Abstract
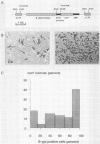
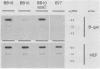
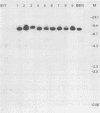
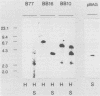
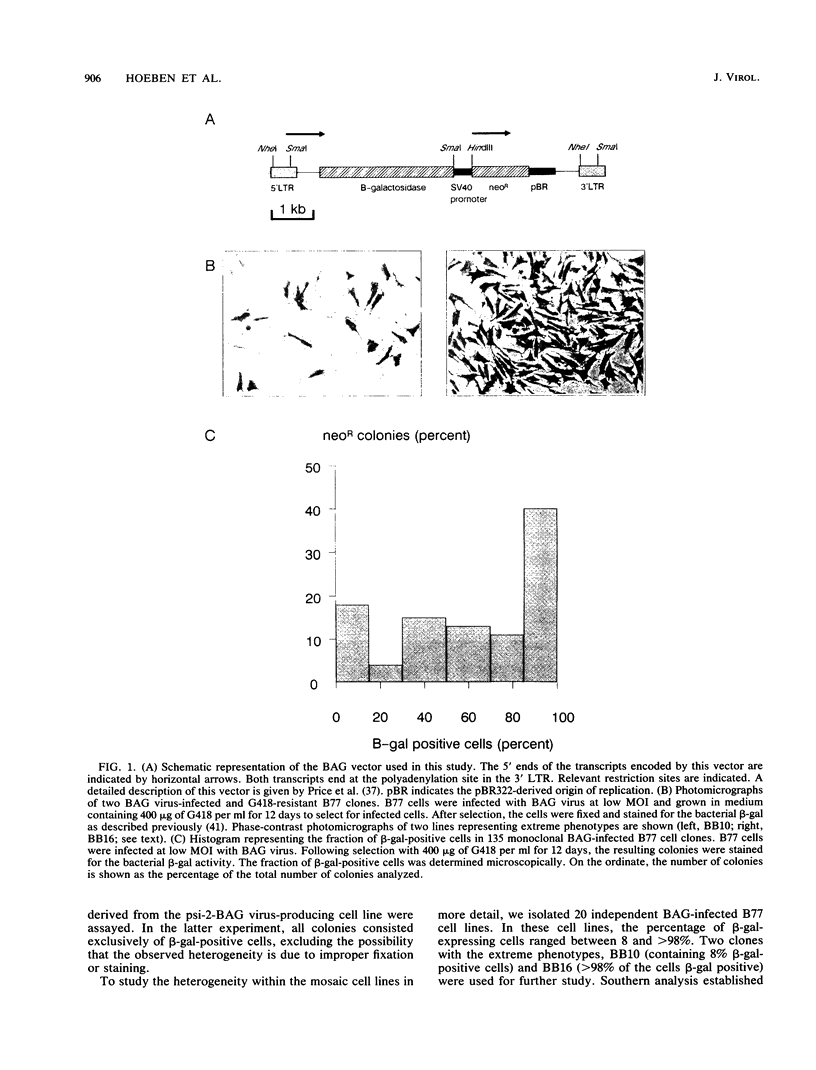
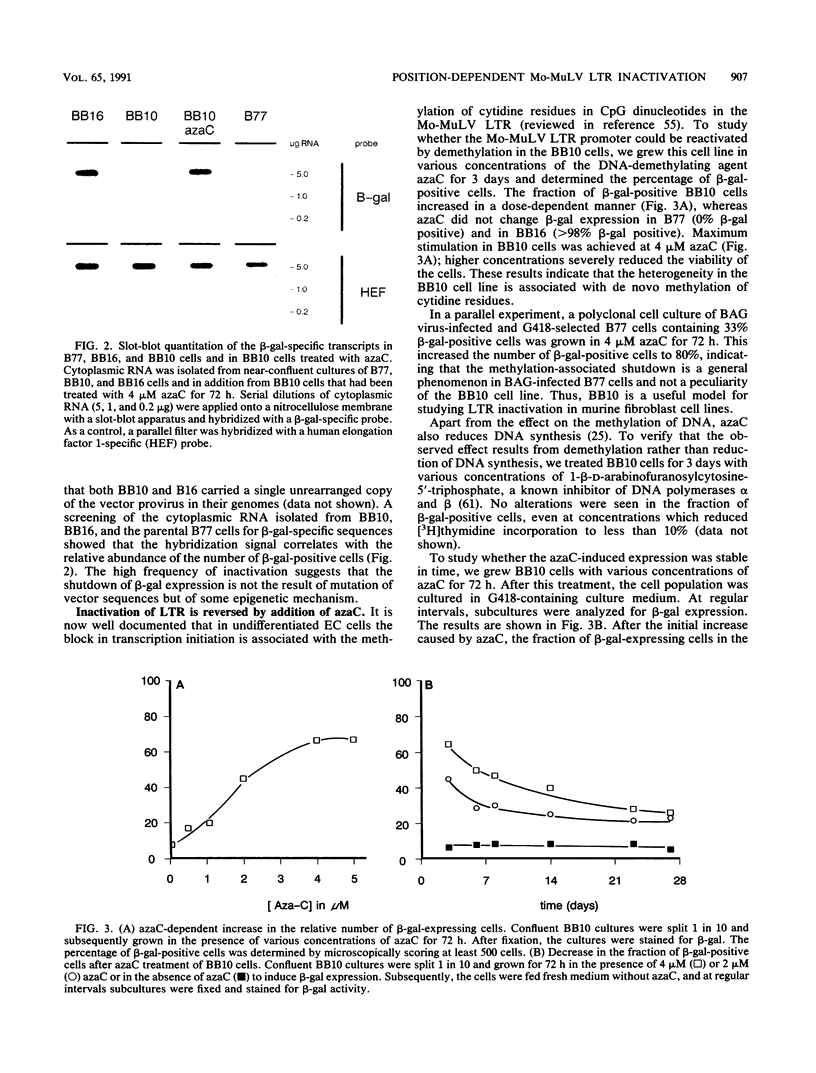
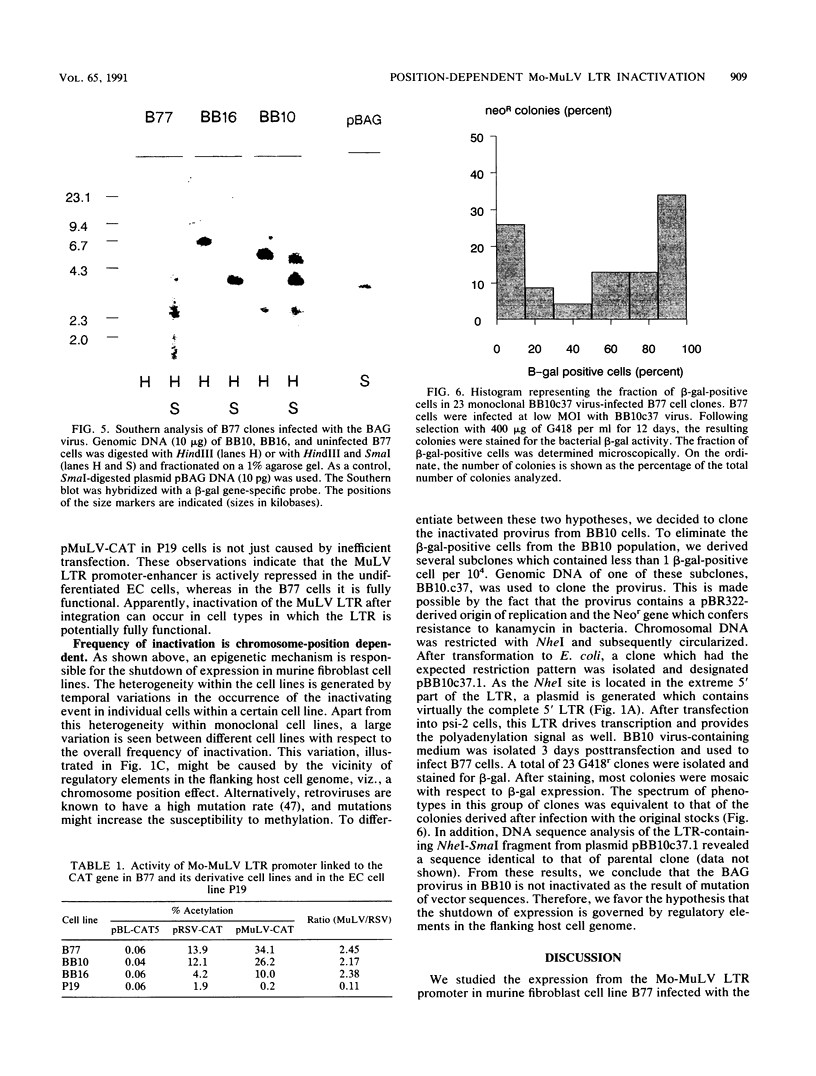
The expression of a retroviral vector with the Moloney murine leukemia virus (Mo-MuLV) long terminal repeat (LTR) promoter after integration into the genome of murine fibroblast cell lines was monitored with the Escherichia coli-derived beta-galactosidase (beta-gal) gene as the reporter. Monoclonal cell lines derived after retroviral infection exhibited a marked heterogeneity in their expression of the reporter gene. We studied two monoclonal cell lines with a single unrearranged copy of the vector provirus integrated into their genome. The first, BB10, expressed the marker enzyme in only 8% of its cell population, whereas in the second, BB16, beta-gal expression could be detected in over 98% of the cells. Treatment of BB10 with the DNA-demethylating agent 5-azacytidine raised the number of beta-gal-positive cells to over 60%. Transfection experiments showed that the Mo-MuLV LTR promoter-enhancer is potentially fully functional in both the BB10 and BB16 cell lines. The inactivated provirus from BB10 cells was cloned and subsequently used to generate retrovirus stocks. The promoter-enhancer activity of its LTR after infection with these BB10-derived viruses showed a variation similar to that of the original virus stocks. Our data showed that (1) inactivation of the Mo-MuLV LTR is a frequent event in murine fibroblast cell lines, (2) inactivation is associated with de novo methylation of cytidine residues, (3) the frequency of inactivation of the provirus must be determined by its chromosomal position, (4) the process of methylation of sequences within the LTR is not necessarily the same as the transcription-repression mechanism that is operating in undifferentiated embryonal carcinoma cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. L. DNA methylation. The effect of minor bases on DNA-protein interactions. Biochem J. 1990 Jan 15;265(2):309–320. doi: 10.1042/bj2650309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barklis E., Mulligan R. C., Jaenisch R. Chromosomal position or virus mutation permits retrovirus expression in embryonal carcinoma cells. Cell. 1986 Nov 7;47(3):391–399. doi: 10.1016/0092-8674(86)90596-9. [DOI] [PubMed] [Google Scholar]
