Abstract
Activation of protein kinase C (PKC) protects the heart from ischemic injury; however, its mechanism of action is unknown, in part because no model for chronic activation of PKC has been available. To test whether chronic, mild elevation of PKC activity in adult mouse hearts results in myocardial protection during ischemia or reperfusion, hearts isolated from transgenic mice expressing a low level of activated PKCβ throughout adulthood (β-Tx) were compared with control hearts before ischemia, during 12 or 28 min of no-flow ischemia, and during reperfusion. Left-ventricular-developed pressure in isolated isovolumic hearts, normalized to heart weight, was similar in the two groups at baseline. However, recovery of contractile function was markedly improved in β-Tx hearts after either 12 (97 ± 3% vs. 69 ± 4%) or 28 min of ischemia (76 ± 8% vs. 48 ± 3%). Chelerythrine, a PKC inhibitor, abolished the difference between the two groups, indicating that the beneficial effect was PKC-mediated. 31P NMR spectroscopy was used to test whether modification of intracellular pH and/or preservation of high-energy phosphate levels during ischemia contributed to the cardioprotection in β-Tx hearts. No difference in intracellular pH or high-energy phosphate levels was found between the β-Tx and control hearts at baseline or during ischemia. Thus, long-term modest increase in PKC activity in adult mouse hearts did not alter baseline function but did lead to improved postischemic recovery. Furthermore, our results suggest that mechanisms other than reduced acidification and preservation of high-energy phosphate levels during ischemia contribute to the improved recovery.
Activation of protein kinase C (PKC) has been postulated to play an important role in modulating cardiac contractile function and cellular growth (1). Furthermore, there are a number of studies showing that PKC inhibitors block, whereas PKC activators mimic, the cardioprotective effect of ischemic preconditioning in a variety of animal models (2–5) and, importantly, in human myocytes (6). However, studies with PKC activators have two major limitations. First, widely used PKC activators, such as phorbol ester, are nonselective and cause significant changes in contractile function in normoxic hearts. Second, experiments with PKC activators can be performed only in an acute setting; they cannot be used as therapeutic agents because of their divergent effects on multiple organ systems.
Recently, by using the binary tetracycline-controlled transactivator (tTA) system, a transgenic mouse line with conditional expression of a constitutively active PKC β-isoform (PKCβ*) has been developed (7, 8). The transgene is targeted to cardiac myocytes with the rat α-myosin heavy-chain promoter. By using this strategy, a modest increase in PKC activity can be achieved in a spatially and temporally restricted fashion (7). If it can be shown that chronic expression of PKCβ* is cardioprotective, we will have an unique model to (i) define the mechanism(s) underlying PKC-mediated myocardial protection and (ii) explore the feasibility of achieving a chronically preconditioned state by gene manipulation.
Accordingly, the purpose of this study is to test whether chronic overexpression of PKCβ* in adult mouse hearts results in myocardial protection during ischemia or reperfusion. In addition, we also test whether two of the mechanisms proposed to explain ischemic preconditioning (9–11)—namely, changes in intracellular pH (pHi) and high-energy phosphate contents—occur during ischemia and reperfusion in these hearts. Our results show that long-term expression of PKCβ* at a low level led to improved postischemic recovery in hearts isolated from the transgenic mice. Expressing PKCβ* did not alter the changes in pHi or high-energy phosphate contents during ischemia, suggesting that mechanisms other than modification of pHi and preservation of high-energy phosphates during ischemia contribute to the improved recovery in these hearts.
Methods
Animals.
The strategy of tTA-regulated transgene expression and characteristics of the tTA/PKCβ* mice have been described (7, 12). Briefly, two lines of mice, both from the C57BL6 background, were generated and crossed. In one line, a rat α-myosin heavy-chain promoter drives cardiac-specific expression of tTA. In the second line, the target gene, a constitutively active bovine PKCβ, was controlled by a heptamerized tetracycline operator/cytomegalovirus minimal promoter. Expression of PKCβ* was suppressed during fetal development and in the first 10 weeks after birth by supplying tetracycline (1 mg/ml) in the drinking water to pregnant animals throughout gestation and to young animals after weaning. PKCβ* expression was turned on by eliminating tetracycline from the drinking water in binary transgenic mice (tTA/PKCβ*, +/+). Previous studies showed that eliminating tetracycline quickly initiated a sustained low-level expression of PKCβ* in the hearts (7). Mice were studied 10–12 months after the transgene expression was turned on. Wild-type (tTA/PKCβ* −/−) and single transgenic PKCβ*-nonexpressing (tTA/PKCβ* −/+ or tTA/PKCβ* +/−) littermates were used as controls. The genotype of each animal was determined by PCR by using standard techniques as described (7).
Isolated Perfused Heart Preparation.
Mice were heparinized (≈100 units, i.p.) 30 min before experiments and were killed by cervical dislocation. The heart was excised, arrested in ice-cold buffer, and connected via the aorta to the perfusion cannula. Retrograde perfusion was maintained at a constant pressure of 70 mmHg (1 mmHg = 133 Pa). A short polyethylene drain was pierced through the apex of the left ventricle (LV) to drain the effluent from the thebesian veins. A water-filled balloon was inserted into the LV for recording of ventricular pressure and heart rate. Balloon volume was adjusted to achieve an end-diastolic pressure of 5–10 mmHg. Coronary flow (CF) rate was monitored by collecting coronary sinus effluent. Hearts were perfused with phosphate-free Krebs–Henseleit buffer containing 118 mM NaCl, 25 mM NaHCO3, 5.3 mM KCl, 2.0 mM CaCl2, 1.2 mM MgSO4, 0.5 mM EDTA, 11 mM glucose, and 0.5 mM pyruvate. The perfusate was equilibrated with 95% O2 and 5% CO2 (pH 7.4). Temperature was maintained at 37°C by using water-jacketing as well as the variable temperature unit in the magnet.
