Abstract
Fungal pathogens perceive and respond to molecules from the plant, triggering pathogenic development. Transduction of these signals may use heterotrimeric G proteins, and it is thought that protein phosphorylation cascades are also important. We have isolated a mitogen-activated protein kinase homolog from the corn pathogen Cochliobolus heterostrophus to test its role as a component of the transduction pathways. The new gene, CHK1, has a deduced amino acid sequence 90% identical to Pmk1 of the rice blast fungus Magnaporthe grisea and 59% identical to Fus3 of Saccharomyces cerevisiae. A series of chk1 deletion mutants has poorly developed aerial hyphae, autolysis, and no conidia. No pseudothecia are formed when a cross between two Δchk1 mutants is attempted. The ability of Δchk1 mutants to infect corn plants is reduced severely. The growth pattern of hyphae on a glass surface is strikingly altered from that of the wild type, forming coils or loops, but no appressoria. This set of phenotypes overlaps only partially with that of pmk1 mutants, the homologous gene of the rice blast fungus. In particular, sexual and asexual sporulation both require Chk1 function in Cochliobolus heterostrophus, in contrast to Pmk1, but perhaps more similar to yeast, where Fus3 transmits the mating signal. Chk1 is required for efficient colonization of leaf tissue, which can be compared with filamentous invasive growth of yeast, modulated through another closely related mitogen-activated protein kinase, Kss1. Ubiquitous signaling elements thus are used in diverse ways in different plant pathogens, perhaps the result of coevolution of the transducers and their targets.
Filamentous fungal pathogens attack plant leaves by a variety of mechanisms (1). Some foliar pathogens penetrate the cuticle and plant cell wall by an appressorium that generates high turgor pressures (2, 3). Other species do not depend on pressure-generating appressoria. Stomata and wounds can provide entry points, and lytic enzymes facilitate fungal attack. The structure of the leaf surface, and unidentified chemical factors on or inside the leaf, are likely to induce pathogenic development of the fungus. Signal transduction pathways in filamentous fungal cells thus are critical to the establishment of disease.
Developmental events in fungal cells, however different they may appear, mirror and counter those by which the plant resists infection and are equally important for the understanding and control of disease. The conservation of eukaryotic signal transduction mechanisms suggests that fungal cells may use these conserved pathways in responding to the host. Indeed, in several fungal species, genes encoding heterotrimeric G protein α (4–8) or β (9) subunits are required for pathogenic development. The Gα subunit genes belong to at least three distinct classes. Adenylyl cyclase may be a downstream target of the activated G proteins, and exogenous cAMP can rescue some, but not all, defects in Gα mutants (10–14). The mitogen-activated protein kinase (MAPK) cascades of budding yeast might be a model for those of filamentous fungal pathogens (15). Mating in Saccharomyces cerevisiae requires a heterotrimeric G protein and a MAPK pathway. Budding yeast has six MAPK homologs in its genome, and deletion of a particular one blocks specific pathways. Furthermore, the lack of a MAPK may allow another one to take its place, resulting in abnormal cross-talk, as shown for filamentous invasive growth and mating (16). The high-osmolarity and pheromone pathways also show abnormal cross-talk in hog1 mutants, probably by a different mechanism (17). The specificity of these cascades thus resides at the input (receptors) and output (MAPK homologs). The FUZ7 gene of the corn smut fungus Ustilago maydis encodes a MAP kinase kinase homolog that is required for both mating and pathogenicity (18). Two distinct MAPK homologs participate in several aspects of pathogenic development in the rice blast fungus Magnaporthe grisea (19, 20). The subtle interchangeability of some yeast MAPKs (16) poses the question of whether the same family members control the same, or different, pathways in different fungal species.
Cochliobolus heterostrophus, the cause of Southern corn leaf blight, is a filamentous, heterothallic ascomycete. Numerous eyespot-like lesions on the leaves are typical of this disease. The disease is most devastating to T cytoplasm corn, whose mitochondria are sensitive to the host-specific polyketide toxin produced by race T of the pathogen (21, 22). In the presence of moisture, spores of the pathogen adhere to a leaf or other surface, germinate, and develop small appressoria, unlike the large appressoria formed by M. grisea and by Colletotrichum species (23). C. heterostrophus appressoria are not considered essential for penetration, which occurs directly or through stomata (1). Mutants in a G protein α-subunit gene, CGA1, are deficient in mating and appressorium formation, but are still able to cause symptoms on corn leaves (24). The pathways to pathogenic development of C. heterostrophus are likely to be different from those of the rice blast fungus. We therefore isolated a homolog of MAPKs from C. heterostrophus, which we have designated CHK1. Deletion of this gene shows that it is required for several developmental processes.
Materials and Methods
PCR Cloning of the CHK1 Gene.
