Abstract
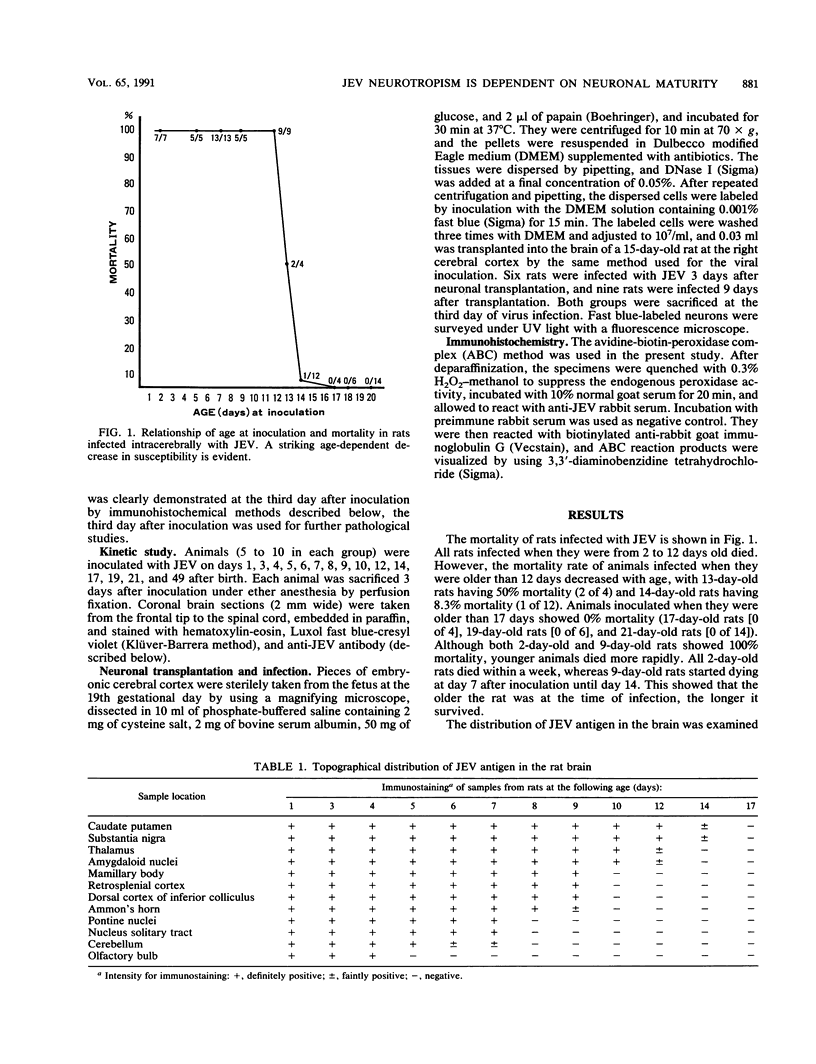
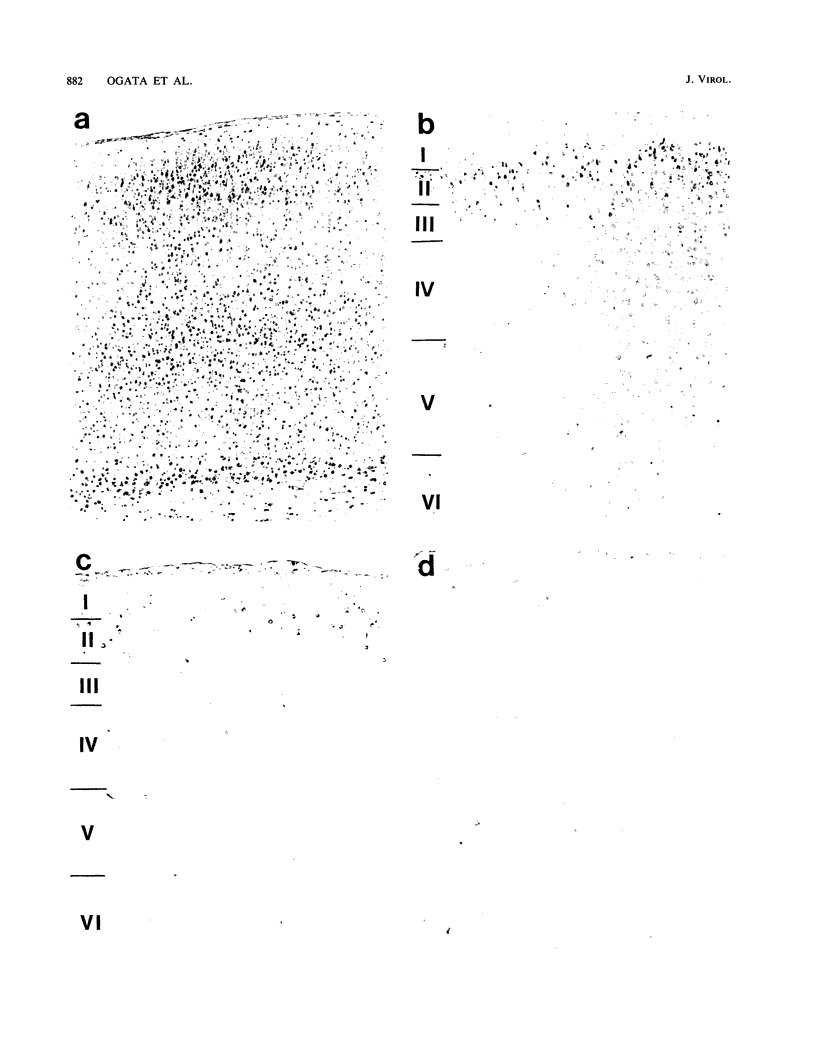
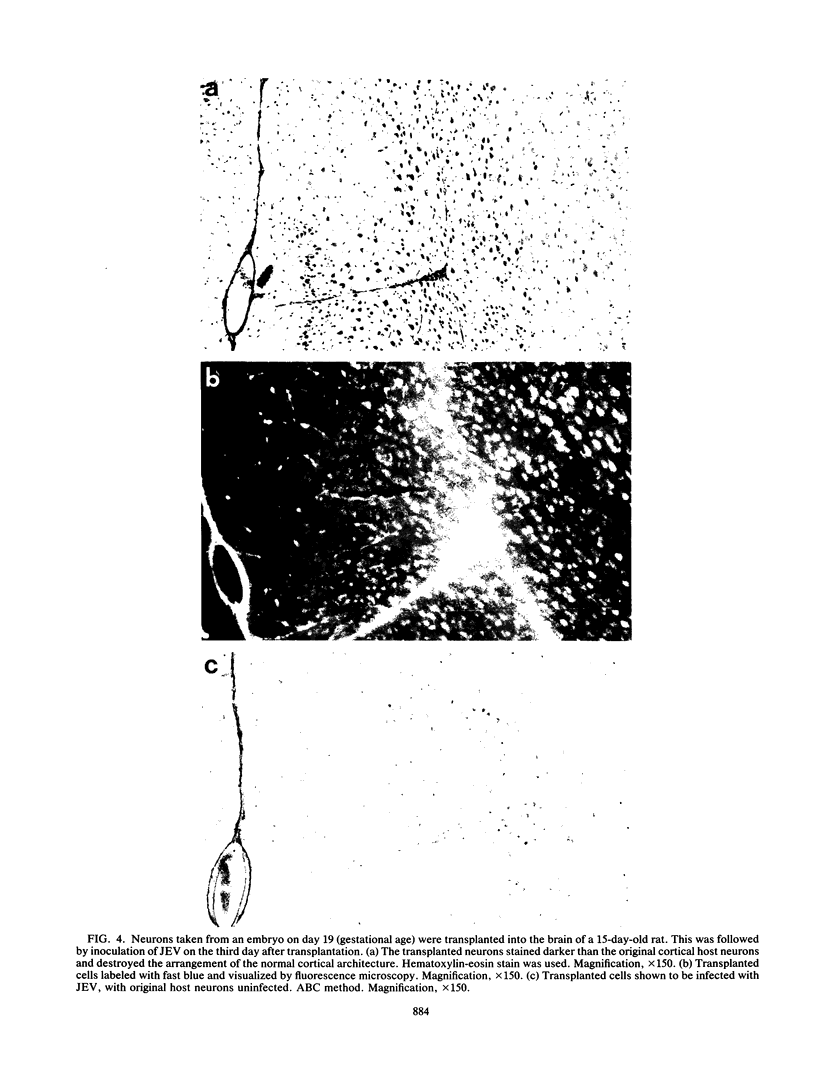
In a study on Fischer rats, all animals infected with Japanese encephalitis virus (JEV) before the age of 13 days died, but animals infected after the age of 14 days did not die, confirming the age-dependent resistance to JEV infection in the rat brain. A study of the kinetics of JEV infection in the developing rat brain disclosed that JEV antigen disappeared in a particular pattern, i.e., from the deeper layers to the upper layers of the motor cortex, which paralleled neuronal maturation in the cortex. Fifteen-day-old rats, which were resistant to JEV infection, received intracerebral transplants of neurons taken from 19-day embryos. When these animals were infected with JEV after transplantation, viral antigen was detected only in the embryonal neurons soon after transplantation. Thus, it can be concluded that the susceptibility to JEV infection in the rat brain is closely associated with neuronal immaturity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angevine J. B., Jr, Sidman R. L. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961 Nov 25;192:766–768. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- EAYRS J. T., GOODHEAD B. Postnatal development of the cerebral cortex in the rat. J Anat. 1959 Oct;93:385–402. [PMC free article] [PubMed] [Google Scholar]