31P NMR Spectroscopy.
The isolated perfused mouse heart was placed in an 10-mm NMR sample tube and inserted into a 1H/31P double-tuned probe situated in a 89-cm bore, 9.4-T superconducting magnet. Spectra were collected by using a 60° flip angle, 15-μs pulse, 2.14-s delay, 6,000-Hz sweep width, and 2 K data points on a GE-400 Omega NMR spectrometer. Spectra were analyzed by using 20-Hz exponential multiplication and zero- and first-order phase corrections, and the resonance peak areas were fitted by Lorentzian line shapes by using NMR1 (New Methods Research, Syracuse, NY). By comparing the peak areas of fully relaxed (recycle time of 15 s) and those of partially saturated (recycle time of 2.14 s) spectra, the correction factors for saturation were calculated for ATP (1.0), phosphocreatine (PCr; 1.2), and inorganic phosphate (Pi; 1.15).
Experimental Protocol.
Two experimental protocols were used to compare the contractile function, high-energy phosphate contents, and pHi in hearts from transgenic mice with chronic expression of PKCβ* (β-Tx) and their controls at baseline, during ischemia, and during reperfusion. Protocol I was used to study the effect of a short-term ischemia (12 min) in hearts paced at 7 Hz, and Protocol II was used to study the effect of a longer term of ischemia (28 min) in unpaced hearts. For each protocol, two baseline NMR spectra were collected simultaneously with measurement of LV pressure after a 20-min stabilization period. Each spectrum was obtained by averaging signals from 208 free-induction decays during a period of 8 min. Global no-flow ischemia was then imposed by clamping the perfusion line; meanwhile, hearts were surrounded by the perfusate, and the temperature was maintained at 37°C. During ischemia, functional measurements and NMR spectra were collected every 4 min to increase time resolution. Hearts were reperfused at the preischemic perfusion pressure for 24 min (Protocol I) or 40 min (Protocol II). Four 4-min NMR spectra were collected during the first 16 min of reperfusion followed by 8-min spectra for the rest of the reperfusion period. At the end of the experiment, the heart was removed from the perfusion apparatus, blotted, and weighed. The atria were cut away, and the ventricles were quickly frozen in liquid nitrogen and stored at −80°C.
To determine whether increased PKC activity was responsible for the increased tolerance to ischemia in β-Tx hearts, chelerythrine (CH), a PKC inhibitor, was infused to three control and three β-Tx hearts in Protocol II. CH was infused before ischemia at 1% of CF (final concentration of 3 μM) for 20 min, followed by an 8-min washout period during which a 31P NMR spectrum was collected. Baseline measurements were made before CH infusion and during the washout period.
Data Analysis.
The ATP content of the isolated perfused mouse heart, analyzed by HPLC assay, is 25.7 ± 3.2 nmol/mg protein for the control group (n = 5) and 24.1 ± 8.1 nmol/mg protein for the β-Tx group (n = 3) at the end of the stabilization period. By using a value of 0.18 mg of protein per mg blotted wet tissue and the value of 0.48 μl of intracellular water per mg blotted wet tissue (13), [ATP] was calculated to be 9.6 ± 1.2 and 9.0 ± 3.0 mM for the control and β-Tx groups, respectively (P = not significant). Therefore, the ATP peak areas of the NMR spectra obtained at baseline were normalized to these values. Concentrations of PCr and Pi for both groups were calculated by using the ratios of their peak areas to ATP peak areas. pHi was determined by comparing the chemical shift of Pi and PCr in each spectrum, because the chemical shift of Pi but not PCr varies with pH.
Statistical Analysis.
Results are presented as means ± SEM. Factorial ANOVA was used to compare the three PKCβ*-nonexpressing genotypes (tTA/PKCβ* −/−, −/+, and +/−). Because no difference was found among these three genotypes (see Results), they were pooled as the control group. Differences between the control group and β-Tx group treated with or without CH were compared by Student’s t test or one-way factorial ANOVA, and changes during ischemia and reperfusion were compared by repeated-measure ANOVA. All statistical analyses were performed with statview (Brain Power, Calabasas, CA), and a value of P < 0.05 was considered significant.
Results
The general characteristics of the mice studied are summarized in Table 1. The control group consisted of three genotypes (tTA/PKCβ* −/−, −/+, and +/−), all of which were PKCβ*-nonexpressing littermates of β-Tx mice. Among these three genotypes, body weights and heart weights were higher in −/− mice compared with the two other genotypes, most likely because they were all males. When heart weight was normalized to body weight, no difference was found among the three genotypes, and they were pooled as controls. Compared with the pooled controls, heart weight in the β-Tx group was higher (176 ± 10 vs. 148 ± 5 mg; P < 0.05), whereas LV chamber volume was not different (42 ± 3 vs. 48 ± 3 μl; P = not significant). When the heart weight was normalized to body weight, the heart weight to body weight ratio in β-Tx hearts was ≈16% higher than that of the controls (5.7 ± 0.3 vs. 4.9 ± 0.1 mg/g; P < 0.05). This result is consistent with previous data showing that β-Tx hearts develop mild concentric hypertrophy after long-term expression of PKCβ* in adulthood (7).