Three degenerate primers were used for PCR. The sense primer MAK2, GT(A/C/G/T)GC(A/C/G/T)AT(A/G)AA(A/G)AA(A/G)AT, corresponds to the highly conserved amino acid sequence VAIKKI in kinase subdomain II. Antisense primers MEK3, GG(C/T)TT(A/C/G/T)A(A/C/G/T)(A/G)TC(A/C/G/T)C(G/T)(A/G)TG, and MAK4, TC(A/C/T/G)GG(A/C/T/G)GC(A/C/T/G)C(G/T)(A/G)TA(A/C/G)(C/T)A, correspond to the conserved sequences HRDLKP and WYRAPE in kinase subdomains VI and VIII (19). Primary PCR used primers MAK2 and MAK4 with genomic DNA of strain C4 as template. Nested PCR was performed with primers MAK2 and MEK3. PCR conditions were: 2 min at 94°C, followed by 30 cycles (1 min, 94°C; 2 min, 52°C; 2 min, 72°C), with a final extension for 5 min at 72°C. The products were cloned into pCR-Script (Stratagene). A product with strong homology to PMK1 of M. grisea (19) was used to screen a cDNA library prepared in pAD-GAL4 (Stratagene) from poly(A)+ mRNA PolyATtract; Promega] from RNA pooled from C4 (MAT-2) and C5 (MAT-1) strains growing in complete (CM) and minimal media (25). Approximately 106 plaques were screened, yielding two positive clones. DNA sequences were determined at the Weizmann Institute of Science, Rehovot, by using TaqCycle automated sequencing with DyeDeoxy Terminators (Applied Biosystems). Homology searches were performed with the blast program (26). DNA sequences were compared by using lalign (genestream network server); amino acid sequence alignments were made with clustal w (27).
DNA Manipulations.
Plasmid DNA was isolated by standard methods (28) or with the High Pure kit (Boehringer Mannheim). Qiaex II (Qiagen) was used for purification from agarose gels. Fungal DNA was isolated from 4- to 5-day-old mycelia grown in CM as described (29), except that mycelium was ground in liquid nitrogen rather than lyophilized, and the chloroform extractions were performed after polyethylene glycol precipitation. Southern blotting followed standard procedures (28). Hybridization was in 7% SDS/0.25 M phosphate buffer at 65°C. Washes were at 65°C: first with 5% SDS, then 1% SDS, in 20 mM phosphate buffer.
Gene Replacement.
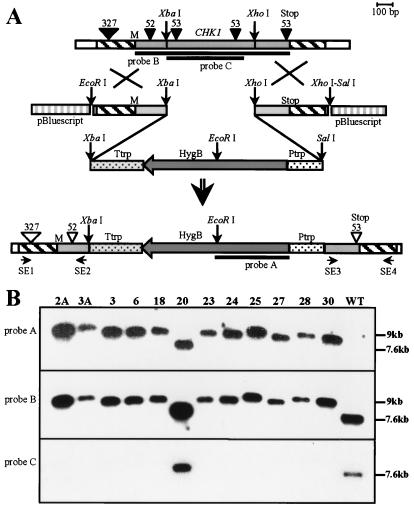
A vector designed to favor double-crossover integration events was made in two steps. First, a 538-bp EcoRI–XbaI fragment of the CHK1 cDNA clone including the first 241 bp of coding sequence was ligated with the XbaI–SalI hygromycin B (hygB) resistance cassette from pUC-ATPH (21) into EcoRI–SalI-digested pBluescript (Stratagene). In the second step, a 501-bp CHK1 XhoI–XhoI fragment including the last 205 bp of coding sequence was inserted downstream of the hygB cassette into the SalI restriction site. The size of the resulting plasmid was 6.1 kb, and it contained CHK1 sequence in which 610 bp of coding sequence was replaced by the hygB resistance cassette (Fig. 2A). Two pairs of primers were designed for amplification of the CHK1 fragments used in construction of the replacement vector: SE1, GAGACGCGGGCAGTCCCAGC; SE2, CGTGGTTGAAGTAGCGCAGC; SE3, GCGCATCTCAGTCGAGGACG; SE4, GCATTTGTGCTTATTGTCATCTCAC (see Fig. 2).
Figure 2.
Strategy for CHK1 gene disruption and analysis of transformants. (A) CHK1 replacement vector contains a 2.1-kb hygB resistance gene cassette. The 538-bp-long left flanking region includes the first 241 bp of CHK1 coding sequence, and the 501-bp-long right flanking region includes the last 205 bp of the CHK1 coding sequence. Homologous recombination through a double-crossover event results in the replacement of part of CHK1 with the hygB resistance cassette. (B) Southern analysis of hygB-resistant transformants. Genomic DNA was digested with SalI. Lanes 1–12 represent 12 different transformants (2A and 3A are MAT-1 and the others are MAT-2); lane 13 is wild type (MAT-2). The blot was sequentially hybridized with probes A, B, and C, which are indicated in A. The 7.6-kb band corresponds to the nondisrupted CHK1, and a 9-kb band results from hygB integration. All transformants except strain 20 show the pattern of hybridization expected for Δchk1 mutants resulting from double-crossover gene replacement; strain 20, the result of an ectopic integration event, retains the wild-type fragment hybridizing with probe C. Sizes and locations of introns are indicated by inverted triangles. Solid triangles indicate introns in the genomic sequence (GenBank accession no. AF178977), and open triangles indicate that these may or may not be present after recombination with the cDNA-derived vector sequences. The locations of primers SE1–4 used for amplifications in the flanking regions are indicated.