- Belmont J. W., MacGregor G. R., Wager-Smith K., Fletcher F. A., Moore K. A., Hawkins D., Villalon D., Chang S. M., Caskey C. T. Expression of human adenosine deaminase in murine hematopoietic cells. Mol Cell Biol. 1988 Dec;8(12):5116–5125. doi: 10.1128/mcb.8.12.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brands J. H., Maassen J. A., van Hemert F. J., Amons R., Möller W. The primary structure of the alpha subunit of human elongation factor 1. Structural aspects of guanine-nucleotide-binding sites. Eur J Biochem. 1986 Feb 17;155(1):167–171. doi: 10.1111/j.1432-1033.1986.tb09472.x. [DOI] [PubMed] [Google Scholar]
- Chumakov I., Stuhlmann H., Harbers K., Jaenisch R. Cloning of two genetically transmitted Moloney leukemia proviral genomes: correlation between biological activity of the cloned DNA and viral genome activation in the animal. J Virol. 1982 Jun;42(3):1088–1098. doi: 10.1128/jvi.42.3.1088-1098.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Feuer G., Taketo M., Hanecak R. C., Fan H. Two blocks in Moloney murine leukemia virus expression in undifferentiated F9 embryonal carcinoma cells as determined by transient expression assays. J Virol. 1989 May;63(5):2317–2324. doi: 10.1128/jvi.63.5.2317-2324.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz T., Hilberg F., Seliger B., Stocking C., Ostertag W. Retroviral mutants efficiently expressed in embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1986 May;83(10):3292–3296. doi: 10.1073/pnas.83.10.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann T. Progress toward human gene therapy. Science. 1989 Jun 16;244(4910):1275–1281. doi: 10.1126/science.2660259. [DOI] [PubMed] [Google Scholar]
- Gautsch J. W., Wilson M. C. Delayed de novo methylation in teratocarcinoma suggests additional tissue-specific mechanisms for controlling gene expression. Nature. 1983 Jan 6;301(5895):32–37. doi: 10.1038/301032a0. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Rigby P. W., Lane D. P. Negative regulation of viral enhancers in undifferentiated embryonic stem cells. Cell. 1985 Sep;42(2):519–526. doi: 10.1016/0092-8674(85)90109-6. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Greaves D. R., Wilson F. D., Lang G., Kioussis D. Human CD2 3'-flanking sequences confer high-level, T cell-specific, position-independent gene expression in transgenic mice. Cell. 1989 Mar 24;56(6):979–986. doi: 10.1016/0092-8674(89)90631-4. [DOI] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Guild B. C., Finer M. H., Housman D. E., Mulligan R. C. Development of retrovirus vectors useful for expressing genes in cultured murine embryonal cells and hematopoietic cells in vivo. J Virol. 1988 Oct;62(10):3795–3801. doi: 10.1128/jvi.62.10.3795-3801.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbers K., Schnieke A., Stuhlmann H., Jähner D., Jaenisch R. DNA methylation and gene expression: endogenous retroviral genome becomes infectious after molecular cloning. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7609–7613. doi: 10.1073/pnas.78.12.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilberg F., Stocking C., Ostertag W., Grez M. Functional analysis of a retroviral host-range mutant: altered long terminal repeat sequences allow expression in embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5232–5236. doi: 10.1073/pnas.84.15.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeben R. C., van der Jagt R. C., Schoute F., van Tilburg N. H., Verbeet M. P., Briët E., van Ormondt H., van der Eb A. J. Expression of functional factor VIII in primary human skin fibroblasts after retrovirus-mediated gene transfer. J Biol Chem. 1990 May 5;265(13):7318–7323. [PubMed] [Google Scholar]
- Jaenisch R., Jähner D. Methylation, expression and chromosomal position of genes in mammals. Biochim Biophys Acta. 1984 May 15;782(1):1–9. doi: 10.1016/0167-4781(84)90099-x. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Jähner D., Nobis P., Simon I., Löhler J., Harbers K., Grotkopp D. Chromosomal position and activation of retroviral genomes inserted into the germ line of mice. Cell. 1981 May;24(2):519–529. doi: 10.1016/0092-8674(81)90343-3. [DOI] [PubMed] [Google Scholar]
- Kaleko M., Garcia J. V., Osborne W. R., Miller A. D. Expression of human adenosine deaminase in mice after transplantation of genetically-modified bone marrow. Blood. 1990 Apr 15;75(8):1733–1741. [PubMed] [Google Scholar]
- Li L. H., Olin E. J., Buskirk H. H., Reineke L. M. Cytotoxicity and mode of action of 5-azacytidine on L1210 leukemia. Cancer Res. 1970 Nov;30(11):2760–2769. [PubMed] [Google Scholar]
- Linney E., Davis B., Overhauser J., Chao E., Fan H. Non-function of a Moloney murine leukaemia virus regulatory sequence in F9 embryonal carcinoma cells. 1984 Mar 29-Apr 4Nature. 308(5958):470–472. doi: 10.1038/308470a0. [DOI] [PubMed] [Google Scholar]