Table 1.
Body weight and heart weight
| Group | Genotype (tTA/PKCβ*) | No. | BW, g | HW, mg | HW/BW, mg/g |
|---|---|---|---|---|---|
| β-Tx | +/+ | 6m, 7f | 31 ± 1 | 176 ± 10† | 5.7 ± 0.3† |
| Control | −/+ | 1m, 9f | 28 ± 1 | 138 ± 7 | 5.0 ± 0.2 |
| +/− | 2m, 2f | 32 ± 4 | 144 ± 8 | 4.5 ± 0.3 | |
| −/− | 7m | 36 ± 2 | 163 ± 6 | 4.6 ± 0.2 | |
| Pooled | 10m, 11f | 31 ± 1 | 148 ± 5 | 4.9 ± 0.1 |
Data are shown as means ± SEM. BW, body weight; HW, heart weight; m, male; f, female. †, P < 0.05 vs. pooled controls.
Baseline Function in β-Tx Hearts.
Table 2 shows LV pressure and heart rate measured at baseline in isolated perfused hearts. Isovolumic contractile performance was also estimated by RPP. RPP was not different among the three genotypes in the control group but was higher in β-Tx hearts compared with that of the controls. The increase in RPP is due to an increase in LVSP in the β-Tx group. When LVEDP was set to 5–10 mmHg and heart rate was paced at 7 Hz, LVSP was 106 ± 6 in the β-Tx group vs. 84 ± 4 mmHg in its controls (P < 0.05). Because LV volume was similar in the two groups and because LV mass was increased in β-Tx hearts, LVSP was normalized by LV weight to assess contractile force per unit of myocardium and the systolic wall stress, assuming a spherical LV for both groups. LVSP per mg was not different between the two groups (0.94 ± 0.07 vs. 0.95 ± 0.08 mmHg/mg; P = not significant), suggesting that contractile force and wall stress were unaltered in β-Tx hearts. Baseline CF was comparable in both groups (15.2 ± 1.1 and 16.8 ± 1.7 ml per min per g for β-Tx and control hearts, respectively; P = not significant).
Table 2.
Baseline function
| Group | Genotype (tTA/PKCβ*) | No. | LVSP, mmHg | LVEDP, mmHg | HR, bpm | RPP, 103 mmHg/min |
|---|---|---|---|---|---|---|
| β-Tx, unpaced | +/+ | 8f | 131 ± 8 | 7 ± 1 | 331 ± 17 | 41 ± 5† |
| Control, unpaced | −/+ | 3f | 84 ± 11 | 6 ± 1 | 314 ± 27 | 25 ± 5 |
| +/− | 2m, 2f | 123 ± 20 | 6 ± 0 | 286 ± 49 | 31 ± 3 | |
| −/− | 4m | 90 ± 4 | 7 ± 1 | 332 ± 29 | 27 ± 2 | |
| Pooled | 6m, 5f | 104 ± 12 | 6 ± 1 | 310 ± 24 | 29 ± 2 | |
| β-Tx, paced | +/+ | 5m, 3f | 106 ± 6 | 8 ± 1 | 420 ± 0 | 45 ± 3† |
| Control, paced | −/+ | 1m, 6f | 84 ± 4 | 8 ± 1 | 420 ± 0 | 35 ± 2 |
Data are shown as means ± SEM. LVSP, left ventricular systolic pressure; LVEDP, LV end-diastolic pressure; HR, heart rate; bpm, beats per min; RPP, rate pressure product; m, male; f, female. †, P < 0.05 vs. controls.
Cardiac Performance During 12-Min Ischemia and 24-Min Reperfusion.
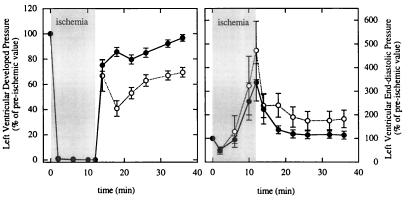
The changes in LV function for β-Tx and controls during ischemia and reperfusion are shown in Fig. 1. All hearts were paced at 7 Hz throughout the experiment. Because the baseline measurements have been shown in Table 2, this figure shows the percentage changes of baseline level during ischemia and reperfusion. Left-ventricular-developed pressure decreased to zero within the first 2 min of ischemia in both groups and remained at zero throughout the ischemic period. LVEDP fell to values less than the preischemic level in the first 6-min period of ischemia but increased substantially in the second 6-min period of ischemia in both groups (P < 0.05 vs. baseline). The increase in LVEDP was variable among individual hearts but, on average, was not different between the two groups (P = not significant, as determined by repeated-measure ANOVA). On reperfusion, eight of eight β-Tx and six of seven control hearts resumed beating within the first 2 min. The median times between reperfusion and recorded rhythmic beating were 41 s (range: 25–120 s) and 40 s (30–483 s) for β-Tx and control hearts, respectively (P = not significant). However, recovery in LV-developed pressure was higher in β-Tx compared with control hearts during the entire reperfusion period (P < 0.05). By the end of reperfusion, LV-developed pressure in β-Tx hearts recovered to 97% of the preischemia value, whereas LV-developed pressure recovered to only 69% in control hearts (P < 0.05). LVEDP returned to the preischemic value in β-Tx hearts at the end of reperfusion (9 ± 2 vs. 8 ± 1 mmHg; P = not significant) but remained higher in the controls (15 ± 3 vs. 9 ± 1 mmHg; P < 0.05). The changes in CF were similar in the two groups (data not shown); by the end of reperfusion, CF was 95 ± 4% and 90 ± 2% of the baseline level for β-Tx and control hearts, respectively (P = not significant).