Strains, Transformation, and Crossing.
Strains C4 (MAT-2), C5 (MAT-1), and CB12 (albino, MAT-2) as well as conditions for fungal growth have been described (25, 29) as were transformation (30, 31) and crossing (25). Single-spore isolates were obtained from transformants that conidiated. If lack of conidia precluded single-spore isolation, transformants were transferred 3–4 times to select against heterokaryons.
Assay of Appressorium Formation.
A small amount of mycelium was suspended in 0.5 ml of CM and placed on sterile glass slides. The slides were incubated at room temperature in dishes lined with moist Whatman 3 paper, covered, and sealed with Parafilm. After 22–28 hr, the appearance of appressoria was observed and photographed by using phase-contrast optics.
Virulence.
Transformants were grown in CM with 50 μg/ml hygB for 3 days, whereas wild type (WT) was grown in CM without hygB. A mycelial suspension at an optical density of about 5 was prepared by using a glass–glass homogenizer. Drops (5 μl) of this suspension (to which Tween 20 was added to a final concentration of 0.1%) were used to inoculate the first two fully developed leaves of 5-day-old corn plants. The plants then were incubated in moist chambers for 24 hr, after which the chambers were opened, and the plants were examined over the following 3 days. Clearing and staining of infected leaves were as described (24).
Results
Isolation of a MAPK Homolog.
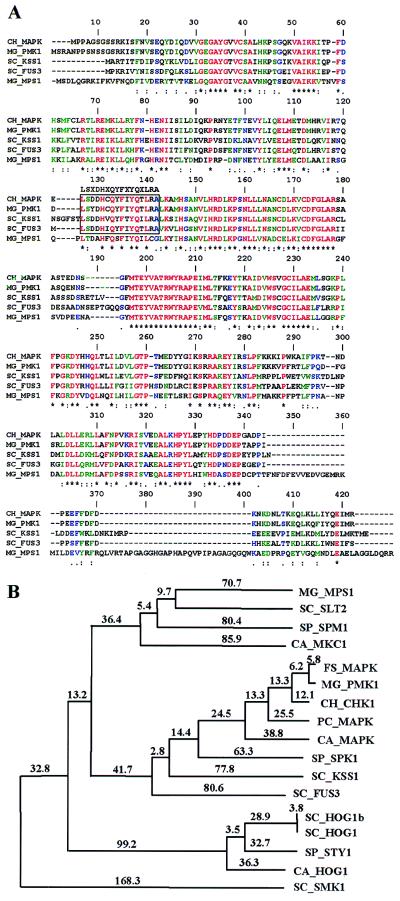
We obtained several products by PCR amplification of genomic DNA from C. heterostrophus by using a nested-primer strategy. Of seven PCR-derived clones sequenced, two were the same and showed 88% identity to PMK1 of M. grisea. The corresponding gene was designated CHK1, and the amplification product was used to screen a cDNA library. A full-length cDNA clone was sequenced, and the ORF encodes a 352-aa protein with 92% identity to MAPK of Nectria haematococca (32) and 90% identity to Pmk1 of M. grisea (Fig. 1). Amino acid sequence alignment showed that the deduced Chk1 protein contains all 11 conserved protein kinase subdomains and the characteristic MAPK phosphorylation sequence TEY in front of subdomain VIII (33). The genomic sequence of CHK1 was determined by PCR amplification by using genomic DNA of WT as a template. The CHK1 coding sequence includes four introns, whose positions were determined by comparison of cDNA and genomic clones and the presence of consensus splice sites. The positions of two introns in CHK1 correspond to those of PMK1 in M. grisea, whereas the third and fourth CHK1 introns do not. Southern analysis indicates that the CHK1 gene is present as a single copy in both mating types.
Figure 1.
(A) Deduced amino acid sequence of the CHK1 gene (CH_MAPK), compared with other fungal MAPK sequences (MG_PMK1, M. grisea Pmk1; SC_KSS1 and SC_FUS3, yeast Kss1 and Fus3, respectively). A consensus signature of the YERK1 subfamily is indicated (box) and is absent in MG_MPS1, Mps1 of M. grisea, which belongs to the YERK2 subfamily. (B) Phylogenetic tree based on amino acid sequence alignments of fungal MAPK homologs. The tree was constructed by using phylip (Phylogeny Inference Package), version 3.57c. The protdist subprogram (Categories distances option) was used to compute the distance matrix, and the tree and the indicated distances (multiplied by 100) were obtained by using the fitch (Fitch–Margoliash method) subprogram.
