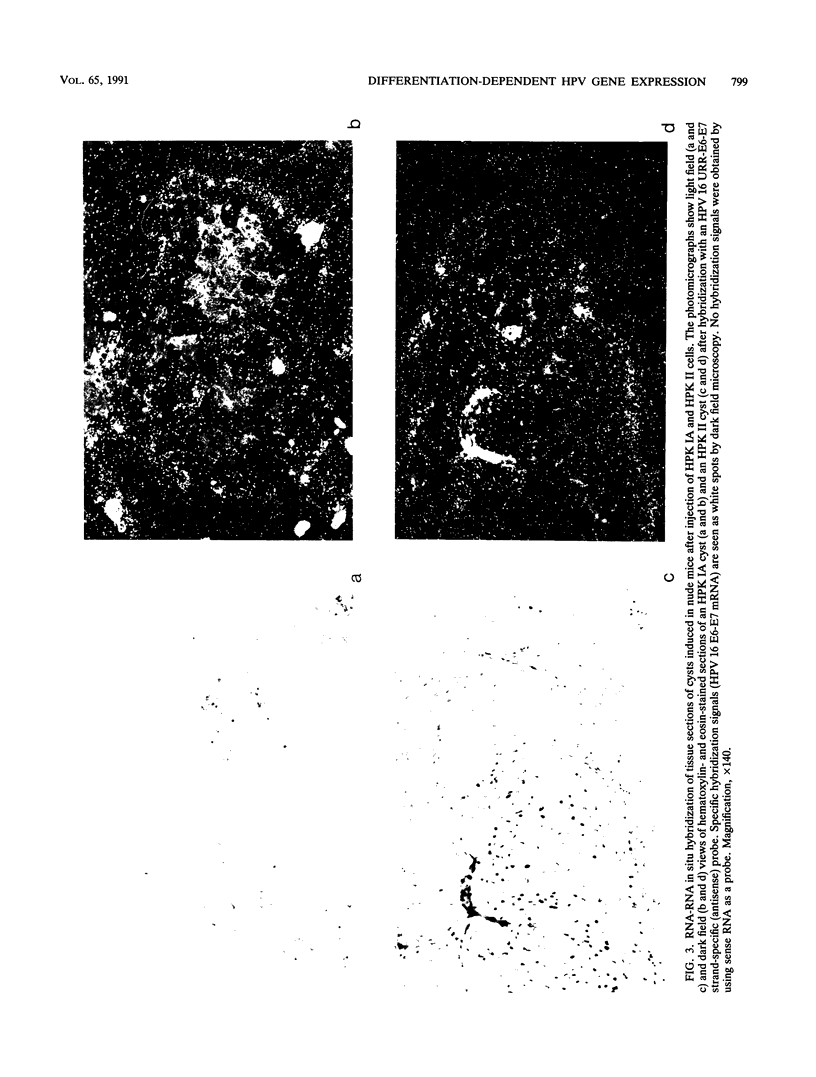
Two human papillomavirus type 16 (HPV 16)-immortalized human keratinocyte cell lines (HPK) were shown to have retained the ability for differentiation after subcutaneous injection into nude mice. These properties were maintained even at late passage. HPK cells gave rise to transiently growing cysts which exhibited an epitheliumlike architecture. Moreover, differentiation-specific markers such as cytokeratin 10, involucrin, and filaggrin were shown to be expressed in an ordered succession. RNA-RNA in situ hybridization revealed heterogeneous and low levels of HPV 16 E6-E7 RNA in the basal layer of the cysts. In contrast, in progressively growing tumors induced by HPK cells containing an activated ras oncogene (EJ-ras) or in tumors induced by the cervical carcinoma cell line CaSki, high levels of E6-E7-specific RNA could be detected. Irrespective of the growth potential of these cell lines in nude mice, viral transcription was always more evident in the basal layer and in proliferatively active cells rather than in differentiated cells. This contrasts with viral gene expression in HPV 16 positive low-grade cervical dysplasia, in which abundant viral transcriptional activity was mapped to the upper third of the epithelium. It is suggested that the physical state of the viral DNA, i.e., integrated viral DNA in the cell lines as opposed to extrachromosomal DNA in low-grade cervical dysplasia, may influence viral gene regulation.