Figure 1.
Percentage changes in LV-developed pressure (Left) and LVEDP (Right) in control (open symbols) and β-Tx (filled symbols) hearts during 12-min ischemia and 24-min reperfusion. Data are shown as means ± SEM. The ischemia period is indicated by the shaded area.
Cardiac Performance During 28-Min Ischemia and 40-Min Reperfusion.
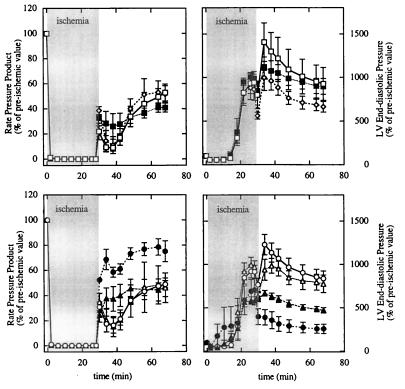
Two comparisons were made in this protocol. First, LVEDP and RPP during ischemia and reperfusion for the three genotypes in control group were compared (Fig. 2 Upper). No significant difference was observed among these three genotypes; thus, they were pooled as the control group. Second, LVEDP and RPP during ischemia and reperfusion were compared for β-Tx and the control group treated with or without CH (Fig. 2 Lower). As shown for the 12-min ischemia, all hearts stopped beating within first 2 min. LVEDP decreased slightly during first 6 min of ischemia and then increased significantly in both β-Tx and control groups. At the end of 28-min ischemia, LVEDP was higher in controls compared with β-Tx hearts (55 ± 1 vs. 44 ± 4 mmHg; P < 0.05). During reperfusion, recovery of RPP was higher in β-Tx hearts throughout the 40-min period and reached 76 ± 8% at the end of reperfusion, whereas control hearts recovered only 48 ± 3% (P < 0.05). LVEDP also decreased markedly in β-Tx hearts during reperfusion; in contrast, LVEDP initially increased in control hearts followed by a slow decline. At the end of reperfusion, LVEDP was substantially higher in controls compared with β-Tx hearts (51 ± 3 vs. 17 ± 3 mmHg; P < 0.05).
Figure 2.
Percentage changes in RPP (Left) and LVEDP (Right) in control and β-Tx hearts during 28-min ischemia and 40-min reperfusion. (Upper) Comparisons among the three genotypes used for controls (open square for −/+; filled square for +/−; open diamond for −/−). (Lower) Comparisons between pooled control (open symbols) and β-Tx (filled symbols) hearts treated with (triangles) or without (circles) CH. Data are shown as means ± SEM. The ischemia period is indicated by the shaded area.
To assess the contribution of PKC activity to the differences observed between β-Tx and control hearts, three β-Tx and three control hearts were treated with CH, a PKC inhibitor, for 20 min before ischemia. Treatment with CH did not alter LVEDP or RPP at baseline in either group. Although CH did not alter the response to ischemia and reperfusion in control hearts, CH significantly decreased the recovery during reperfusion in β-Tx hearts. At the end of reperfusion, RPP in β-Tx hearts treated with CH recovered to the same low extent as did controls (45 ± 7% vs. 47 ± 11%; P = not significant). Treatment with CH did not alter the changes in LVEDP during ischemia for β-Tx hearts but resulted in a higher LVEDP during reperfusion compared with untreated β-Tx hearts (P < 0.05, as determined by repeated-measure ANOVA). At the end of reperfusion, LVEDP was 17 ± 3 mmHg for β-Tx hearts, 36 ± 4 mmHg for β-Tx hearts treated with CH, and 44 ± 7 mmHg for control hearts treated with CH. Because baseline LVEDP was 7 ± 1, 8 ± 1, and 6 ± 1 mmHg for these three groups, LVEDP was 2.6 ± 0.5, 4.7 ± 0.3, and 7.8 ± 1 times baseline values for these three groups at the end of reperfusion, respectively (Fig. 2).
31P NMR Measurements.
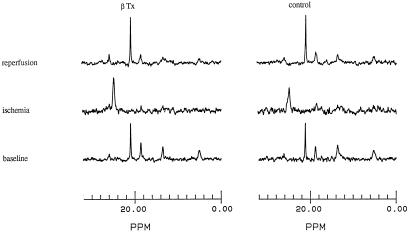
Representative 31P NMR spectra at baseline, at the end of ischemia, and at the end of reperfusion for a control heart and a β-Tx heart from the short-term ischemia protocol are shown in Fig. 3. From left to right, the peaks are for Pi, PCr, and γ-, α-, and β-phosphates of ATP. The area under the peak represents the amount of each compound in the heart. The baseline spectra were similar for the β-Tx and the control heart. Adequate perfusion was evidenced by a high level of PCr relative to ATP and normal pHi in both hearts. During ischemia, PCr and ATP were depleted, whereas Pi increased substantially in both hearts. The chemical shift of Pi was moved toward the right, indicating a marked intracellular acidosis during ischemia. During reperfusion, PCr recovered completely, but ATP recovered by only ≈50% in both hearts. The chemical shift of Pi returned to the preischemia position, indicating a full recovery of pHi. The changes were similar in the β-Tx heart compared with the control heart.