- Loh T. P., Sievert L. L., Scott R. W. Negative regulation of retrovirus expression in embryonal carcinoma cells mediated by an intragenic domain. J Virol. 1988 Nov;62(11):4086–4095. doi: 10.1128/jvi.62.11.4086-4095.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh T. P., Sievert L. L., Scott R. W. Proviral sequences that restrict retroviral expression in mouse embryonal carcinoma cells. Mol Cell Biol. 1987 Oct;7(10):3775–3784. doi: 10.1128/mcb.7.10.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIvor R. S., Johnson M. J., Miller A. D., Pitts S., Williams S. R., Valerio D., Martin D. W., Jr, Verma I. M. Human purine nucleoside phosphorylase and adenosine deaminase: gene transfer into cultured cells and murine hematopoietic stem cells by using recombinant amphotropic retroviruses. Mol Cell Biol. 1987 Feb;7(2):838–846. doi: 10.1128/mcb.7.2.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Eckner R. J., Jolly D. J., Friedmann T., Verma I. M. Expression of a retrovirus encoding human HPRT in mice. Science. 1984 Aug 10;225(4662):630–632. doi: 10.1126/science.6377498. [DOI] [PubMed] [Google Scholar]
- Montandon P. E., Montandon F., Fan H. Methylation state and DNase I sensitivity of chromatin containing Moloney murine leukemia virus DNA in exogenously infected mouse cells. J Virol. 1982 Nov;44(2):475–486. doi: 10.1128/jvi.44.2.475-486.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. A., Fletcher F. A., Villalon D. K., Utter A. E., Belmont J. W. Human adenosine deaminase expression in mice. Blood. 1990 May 15;75(10):2085–2092. [PubMed] [Google Scholar]
- Mooslehner K., Karls U., Harbers K. Retroviral integration sites in transgenic Mov mice frequently map in the vicinity of transcribed DNA regions. J Virol. 1990 Jun;64(6):3056–3058. doi: 10.1128/jvi.64.6.3056-3058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery C. L., Feijen A., van der Saag P. T., van den Brink C. E., de Laat S. W. Clonal variants of differentiated P19 embryonal carcinoma cells exhibit epidermal growth factor receptor kinase activity. Dev Biol. 1985 Jun;109(2):402–410. doi: 10.1016/0012-1606(85)90466-x. [DOI] [PubMed] [Google Scholar]