Full text
PDF

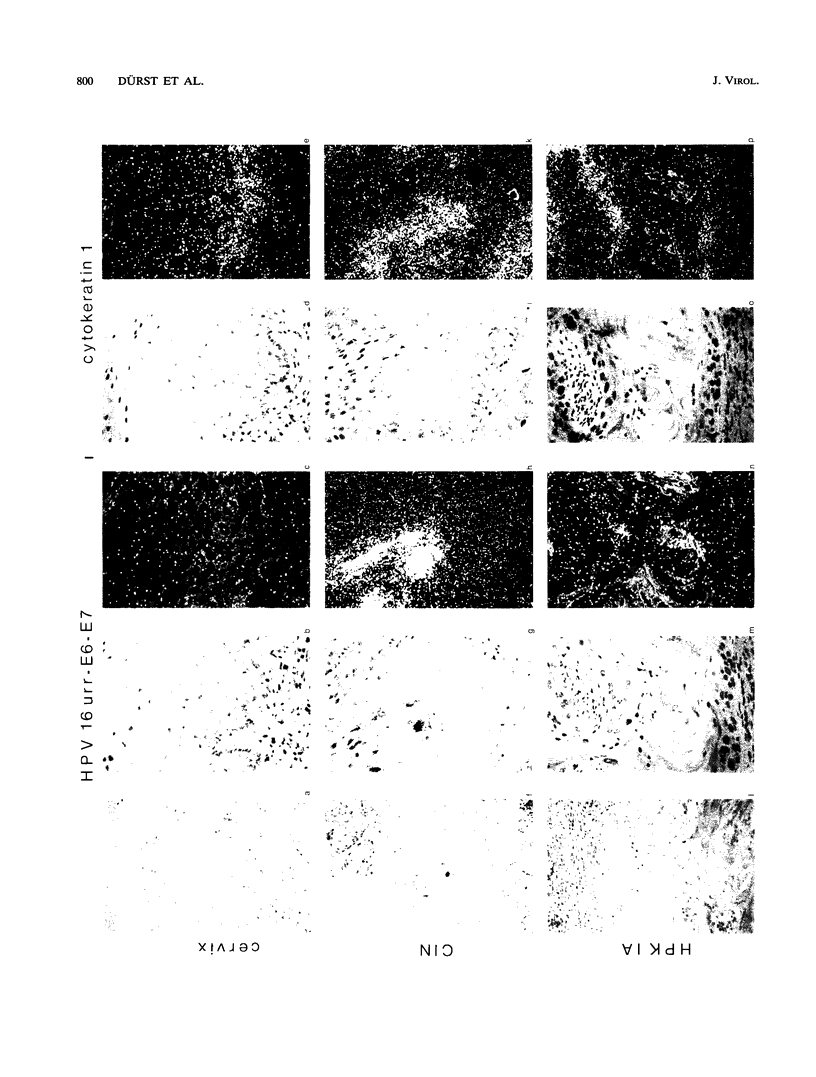

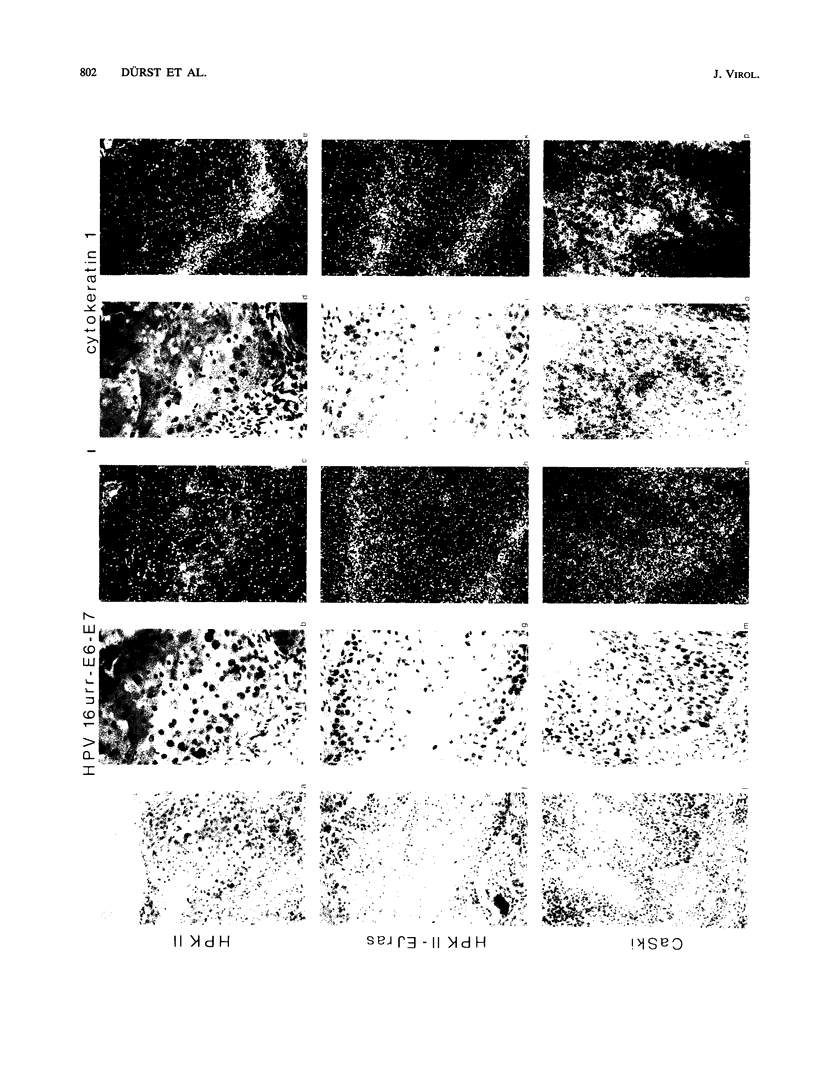


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asselineau D., Bernard B. A., Bailly C., Darmon M., Pruniéras M. Human epidermis reconstructed by culture: is it "normal"? J Invest Dermatol. 1986 Feb;86(2):181–186. doi: 10.1111/1523-1747.ep12284237. [DOI] [PubMed] [Google Scholar]
- Bohnert A., Hornung J., Mackenzie I. C., Fusenig N. E. Epithelial-mesenchymal interactions control basement membrane production and differentiation in cultured and transplanted mouse keratinocytes. Cell Tissue Res. 1986;244(2):413–429. doi: 10.1007/BF00219217. [DOI] [PubMed] [Google Scholar]
- Bosch F. X., Ouhayoun J. P., Bader B. L., Collin C., Grund C., Lee I., Franke W. W. Extensive changes in cytokeratin expression patterns in pathologically affected human gingiva. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;58(1):59–77. doi: 10.1007/BF02890059. [DOI] [PubMed] [Google Scholar]
- Bosch F. X., Schwarz E., Boukamp P., Fusenig N. E., Bartsch D., zur Hausen H. Suppression in vivo of human papillomavirus type 18 E6-E7 gene expression in nontumorigenic HeLa X fibroblast hybrid cells. J Virol. 1990 Oct;64(10):4743–4754. doi: 10.1128/jvi.64.10.4743-4754.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukamp P., Breitkreutz D., Stark H. J., Fusenig N. E. Mesenchyme-mediated and endogenous regulation of growth and differentiation of human skin keratinocytes derived from different body sites. Differentiation. 1990 Aug;44(2):150–161. doi: 10.1111/j.1432-0436.1990.tb00548.x. [DOI] [PubMed] [Google Scholar]
- Buckley C. H., Butler E. B., Fox H. Cervical intraepithelial neoplasia. J Clin Pathol. 1982 Jan;35(1):1–13. doi: 10.1136/jcp.35.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M. L., Sklar J. Lymphoproliferative disorders in cardiac transplant recipients are multiclonal lymphomas. Lancet. 1984 Sep 1;2(8401):489–493. doi: 10.1016/s0140-6736(84)92566-2. [DOI] [PubMed] [Google Scholar]
- Cox K. H., DeLeon D. V., Angerer L. M., Angerer R. C. Detection of mrnas in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol. 1984 Feb;101(2):485–502. doi: 10.1016/0012-1606(84)90162-3. [DOI] [PubMed] [Google Scholar]
- Crum C. P., Nuovo G., Friedman D., Silverstein S. J. Accumulation of RNA homologous to human papillomavirus type 16 open reading frames in genital precancers. J Virol. 1988 Jan;62(1):84–90. doi: 10.1128/jvi.62.1.84-90.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPaolo J. A., Woodworth C. D., Popescu N. C., Notario V., Doniger J. Induction of human cervical squamous cell carcinoma by sequential transfection with human papillomavirus 16 DNA and viral Harvey ras. Oncogene. 1989 Apr;4(4):395–399. [PubMed] [Google Scholar]