Figure 3.
Representative 31P NMR spectra for a β-Tx (Left) and a control (Right) heart at baseline (Bottom), at the end of 12-min ischemia (Middle), and at the end of 24-min reperfusion (Top). For each spectrum, from left to right, the peaks represent Pi, PCr, and γ-, α-, and β-phosphates of ATP. The difference in the chemical shift of Pi and PCr is proportional to the pHi. Note the shift of Pi peak during ischemia, indicating intracellular acidosis.
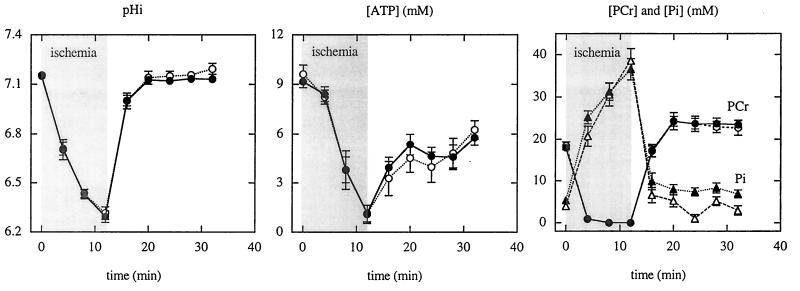
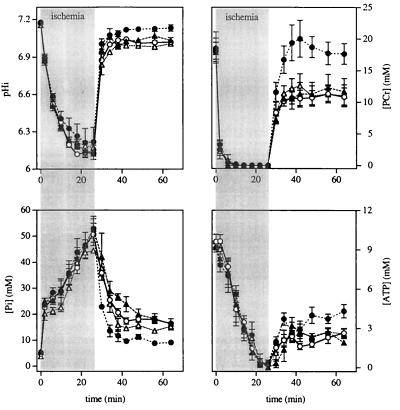
As shown in Fig. 4 and Fig. 5, NMR measurements before ischemia were not different in β-Tx hearts compared with controls. pHi was 7.15 ± 0.01 in β-Tx hearts and 7.16 ± 0.01 in controls (P = not significant). Preischemia levels of [ATP], [PCr], and [Pi] were also similar in the two groups. The PCr/ATP ratio was 1.76 and 1.84 for β-Tx and control hearts, respectively. Fig. 4 shows the time-dependent changes in pHi, [ATP], [PCr], and [Pi] during 12-min ischemia and 24-min reperfusion in the two groups. pHi decreased to 6.29 ± 0.04 and 6.31 ± 0.03 during ischemia and recovered to 7.13 ± 0.01 and 7.18 ± 0.03 during reperfusion in β-Tx and control hearts, respectively. The time course for the changes in pHi was not different in the two groups (P = not significant, as determined by repeated-measure ANOVA). For both groups, [ATP] was decreased by ≈20% during the first 4 min of ischemia, whereas PCr was rapidly depleted to an undetectable level; Pi was markedly increased. As ischemia progressed, [ATP] decreased, and [Pi] increased even further. By the end of 12-min ischemia, [PCr] was undetectable; [ATP] was barely detectable (≈1 mM); and [Pi] reached ≈40 mM. During reperfusion, PCr recovered quickly and “overshot” the preischemia value in both groups. Pi returned to preischemia levels in the control and was slightly higher than the preischemic level in β-Tx hearts (P < 0.05). ATP, however, recovered to only 50–60% of the preischemia level in both groups. The changes in high-energy phosphate contents were not different in the two groups (P = not significant, as determined by repeated-measure ANOVA). The total intracellular phosphate, assessed by adding 3× [ATP], [PCr], and [Pi], was unaltered. At the end of the reperfusion period, the total phosphate was 108 ± 2% and 99 ± 8% of baseline values for β-Tx and control hearts, respectively.
Figure 4.
Group means for pHi (Left), [ATP] (Center), and [PCr] and [Pi] (Right) at baseline, during 12-min ischemia and 24-min reperfusion for control (open symbols) and β-Tx (filled symbols) hearts. Data are shown as means ± SEM. The ischemia period is indicated by the shaded area.
Figure 5.
Group means for pHi, [Pi], [PCr], and [ATP] at baseline, during 28-min ischemia and 40-min reperfusion for control (open symbols) and β-Tx (filled symbols) hearts treated with (triangles) or without (circles) CH. Data are shown as means ± SEM. The ischemia period is indicated by the shaded area.