- Niwa O. Suppression of the hypomethylated Moloney leukemia virus genome in undifferentiated teratocarcinoma cells and inefficiency of transformation by a bacterial gene under control of the long terminal repeat. Mol Cell Biol. 1985 Sep;5(9):2325–2331. doi: 10.1128/mcb.5.9.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa O., Yokota Y., Ishida H., Sugahara T. Independent mechanisms involved in suppression of the Moloney leukemia virus genome during differentiation of murine teratocarcinoma cells. Cell. 1983 Apr;32(4):1105–1113. doi: 10.1016/0092-8674(83)90294-5. [DOI] [PubMed] [Google Scholar]
- Price J., Turner D., Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohdewohld H., Weiher H., Reik W., Jaenisch R., Breindl M. Retrovirus integration and chromatin structure: Moloney murine leukemia proviral integration sites map near DNase I-hypersensitive sites. J Virol. 1987 Feb;61(2):336–343. doi: 10.1128/jvi.61.2.336-343.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J. R., Rubenstein J. L., Nicolas J. F. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986 Dec 1;5(12):3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherdin U., Rhodes K., Breindl M. Transcriptionally active genome regions are preferred targets for retrovirus integration. J Virol. 1990 Feb;64(2):907–912. doi: 10.1128/jvi.64.2.907-912.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C. C., Stoye J. P., Coffin J. M. Highly preferred targets for retrovirus integration. Cell. 1988 May 20;53(4):531–537. doi: 10.1016/0092-8674(88)90569-7. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Simon D., Stuhlmann H., Jähner D., Wagner H., Werner E., Jaenisch R. Retrovirus genomes methylated by mammalian but not bacterial methylase are non-infectious. Nature. 1983 Jul 21;304(5923):275–277. doi: 10.1038/304275a0. [DOI] [PubMed] [Google Scholar]
- Soriano P., Cone R. D., Mulligan R. C., Jaenisch R. Tissue-specific and ectopic expression of genes introduced into transgenic mice by retroviruses. Science. 1986 Dec 12;234(4782):1409–1413. doi: 10.1126/science.3024318. [DOI] [PubMed] [Google Scholar]
- Stein B., Rahmsdorf H. J., Steffen A., Litfin M., Herrlich P. UV-induced DNA damage is an intermediate step in UV-induced expression of human immunodeficiency virus type 1, collagenase, c-fos, and metallothionein. Mol Cell Biol. 1989 Nov;9(11):5169–5181. doi: 10.1128/mcb.9.11.5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J. C., Dower N. A., Siminovitch L. Stability of retrovirally transduced markers in a rat cell line. Somat Cell Mol Genet. 1986 Nov;12(6):575–583. doi: 10.1007/BF01671943. [DOI] [PubMed] [Google Scholar]