- Greve J. M., Davis G., Meyer A. M., Forte C. P., Yost S. C., Marlor C. W., Kamarck M. E., McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989 Mar 10;56(5):839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- Grossberg S. E., Scherer W. F. The effect of host age, virus dose and route of inoculation on inapparent infection in mice with Japanese encephalitis virus. Proc Soc Exp Biol Med. 1966 Oct;123(1):118–124. doi: 10.3181/00379727-123-31418. [DOI] [PubMed] [Google Scholar]
- Ichikawa M., Hirata Y. Morphology and distribution of postnatally generated glial cells in the somatosensory cortex of the rat: an autoradiographic and electron microscopic study. Brain Res. 1982 Aug;256(4):369–377. doi: 10.1016/0165-3806(82)90180-8. [DOI] [PubMed] [Google Scholar]
- Iwasaki Y., Zhao J. X., Yamamoto T., Konno H. Immunohistochemical demonstration of viral antigens in Japanese encephalitis. Acta Neuropathol. 1986;70(1):79–81. doi: 10.1007/BF00689518. [DOI] [PubMed] [Google Scholar]
- Jaeger C. B., Lund R. D. Transplantation of embryonic occipital cortex to the brain of newborn rats: a Golgi study of mature and developing transplants. J Comp Neurol. 1981 Aug 1;200(2):213–230. doi: 10.1002/cne.902000204. [DOI] [PubMed] [Google Scholar]
- Johnson R. T., Burke D. S., Elwell M., Leake C. J., Nisalak A., Hoke C. H., Lorsomrudee W. Japanese encephalitis: immunocytochemical studies of viral antigen and inflammatory cells in fatal cases. Ann Neurol. 1985 Nov;18(5):567–573. doi: 10.1002/ana.410180510. [DOI] [PubMed] [Google Scholar]
- Kenney S., Natarajan V., Strike D., Khoury G., Salzman N. P. JC virus enhancer-promoter active in human brain cells. Science. 1984 Dec 14;226(4680):1337–1339. doi: 10.1126/science.6095453. [DOI] [PubMed] [Google Scholar]