Fig. 5 shows the time-dependent changes in pHi, [ATP], [PCr], and [Pi] during 28-min ischemia and 40-min reperfusion in β-Tx and control hearts treated with or without CH. pHi decreased to 6.22 ± 0.12 and 6.13 ± 0.02 (P = not significant) during ischemia and recovered to 7.13 ± 0.02 and 7.02 ± 0.01 (P < 0.05) during reperfusion in β-Tx and control hearts, respectively. Analysis by repeated-measure ANOVA showed that the decline in pHi during ischemia was not different between the two groups, but the recovery of pHi during reperfusion was faster in the β-Tx group (P < 0.05). During ischemia, [PCr] and [ATP] decreased, whereas [Pi] markedly increased in both groups. By the end of 28-min ischemia, [PCr] and [ATP] were undetectable and [Pi] reached ≈50 mM. There was no difference in the time course for these changes between the two groups. During reperfusion, PCr in β-Tx hearts recovered to the preischemia value in the first 10 min and was maintained at that level throughout the reperfusion period. In contrast, [PCr] in control hearts recovered to only 60% of the preischemia level. Furthermore, β-Tx hearts also showed improved recovery in Pi (9.1 ± 0.7 vs. 16.4 ± 0.7 mM) and ATP (4.3 ± 0.5 vs. 2.6 ± 0.3 mM) compared with the controls (P < 0.05). Total intracellular phosphate, assessed by summing 3× [ATP], [PCr], and [Pi], was decreased in both groups. Before ischemia, total intracellular phosphate was 53 ± 1 mM for both groups, and it decreased to 40 ± 3 and 34 ± 3 mM for β-Tx and control hearts, respectively, at the end of the reperfusion period. The difference between β-Tx and control hearts did not reach statistical significance (P = 0.08, as determined by repeated-measure ANOVA). Treatment with CH did not alter the metabolic profile before ischemia in either group but abolished the differences observed between β-Tx hearts and the controls during ischemia and reperfusion (Fig. 5).
Discussion
Postischemic Recovery in Hearts Expressing PKCβ*.
The major finding of this study is that hearts with long-term low-level increase of PKC activity (β-Tx) showed improved functional recovery after ischemia. Using two different durations of ischemia, we found that the recovery of LV contractile function was improved in β-Tx hearts compared with that of the controls after either 12-min ischemia (97% vs. 69%) or 28-min ischemia (76% vs. 48%). This improvement was abolished by acutely applying a PKC inhibitor, CH.
Before we attribute our finding to the PKC-related myocardial protection, alternative explanations need to be considered. One explanation for improved recovery in β-Tx hearts is that these hearts developed cardiac hypertrophy, which might contribute to a higher postischemic contractile function. This explanation is unlikely for two reasons. First, previous studies have shown that hypertrophied hearts are more, not less susceptible to ischemic injury (14, 15). For example, hearts hypertrophied because of pressure overload developed earlier and more severe diastolic dysfunction during ischemia and reperfusion, whereas systolic function was not different from that of the controls (15). In contrast, we found that both systolic and diastolic function improved in β-Tx hearts during reperfusion. Second, we showed, in this study, that treating β-Tx hearts with CH acutely, which would not alter cardiac hypertrophy, abolished the difference in postischemic recovery between β-Tx hearts and the controls.
Activation of PKC has been implicated in the signaling pathway leading to ischemic preconditioning (3). In acute experimental settings, pharmacological activation of PKC results in myocardial protection, whereas PKC inhibition abolishes the effect (2–6, 16, 17). In this study, we found that long-term expression of PKCβ* in adult mouse hearts also resulted in myocardial protection. This finding is important, because our results suggest that chronic myocardial protection can be achieved by mild elevation of PKC activity in the heart. Thus, this transgenic mouse provides us with a chronic model for studying the mechanism(s) of PKC-related myocardial protection.
PKC Isoforms and Myocardial Protection.
Several recent studies have suggested that myocardial protection achieved by ischemic preconditioning is associated with selective translocations of specific isoforms of PKC without a significant change in the total myocardial PKC activity (2, 18–20). Translocation of several PKC isoforms, e.g., α, δ, ɛ, and η, has been reported in preconditioned hearts. PKC β-isoform has not been implicated previously in the cardioprotection by ischemic preconditioning. In this study, we observed that chronic expression of PKCβ*, an activated β-isoform, also results in myocardial protection during ischemia.
There may be several explanations for this surprising finding. First, the transgene product in β-Tx hearts is constitutively active and may be independent of the isoform-specific activation and translocation. Second, because the genetic manipulation rendering PKCβ constitutively active involved a large internal deletion (amino acids 6–159) in the protein (7), the activated PKCβ may have lost some of its isoform-specific properties. Third, involvement of PKCβ in ischemic preconditioning may not have been identified in previous studies because of a low-level expression of this isoform in normal heart. Fourth, chronic expression of an activated PKC β-isoform might lead to alteration in the expression of other PKC isoforms. Because results from the present study do not confirm or exclude any of these possibilities, we cannot conclude that the observations made here are PKC β-isoform-specific. Nevertheless, our results show that one consequence of chronic expression of PKCβ* is improved postischemic recovery, and this effect depends on PKC activity. We suggest that this model will be useful for studying the effects of chronic activation of PKC in the heart as well as its mechanism(s) of myocardial protection.
pHi and High-Energy Phosphates.
It has been suggested, but not proven, that attenuating intracellular acidification during ischemia contributes to the beneficial effect of ischemic preconditioning (9, 10, 16). Although supplying PKC activators has been shown to stimulate Na+/H+ exchange (21), it remains to be established whether PKC activation results in reduced intracellular acidification and consequently contributes to the beneficial effect of preconditioning. By using the noninvasive tool of 31P NMR spectroscopy to monitor pHi, we found that long-term, low-level expression of PKCβ* in the adult mouse heart did not modify pHi either at baseline or during ischemia. Thus, decreased proton accumulation during ischemia is an unlikely explanation for improved postischemic recovery in β-Tx hearts. Our results support studies on other species showing that reduced acidification is not necessary for myocardial protection by PKC activation (16, 17, 22, 23). Our results show a previously uncharacterized aspect of pHi control in β-Tx hearts. Although the recovery of pHi was identical in all hearts after 12-min ischemia, recovery was faster in β-Tx hearts after 28-min ischemia.