Disruption of CHK1.
To assign functions to the CHK1 gene, we used homologous recombination to mutate this locus. We designed a construct to replace part of the CHK1 coding sequence with the E. coli hph gene (hygB), providing resistance to hygB (Fig. 2A). MAT-2 strain C4 and MAT-1 strain C5 were transformed with this construct. In most of the hygB-resistant transformants, the WT copy was replaced by the deletion mutation, whereas several transformants resulted from ectopic single-crossover integrations. The disruption event was confirmed by Southern analysis (Fig. 2B). There are small differences in the sizes of the bands corresponding to the homologous integrations. Genomic DNA of WT and transformants 2A, 25, 27, 28, and 30 was amplified by using primers SE1–4, resulting in products of varying size. The sizes were consistent with the differences in migration shown by Southern analysis. Sequencing of the PCR products demonstrated that recombination between endogenous CHK1 and cDNA-derived homologous regions of vector occurred at different sites, before or after introns (Fig. 2A). There was no correlation between any of the phenotypes (see below) and the size of the fragment carrying the double-crossover replacement.
All of the homologous integrants displayed characteristic colony morphology different from WT and completely lacked conidia. Because these result from similar events in which most of the coding region was deleted and replaced by the hygB resistance cassette, the homologous integrant transformants are described collectively as Δchk1 mutants. Individual mutant chk1 alleles are designated by the original transformant numbers (Fig. 2B). Strain 20, a transformant in which the construct was integrated ectopically, had WT morphology and produced abundant conidia.
Colony Morphology and Appressorium Formation.
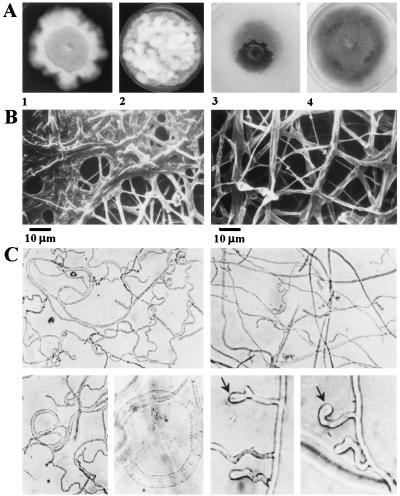
Colonies of Δchk1 mutants showed altered morphology: mutants have poorly developed aerial hyphae uniformly distributed over the entire colony surface, in contrast to WT colonies, which have well-developed, long aerial hyphae. Mutant colonies often acquire a smooth spot in part or all of the central region. Aerial hyphae in this area visibly disappeared, falling down and fusing with or adhering to each other and the underlying mat (Fig. 3 A and B). Microscopic observation demonstrated that, although some normal hyphae were present in this central region, many showed signs of internal autolysis or breakdown that was clearly abnormal, even compared with senescent regions of WT mycelial colonies. Under white light enriched with UV (320–400 nm), aerial hyphae of the WT quickly differentiated into conidiophores bearing conidia. In contrast, at the center of Δchk1 colonies, a region of smooth and translucent surface appeared, and no conidia were formed (Fig. 3A).
Figure 3.
Effects of targeted deletions at CHK1 on colony and hyphal development. (A) Colony morphology of Δchk1 mutants. (1 and 2) Colonies grown on CM. Plates were incubated at 30°C in the dark for 1 week. (3 and 4) Colonies grown on minimal medium. Plates were incubated at 25°C in UVA-enriched white light for 1 week. (1 and 3) chk1–3A and chk1–30, grown with hygB. (2 and 4) Wild-type controls. (B) Altered ultrastructural morphology of fungal colonies as a consequence of disruption of CHK1. Scanning electron micrographs show an apparent fusion or adhesion of hyphae in the central region of mutant colonies. (Left) chk1–30. (Right) WT. (C) Appressorium formation on a glass surface. (Left) chk1–30. (Right) Ectopic control, strain 20. Small appressoria appear as enlarged hyphal tips (arrows).
Hyphal tips of the WT respond to the presence of a glass or plastic surface by swelling to form small appressoria. We investigated whether CHK1 is required for this developmental response. In the usual assay for appressorium formation (23, 24), conidia are allowed to germinate on a glass slide. Because the chk1 deletion mutants do not conidiate, mycelium was inoculated on glass slides, and CM was used for incubation, rather than water. WT, and a transformant in which the construct was integrated at an ectopic location (strain 20, Fig. 2B), formed appressoria in these conditions, though less than in the usual assay. Δchk1 mutants, in contrast, did not form appressoria at all: the hyphae continued to grow, often forming loops and coils (Fig. 3C).
Mating Ability.