- Stuhlmann H., Jähner D., Jaenisch R. Infectivity and methylation of retroviral genomes is correlated with expression in the animal. Cell. 1981 Oct;26(2 Pt 2):221–232. doi: 10.1016/0092-8674(81)90305-6. [DOI] [PubMed] [Google Scholar]
- Taketo M., Tanaka M. A cellular enhancer of retrovirus gene expression in embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3748–3752. doi: 10.1073/pnas.84.11.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiyama T., Niwa O., Yokoro K. Mechanism of suppression of the long terminal repeat of Moloney leukemia virus in mouse embryonal carcinoma cells. Mol Cell Biol. 1989 Nov;9(11):4670–4676. doi: 10.1128/mcb.9.11.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio D., Einerhand M. P., Wamsley P. M., Bakx T. A., Li C. L., Verma I. M. Retrovirus-mediated gene transfer into embryonal carcinoma and hemopoietic stem cells: expression from a hybrid long terminal repeat. Gene. 1989 Dec 14;84(2):419–427. doi: 10.1016/0378-1119(89)90516-7. [DOI] [PubMed] [Google Scholar]
- Vijaya S., Steffen D. L., Robinson H. L. Acceptor sites for retroviral integrations map near DNase I-hypersensitive sites in chromatin. J Virol. 1986 Nov;60(2):683–692. doi: 10.1128/jvi.60.2.683-692.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiher H., Barklis E., Ostertag W., Jaenisch R. Two distinct sequence elements mediate retroviral gene expression in embryonal carcinoma cells. J Virol. 1987 Sep;61(9):2742–2746. doi: 10.1128/jvi.61.9.2742-2746.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. A., Orkin S. H., Mulligan R. C. Retrovirus-mediated transfer of human adenosine deaminase gene sequences into cells in culture and into murine hematopoietic cells in vivo. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2566–2570. doi: 10.1073/pnas.83.8.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. M., Danos O., Grossman M., Raulet D. H., Mulligan R. C. Expression of human adenosine deaminase in mice reconstituted with retrovirus-transduced hematopoietic stem cells. Proc Natl Acad Sci U S A. 1990 Jan;87(1):439–443. doi: 10.1073/pnas.87.1.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Yee J. K., Wolff J. A., Friedmann T. Factors affecting long-term stability of Moloney murine leukemia virus-based vectors. Virology. 1989 Aug;171(2):331–341. doi: 10.1016/0042-6822(89)90600-4. [DOI] [PubMed] [Google Scholar]