Previous studies have also suggested that ischemic preconditioning slows the rate of ATP depletion and, thus, delays cellular damage during a prolonged ischemic insult (11, 17). Ischemic preconditioning decreases myocardial ATP content before the prolonged ischemia; PKC activation does not change the ATP content (16). When hearts treated with PKC activators were compared with untreated hearts, the rates of ATP depletion and recovery were similar during ischemia and reperfusion, but the functional recovery was improved in hearts with activated PKC (16). Consistent with these observations, we found that the rate of ATP depletion during ischemia was not different between β-Tx hearts and the control. Furthermore, improved postischemic recovery in function after 12-min ischemia in β-Tx hearts was not associated with higher [ATP]. For hearts subjected to 28-min ischemia, the recovery of [ATP] after reperfusion was higher in the β-Tx group, despite a similar pattern of ATP depletion during ischemia, which showed improved preservation of the purine pool. Taken together, these results indicate, first, that cardioprotection by PKC activation does not require a slower rate of ATP depletion during ischemia and, second, that the decreased rate of fall in ATP observed in preconditioned hearts may involve a mechanism other than PKC activation.
It is of interest to note that the postischemic recovery of PCr was only 60% in control hearts subjected to 28-min ischemia, whereas the recovery in β-Tx hearts was 100%. These results could not be explained by a greater loss of phosphate in the controls, because [Pi] was nearly twice as much in the control than in β-Tx hearts at the end of reperfusion. It is likely that the difference in [PCr] reflects an impairment in ATP synthesis and/or creatine kinase reaction rate. Thus, the ability to resynthesize high-energy phosphates during reperfusion was better preserved in β-Tx hearts, even though the rates and extents of depletion of PCr and ATP in these hearts during ischemia was not different from that of the controls.
Effect of Chronic PKC Activation on Contractile Function.
Previous studies with PKC activators have yielded major inconsistencies with regard to the effect of PKC activation on myocardial contractility (1, 17, 21). In the model we used here, expressing PKCβ* during early postnatal life results in alterations in the cytosolic Ca2+ transient and cell shortening in cardiac myocytes (7, 8). Initiating expression of a low level of PKCβ* in adult hearts resulted in a modest hypertrophy without significant pathological changes (7). In contrast to this model, a recent report showed that increasing cardiac PKCβ level by more than 10-fold in adult hearts led to hypertrophic cardiomyopathy and decreased contractility (24, 25). These results suggest that different timing and/or different levels of PKC expression have very different effects on cardiac structure and function.
With the same model used here, a previous study found that systolic pressure generation in vivo was not altered, but diastolic relaxation rates were blunted (7). In the present study, which used an isolated perfused heart preparation free of neurohormonal factors, we also found that baseline systolic function was not markedly altered. Furthermore, no LV dilatation was observed in these hearts even after expressing PKCβ* for 10 months. We observed a higher systolic pressure in β-Tx hearts by using isolated isovolumic heart preparations and setting LVEDP to 5–10 mmHg. However, LVSP per milligram of LV was similar in β-Tx and control hearts, suggesting an equivalent systolic wall stress. Because LVEDP was preset to a fixed level in our isovolumic heart preparation, baseline diastolic function could not be assessed in this study. Thus, we found that long-term low-level activation of PKCβ in adult hearts improves postischemic recovery without marked alterations in baseline isovolumic contractile function.
Limitations of the Study.
Given that the time of ischemia was short and that the total intracellular Pi was unaltered throughout the experiment, it is likely that 12-min ischemia did not cause significant myocyte necrosis in this study. Using this protocol, we test the effect of chronic PKC activation against “stunning.” In the second protocol, we used a longer period of ischemia, i.e., 28 min. This duration was chosen based on previous studies that used isolated perfused rat hearts in which a ischemia times of 20–40 min were used to study the mechanism(s) of cardioprotection by PKC activation or preconditioning (2, 5, 16, 20). Because we did not perform any assay for the markers of cell death, our study assesses only functional recovery and not cell necrosis.
We found that long-term, low-level expression of PKCβ* isoform in adult mouse hearts did not significantly alter baseline function but led to improved postischemic recovery in function. Furthermore, expressing PKCβ* did not alter either pHi or high-energy phosphate levels at baseline or during ischemia, suggesting that mechanisms other than reduced acidification and preservation of high-energy phosphate levels during ischemia contribute to the improved recovery.
Acknowledgments
We thank Heather Molleur for technical assistance. This study was supported by National Institutes of Health Grants SCOR HL 52350 (to J.S.I.), R01 HL15498 (to P.M.B.), R01 HL50594 (to J.S.I.), and R29 HL59246 (to R.T.), by American Heart Association National Scientist Development Grant (to R.T.), by a New York City Affiliate Grant-in-Aid (to P.M.B.). M.S. was supported by a Research Fellowship from the Deutsche Forschungsgemeinschaft.
Footnotes
Abbreviations: PKC, protein kinase C; PKCβ*, activated PKC β-isoform; tTA, tetracycline transactivator; Pi, inorganic phosphate; β-Tx, expressing PKCβ*; CH, chelerythrine; LV, left ventricle/ventricular; RPP, rate pressure product; LVEDP, LV end-diastolic pressure; LVSP, LV systolic pressure; CF, coronary flow; pHi, intracellular pH; PCr, phosphocreatine.