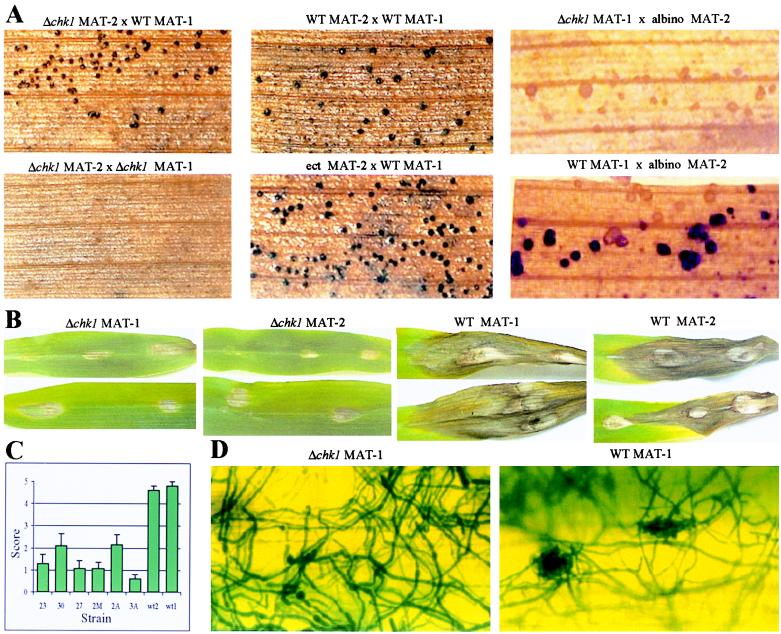
Ascomycete signal transduction mutants often are affected in their ability to mate and in the fertility of the cross. Mating in C. heterostrophus is defined as the production of pseudothecia, structures formed primarily from parental mycelium. Asci develop from ascogeneous hyphae within the pseudothecium. A fully fertile cross is one that produces pseudothecia of both parental types containing viable ascospores. To determine whether Δchk1 mutants are altered in their ability to mate and to complete the development of ascospores, we crossed the mutants with WT strains of opposite mating type. In crosses of Δchk1 mutants to WT of opposite mating type, pseudothecia were produced (Fig. 4A). Many of these were abortive round structures that did not contain asci, and 10–20% were intact fruiting bodies from which ascospores could be isolated. Furthermore, in crosses between two Δchk1 strains of opposite mating type, no pseudothecia were formed (Fig. 4A). Chk1 thus is involved in sexual reproduction. Pseudothecia, all of which were white, were produced in crosses between Δchk1 (MAT-1) and an albino (MAT-2) strain (Fig. 4A). The production of only white pseudothecia shows that these structures are formed by the parent with an intact CHK1 allele, indicating that the mutant is female sterile. Dissection of individual pseudothecia produced in crosses of chk1–30 (MAT-2) and chk1–23 (MAT-2) to WT C5 (MAT-1) resulted in 25 colonies grown from single, randomly isolated ascospores. Thirteen of 25 were HygB-resistant and showed the Δchk1 phenotypes, whereas the remainder were HygB-sensitive and WT. The mutant phenotypes thus cosegregated completely with HygB resistance. Cosegregation of the replacement event and HygB resistance was confirmed by Southern analysis.
Figure 4.
Effect of deletions at CHK1 on the ability to mate and to cause disease symptoms. (A) Mating assays. (Upper Left) chk1–23 (MAT-2) × WT (MAT-1). (Upper Center) Control WT cross. (Upper Right) chk1–2A (MAT-1) × CB12 (albino, MAT-2). (Lower Left) chk1–23 (MAT-2) × chk1–2A (MAT-1). (Lower Center) Ectopic integration strain 20 (MAT-2) × WT. (Lower Right) WT (MAT-1) × CB12. Pseudothecia are visible as dark spots or lacking pigment (albino). (B) Pathogenicity assays on corn leaves. Photographs show typical lesions formed by WT and Δchk1 strains at 8 days after inoculation. First and second photographs show Δchk1 mutants: strain 2M, an ascospore isolate of mating-type MAT-1 from a backcross of strain 30 (chk1–30 MAT-2) to WT; and strain 27 (chk1–27 MAT-2). Third and fourth photographs show WT. (C) Quantitative analysis of disease symptoms: lesions were scored on a scale of 0–5 (0 = no lesion; 1 = decolorization or necrotic lesion <1 mm; 2 = necrotic lesion 1–2 mm; 3 = 2–3 mm; 4 = 4–5 mm; 5 = severe lesion >5 mm) at 3 days after inoculation. Bars indicate SEM of a total of 15–18 replicate spots from three different plants. Differences between each mutant and wild type are significant (Student’s t test, P < 0.0002). Numbers on the x axis are strains in Fig. 2B, except wt2, wt1 (MAT-2 and MAT-1), and strain 2M, which is described in B. (D) Visualization of hyphal growth on the leaf. Corn leaves were inoculated with mycelial homogenates as in B. After 48 hr, samples were fixed, stained, and photographed at ×200. (Left) Strain 3A (chk1–3A MAT-1). (Right) WT MAT-1.