- Kristt D. A. Neuronal differentiation in somatosensory cortex of the rat. I. Relationship to synaptogenesis in the first postnatal week. Brain Res. 1978 Jul 21;150(3):467–486. doi: 10.1016/0006-8993(78)90814-4. [DOI] [PubMed] [Google Scholar]
- Lentz T. L. Binding of viral attachment protein to host-cell receptor: the Achilles heel of infectious viruses. Trends Pharmacol Sci. 1988 Jul;9(7):247–252. doi: 10.1016/0165-6147(88)90154-X. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C. L., Wimmer E., Racaniello V. R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989 Mar 10;56(5):855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- Sabin A. B. Nature of Inherited Resistance to Viruses Affecting the Nervous System. Proc Natl Acad Sci U S A. 1952 Jun;38(6):540–546. doi: 10.1073/pnas.38.6.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staunton D. E., Merluzzi V. J., Rothlein R., Barton R., Marlin S. D., Springer T. A. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell. 1989 Mar 10;56(5):849–853. doi: 10.1016/0092-8674(89)90689-2. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi H., Mori C., Fuke I., Morita K., Kuhara S., Kondou J., Kikuchi Y., Nagamatu H., Igarashi A. Complete nucleotide sequence of the Japanese encephalitis virus genome RNA. Virology. 1987 Dec;161(2):497–510. doi: 10.1016/0042-6822(87)90144-9. [DOI] [PubMed] [Google Scholar]
- White J. M., Littman D. R. Viral receptors of the immunoglobulin superfamily. Cell. 1989 Mar 10;56(5):725–728. doi: 10.1016/0092-8674(89)90674-0. [DOI] [PubMed] [Google Scholar]
- Zimmerman H. M. The Pathology of Japanese B Encephalitis. Am J Pathol. 1946 Sep;22(5):965–991. [PMC free article] [PubMed] [Google Scholar]