References
- 1.Puceat M, Brown J H. In: Protein Kinase C. Kuo J F, editor. Oxford: Oxford Univ. Press; 1994. pp. 249–268. [Google Scholar]
- 2.Mitchell M B, Meng X, Ao L, Brown J M, Harken A H, Banerjee A. Circ Res. 1995;76:73–81. doi: 10.1161/01.res.76.1.73. [DOI] [PubMed] [Google Scholar]
- 3.Cohen M V, Downey J M. Annu Rev Med. 1996;47:21–29. doi: 10.1146/annurev.med.47.1.21. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Tsuchida A, Cohen M V, Downey J M. J Mol Cell Cardiol. 1995;27:883–892. doi: 10.1016/0022-2828(95)90038-1. [DOI] [PubMed] [Google Scholar]
- 5.Bugge E, Ytrehus K. Cardiovasc Res. 1996;32:920–929. [PubMed] [Google Scholar]
- 6.Ikonomidis J S, Shirai T, Weisel R D, Derylo B, Rao V, Whiteside C I, Mickle D A, Li R K. Am J Physiol. 1997;272:H1220–H1230. doi: 10.1152/ajpheart.1997.272.3.H1220. [DOI] [PubMed] [Google Scholar]
- 7.Bowman J C, Steinberg S F, Jiang T, Geenen D L, Fishman G I, Buttrick P M. J Clin Invest. 1997;100:2189–2195. doi: 10.1172/JCI119755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinberg S F, Jiang T, Bowman J C, Fishman G I, Buttrick P M. Circulation. 1996;94:I159. (abstr.). [Google Scholar]
- 9.Steenbergen C, Perlman M E, London R E, Murphy E. Circ Res. 1993;72:112–125. doi: 10.1161/01.res.72.1.112. [DOI] [PubMed] [Google Scholar]
- 10.Wolfe C L, Sievers R E, Visseren F L J, Donnelly T J. Circulation. 1993;87:881–892. doi: 10.1161/01.cir.87.3.881. [DOI] [PubMed] [Google Scholar]
- 11.Murry C E, Richard V J, Reimer K A, Jennings R B. Circ Res. 1990;66:913–931. doi: 10.1161/01.res.66.4.913. [DOI] [PubMed] [Google Scholar]
- 12.Yu Z, Redfern C S, Fishman G I. Circ Res. 1996;75:691–697. doi: 10.1161/01.res.79.4.691. [DOI] [PubMed] [Google Scholar]
- 13.Polimeni P I, Buraczewski S I. J Mol Cell Cardiol. 1988;20:15–22. doi: 10.1016/s0022-2828(88)80175-5. [DOI] [PubMed] [Google Scholar]
- 14.Wexler L F, Lorell B H, Momomura S, Weinberg E O, Ingwall J S, Apstein C S. Circ Res. 1988;62:766–775. doi: 10.1161/01.res.62.4.766. [DOI] [PubMed] [Google Scholar]
- 15.Mochizuki T, Eberli F R, Ngoy S, Apstein C S, Lorell B H. Circ Res. 1993;73:550–558. doi: 10.1161/01.res.73.3.550. [DOI] [PubMed] [Google Scholar]
- 16.Chen W, Wetsel W, Steenbergen C, Murrphy E. J Mol Cell Cardiol. 1996;28:871–880. doi: 10.1006/jmcc.1996.0082. [DOI] [PubMed] [Google Scholar]
- 17.Watson J E, Karmazyn M. Circ Res. 1991;69:1114–1131. doi: 10.1161/01.res.69.4.1114. [DOI] [PubMed] [Google Scholar]
- 18.Gary M O, Mochly-Rosen D, Honbo N Y, Karliner J S. Circulation. 1995;92:I137. (abstr.). [Google Scholar]
- 19.Ping P, Zhang J, Qiu Y, Tang X-L, Manchikalapudi S, Cao X, Bolli R. Circ Res. 1997;81:404–414. doi: 10.1161/01.res.81.3.404. [DOI] [PubMed] [Google Scholar]
- 20.Miyawaki H, Ashraf M. Circ Res. 1997;80:790–799. doi: 10.1161/01.res.80.6.790. [DOI] [PubMed] [Google Scholar]
- 21.Steinberg S F, Goldberg M, Rybin V O. J Mol Cell Cardiol. 1995;27:141–153. doi: 10.1016/s0022-2828(08)80014-4. [DOI] [PubMed] [Google Scholar]
- 22.Ladilov Y V, Balser C, Piper H M. Circ Res. 1998;82:451–457. doi: 10.1161/01.res.82.4.451. [DOI] [PubMed] [Google Scholar]
- 23.Cave A C, Garlick P B. Am J Physiol. 1997;272:H544–H552. doi: 10.1152/ajpheart.1997.272.1.H544. [DOI] [PubMed] [Google Scholar]
- 24.Wakasaki H, Koya D, Schoen F J, Jirousek M R, Ways D K, Hoit B D, Walsh R A, King G L. Proc Natl Acad Sci USA. 1997;94:9320–9325. doi: 10.1073/pnas.94.17.9320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeishi Y, Chu G, Kirkpatrick D M, Li Z, Wakasaki H, Kranias E G, King G L, Walsh R A. J Clin Invest. 1998;102:72–78. doi: 10.1172/JCI2709. [DOI] [PMC free article] [PubMed] [Google Scholar]