Ability to Cause Disease on Corn Plants.
To determine whether CHK1 is essential for pathogenicity on corn, we inoculated corn plants with mycelial suspensions of Δchk1 strains and WT controls. Typical spreading necrotic lesions were formed upon inoculation with WT mycelia. The Δchk1 mutants were consistently less virulent, often causing almost no signs of disease or slight discoloration of the leaf. Occasionally, necrotic lesions were formed, but failed to develop into a large necrotic area. In the WT, in contrast, virtually every inoculation led to severe necrosis (Fig. 4B). Upon quantitative estimation of the extent of symptoms (Fig. 4C), we found that all the mutants were reduced significantly in virulence. Pathogenicity was not completely lost in any of the Δchk1 mutants. The fungus was reisolated on nonselective medium from several lesions formed by Δchk1 mutants (strains 3A and 2A). The resulting colonies showed mutant phenotypes and were hygB-resistant, indicating that the ability of the mutants to cause symptoms did not result from contamination of the transformants with WT spores or nuclei. Mycelia of Δchk1 grew extensively on the leaf surface, but could not be detected in internal tissues of the leaf 48 hr after inoculation, when mycelia of the WT were already clearly visible inside the leaf. Small clusters of hyphae are seen, often over stomata, when the WT grows on the leaf surface. These are absent from the mutant, which seems to have less ability to sense the features of the leaf surface (Fig. 4D). No appressoria were formed by the mutant on the leaf surface, though we note that well-developed appressoria were also rarely made by the WT under these conditions. This can be taken as support for the observation that in C. heterostrophus, appressoria are not necessary for successful colonization of the leaf (1).
Discussion
The Southern corn leaf blight fungus MAPK gene CHK1 belongs to the extracellular signal-regulated kinase (ERK) family of the MAPK superfamily, whose main function in other eukaryotes is the transduction of extracellular signals. According to the signature sequence, Chk1 is related to the YERK1 (yeast and fungal ERK) subfamily of the ERK family (Fig. 1) (33). Members of the YERK1 subfamily are activated by pheromones and mating signals (Fus3, S. cerevisiae; Spk1, Schizosaccharomyces pombe) and are important for cell cycle regulation and conjugation. Nutrient deprivation also induces kinases of this subfamily to trigger morphological modifications (Kss1, S. cerevisiae). A Candida albicans YERK1, Cek1, is involved in virulence (34). The M. grisea member of this subfamily, Pmk1, is required for infection-related morphogenesis (19), whereas another MAPK in this fungus, Mps1 (20), belongs to the YERK2 subfamily, whose members regulate cell morphology (Slt2, S. cerevisiae). Chk1 is the only MAPK homolog isolated so far from C. heterostrophus, but it seems likely that it has more than one MAPK gene, as in budding and fission yeast, C. albicans and M. grisea.
To understand Chk1 function, we have generated Δchk1 mutants and characterized their phenotypes. They do not conidiate under normal conditions, are unable to produce appressoria, have a greatly reduced ability to infect corn leaves, and are defective in mating when both partners lack a functional copy of the gene. This pleiotropic phenotype implies that Chk1 is involved in several developmental pathways, each responsive to different signal(s). When a signaling molecule is involved in multiple pathways, additional distinct components or even pathways are necessary to confer the specific responses to particular signals. There is precedent from yeast for one signal eliciting two pathways. Yeast pheromones activate the Fus3 pathway. At the same time, through Ste20, pheromones activate Slt2, whose primary function is to transduce stresses (hypoosmotic, nutrient limitation, and heat) (15). Specificity in this case is achieved through the Fus3 pathway.
G proteins are generally upstream of MAPK pathways or other effectors such as adenylate cyclase. A C. heterostrophus heterotrimeric G protein α-subunit gene, CGA1 (24), is required for some of the same developmental routes as the MAPK CHK1. CGA1, like CHK1, is required for appressorium formation and mating. No viable cga1 mutant ascospores are produced in crosses of cga1 with WT, whereas chk1 mutant progeny can be isolated easily from backcrosses. Furthermore, CGA1 is needed for the normal wavy growth pattern on a hard surface, whereas the chk1 mutants have a wavy or coiled growth pattern. cga1 mutants produce abundant conidia, whereas chk1 mutants do not conidiate. A number of possible schemes could explain these findings. Appressorium formation, for example, might depend on a cAMP-dependent pathway through Cga1, together with the Chk1 MAPK pathway, as proposed for M. grisea (13, 19). On the other hand, conidiation appears to be induced through Chk1 without need for Cga1. Mutants of Aspergillus nidulans in which the Gαi pathway is not down-regulated (FadA, FlbA) are nonconidiating (fluffy) and autolytic (35). This phenotype is reminiscent of Δchk1, suggesting that defective functioning of the signaling pathways for sporulation might lead to development of abnormal aerial hyphae and to cell death.