- Dyson N., Howley P. M., Münger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989 Feb 17;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Dürst M., Dzarlieva-Petrusevska R. T., Boukamp P., Fusenig N. E., Gissmann L. Molecular and cytogenetic analysis of immortalized human primary keratinocytes obtained after transfection with human papillomavirus type 16 DNA. Oncogene. 1987;1(3):251–256. [PubMed] [Google Scholar]
- Dürst M., Gallahan D., Jay G., Rhim J. S. Glucocorticoid-enhanced neoplastic transformation of human keratinocytes by human papillomavirus type 16 and an activated ras oncogene. Virology. 1989 Dec;173(2):767–771. doi: 10.1016/0042-6822(89)90595-3. [DOI] [PubMed] [Google Scholar]
- Hawley-Nelson P., Vousden K. H., Hubbert N. L., Lowy D. R., Schiller J. T. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 1989 Dec 1;8(12):3905–3910. doi: 10.1002/j.1460-2075.1989.tb08570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson J. B., Bedell M. A., McCance D. J., Laiminis L. A. Immortalization and altered differentiation of human keratinocytes in vitro by the E6 and E7 open reading frames of human papillomavirus type 18. J Virol. 1990 Feb;64(2):519–526. doi: 10.1128/jvi.64.2.519-526.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp A. C., Franke W. W. Spontaneous losses of control of cytokeratin gene expression in transformed, non-epithelial human cells occurring at different levels of regulation. Cell. 1989 Oct 6;59(1):67–79. doi: 10.1016/0092-8674(89)90870-2. [DOI] [PubMed] [Google Scholar]
- McCance D. J., Kopan R., Fuchs E., Laimins L. A. Human papillomavirus type 16 alters human epithelial cell differentiation in vitro. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7169–7173. doi: 10.1073/pnas.85.19.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisels A., Fortin R., Roy M. Condylomatous lesions of the cervix. II. Cytologic, colposcopic and histopathologic study. Acta Cytol. 1977 May-Jun;21(3):379–390. [PubMed] [Google Scholar]
- Mincheva A., Gissmann L., zur Hausen H. Chromosomal integration sites of human papillomavirus DNA in three cervical cancer cell lines mapped by in situ hybridization. Med Microbiol Immunol. 1987;176(5):245–256. doi: 10.1007/BF00190531. [DOI] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Münger K., Phelps W. C., Bubb V., Howley P. M., Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989 Oct;63(10):4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münger K., Werness B. A., Dyson N., Phelps W. C., Harlow E., Howley P. M. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 1989 Dec 20;8(13):4099–4105. doi: 10.1002/j.1460-2075.1989.tb08594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecoraro G., Morgan D., Defendi V. Differential effects of human papillomavirus type 6, 16, and 18 DNAs on immortalization and transformation of human cervical epithelial cells. Proc Natl Acad Sci U S A. 1989 Jan;86(2):563–567. doi: 10.1073/pnas.86.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirisi L., Yasumoto S., Feller M., Doniger J., DiPaolo J. A. Transformation of human fibroblasts and keratinocytes with human papillomavirus type 16 DNA. J Virol. 1987 Apr;61(4):1061–1066. doi: 10.1128/jvi.61.4.1061-1066.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rader J. S., Golub T. R., Hudson J. B., Patel D., Bedell M. A., Laimins L. A. In vitro differentiation of epithelial cells from cervical neoplasias resembles in vivo lesions. Oncogene. 1990 Apr;5(4):571–576. [PubMed] [Google Scholar]
- Rice R. H., Green H. Presence in human epidermal cells of a soluble protein precursor of the cross-linked envelope: activation of the cross-linking by calcium ions. Cell. 1979 Nov;18(3):681–694. doi: 10.1016/0092-8674(79)90123-5. [DOI] [PubMed] [Google Scholar]
- Richart R. M. Cervical intraepithelial neoplasia. Pathol Annu. 1973;8:301–328. [PubMed] [Google Scholar]
- Schlegel R., Phelps W. C., Zhang Y. L., Barbosa M. Quantitative keratinocyte assay detects two biological activities of human papillomavirus DNA and identifies viral types associated with cervical carcinoma. EMBO J. 1988 Oct;7(10):3181–3187. doi: 10.1002/j.1460-2075.1988.tb03185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seedorf K., Krämmer G., Dürst M., Suhai S., Röwekamp W. G. Human papillomavirus type 16 DNA sequence. Virology. 1985 Aug;145(1):181–185. doi: 10.1016/0042-6822(85)90214-4. [DOI] [PubMed] [Google Scholar]
- Werness B. A., Levine A. J., Howley P. M. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990 Apr 6;248(4951):76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- Woodworth C. D., Doniger J., DiPaolo J. A. Immortalization of human foreskin keratinocytes by various human papillomavirus DNAs corresponds to their association with cervical carcinoma. J Virol. 1989 Jan;63(1):159–164. doi: 10.1128/jvi.63.1.159-164.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth C. D., Waggoner S., Barnes W., Stoler M. H., DiPaolo J. A. Human cervical and foreskin epithelial cells immortalized by human papillomavirus DNAs exhibit dysplastic differentiation in vivo. Cancer Res. 1990 Jun 15;50(12):3709–3715. [PubMed] [Google Scholar]