The multiple developmental pathways that require Chk1 (appressoria, conidia, aerial hyphae, and pseudothecia) are likely to share a requirement for cytoskeleton reorganization and alterations of polarized tip growth. Evidence for this comes from studies of cAMP-dependent protein kinase (PKA) in Neurospora crassa (36). The loss of growth polarity conferred by unregulated PKA looks structurally similar to the surface-induced tip swelling (appressoria) studied here (Fig. 3C). Thus, MAPK pathways may be involved in the control of cell polarity. This idea is supported by the findings that the yeast MAPK Fus3 mediates Bem1 and Cdc42-related cytoskeleton rearrangement in response to pheromone (37) and that Slt2 is required for polarized cell growth (38).
The close homolog of CHK1, PMK1 of M. grisea, also is required for appressorium formation and pathogenicity, but not for other developmental functions conferred by CHK1. This finding emphasizes the different use of similar signaling components, even in two ascomycete foliar pathogens that cause diseases that appear, at least superficially, similar. MPS1 of the rice blast fungus is involved in pathogenicity, conidiation, mating, aerial hypha formation, and cell wall integrity (20). Thus, although CHK1 is more similar to PMK1 than to MPS1 (Fig. 1), it has some of the functions of both these genes: sexual and asexual sporulation (MPS1); pathogenicity (both); appressorium formation (PMK1); aerial hypha formation, and a function related to viability or cell wall integrity (MPS1). If there is indeed a MAPK family, as seems likely in view of what is known from other eukaryotes, the “division of labor” among the MAPK subfamilies depends on the species.
MAPKs may have coevolved with their targets, scaffold proteins, and the kinases that activate them. A MAPK belonging to a particular subfamily could have been recruited for a distinct set of functions in different species. Formation of the large, melanized appressorium in rice blast represents an evolutionarily specialized developmental route. In C. heterostrophus this is not necessarily so. The development of its small appressoria does not represent a high level of cell differentiation. PMK1 thus acquired a specialized function in M. grisea, whereas MPS1 retained a more general set of functions. CHK1 became, or remained, functionally more similar to MPS1, which has a wide set of developmental roles. Support for such an interpretation comes from studies of a fungal transcription factor with a Myb-like DNA-binding domain (39). The Neurospora homolog of an Aspergillus regulator of conidiation can complement Aspergillus mutants in this gene, flbD, whereas deletion of the Neurospora gene had no major effects on development. The Myb-like DNA-binding domain is shared between the two species, whereas the remainder of the protein is more divergent. Recruitment of homologous genes for divergent sets of functions may be a general phenomenon in the evolution of ascomycetes.
Acknowledgments
We thank Olen Yoder and B. Gillian Turgeon for strains and plasmids and Rudy Maor for help with the plant assays. This work was supported by the Israel Academy of Sciences and by a United States–Israel Binational Science Foundation grant to B.A.H. and H.M.
Abbreviations
- MAPK
mitogen-activated protein kinase
- ERK
extracellular signal-regulated kinase
- YERK
yeast and fungal ERK
- hygB
hygromycin B
- CM
complete medium
- WT
wild type
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF178977).
References
- 1.Mendgen K, Hahn M, Deising H. Annu Rev Phytopathol. 1996;34:367–386. doi: 10.1146/annurev.phyto.34.1.367. [DOI] [PubMed] [Google Scholar]
- 2.Howard R J, Valent B. Annu Rev Microbiol. 1996;50:491–512. doi: 10.1146/annurev.micro.50.1.491. [DOI] [PubMed] [Google Scholar]
- 3.Takano Y, Kubo Y, Kawamura C, Tsuge T, Furusawa I. Fungal Genet Biol. 1997;21:131–140. [PubMed] [Google Scholar]
- 4.Regenfelder E, Spellig T, Hartmann A, Lauenstein S, Bölker M, Kahmann R. EMBO J. 1997;16:1934–1942. doi: 10.1093/emboj/16.8.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu S, Dean R A. Mol Plant–Microbe Interact. 1997;10:1075–1086. doi: 10.1094/MPMI.1997.10.9.1075. [DOI] [PubMed] [Google Scholar]
- 6.Gao S, Nuss D. Proc Natl Acad Sci USA. 1996;93:14122–14127. doi: 10.1073/pnas.93.24.14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao S, Nuss D L. Mol Plant–Microbe Interact. 1998;11:1130–1135. doi: 10.1094/MPMI.1998.11.11.1130. [DOI] [PubMed] [Google Scholar]
- 8.Bölker M. Fungal Genet Biol. 1998;25:143–156. doi: 10.1006/fgbi.1998.1102. [DOI] [PubMed] [Google Scholar]
- 9.Kasahara S, Nuss D L. Mol Plant–Microbe Interact. 1997;10:984–993. doi: 10.1094/MPMI.1997.10.8.984. [DOI] [PubMed] [Google Scholar]
- 10.Loubradou G, Begueret J, Turcq B. Genetics. 1999;152:519–528. doi: 10.1093/genetics/152.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krüger J, Loubradou G, Regenfelder E, Hartmann A, Kahmann R. Mol Gen Genet. 1998;260:193–198. doi: 10.1007/s004380050885. [DOI] [PubMed] [Google Scholar]
- 12.Ivey F D, Yang Q, Borkovich K A. Fungal Genet Biol. 1999;26:48–61. doi: 10.1006/fgbi.1998.1101. [DOI] [PubMed] [Google Scholar]
- 13.Choi W, Dean R A. Plant Cell. 1997;9:1973–1983. doi: 10.1105/tpc.9.11.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alspaugh J, Perfect J, Heitman J. Genes Dev. 1997;11:3206–3217. doi: 10.1101/gad.11.23.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banuett F. Microbiol Mol Biol Rev. 1998;62:249–274. doi: 10.1128/mmbr.62.2.249-274.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madhani H D, Styles C A, Fink G R. Cell. 1997;91:673–684. doi: 10.1016/s0092-8674(00)80454-7. [DOI] [PubMed] [Google Scholar]
- 17.O’Rourke S M, Herskowitz I. Genes Dev. 1998;12:2874–2886. doi: 10.1101/gad.12.18.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banuett F, Herskowitz I. Genes Dev. 1994;8:1367–1378. doi: 10.1101/gad.8.12.1367. [DOI] [PubMed] [Google Scholar]
- 19.Xu J R, Hamer J E. Genes Dev. 1996;10:2696–2706. doi: 10.1101/gad.10.21.2696. [DOI] [PubMed] [Google Scholar]
- 20.Xu J R, Staiger C J, Hamer J E. Proc Natl Acad Sci USA. 1998;95:12713–12718. doi: 10.1073/pnas.95.21.12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu S, Lyngholm L, Yang G, Bronson C, Yoder O C, Turgeon B G. Proc Natl Acad Sci USA. 1994;91:12649–12653. doi: 10.1073/pnas.91.26.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoder O C. Adv Plant Pathol. 1988;6:93–112. [Google Scholar]
- 23.Braun E, Howard R. Exp Mycol. 1994;18:211–220. [Google Scholar]
- 24.Horwitz B A, Sharon A, Lu S W, Ritter V, Sandrock T M, Yoder O C, Turgeon B G. Fungal Genet Biol. 1999;26:19–32. doi: 10.1006/fgbi.1998.1094. [DOI] [PubMed] [Google Scholar]
- 25.Leach J, Lang B, Yoder O. J Gen Microbiol. 1982;128:1719–1729. [Google Scholar]
- 26.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 27.Thompson J D, Higgins D G, Gibson T J. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 29.Wirsel S, Turgeon B G, Yoder O C. Curr Genet. 1996;29:241–249. [PubMed] [Google Scholar]
- 30.Turgeon B G, Garber R C, Yoder O C. Mol Cell Biol. 1987;7:3297–3305. doi: 10.1128/mcb.7.9.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turgeon B G, Bohlmann H, Ciuffetti L M, Christiansen S K, Yang G, Schäfer W, Yoder O C. Mol Gen Genet. 1993;238:270–284. doi: 10.1007/BF00279556. [DOI] [PubMed] [Google Scholar]
- 32.Li D, Rogers L, Kolattukudy P E. Gene. 1997;195:161–166. doi: 10.1016/s0378-1119(97)00124-8. [DOI] [PubMed] [Google Scholar]
- 33.Kültz D. J Mol Evol. 1998;46:571–588. doi: 10.1007/pl00006338. [DOI] [PubMed] [Google Scholar]
- 34.Csank C, Schroppel K, Leberer E, Harcus D, Mohamed O, Meloche S, Thomas D Y, Whiteway M. Infect Immunol. 1998;66:2713–2721. doi: 10.1128/iai.66.6.2713-2721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu J-H, Wieser J, Adams T H. EMBO J. 1996;15:5184–5190. [PMC free article] [PubMed] [Google Scholar]
- 36.Bruno K, Aramayo R, Minke P, Metzenberg R, Plamann M. EMBO J. 1996;21:5772–5782. [PMC free article] [PubMed] [Google Scholar]
- 37.Butty A-C, Pryciak P M, Huang L S, Herskowitz I, Peter M. Science. 1998;282:1511–1516. doi: 10.1126/science.282.5393.1511. [DOI] [PubMed] [Google Scholar]
- 38.Mazzoni C, Zarov P, Rambourg A, Mann C. J Cell Biol. 1993;123:1821–1833. doi: 10.1083/jcb.123.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen W-C, Wieser J, Adams T, Ebbole D. Genetics. 1998;148:1031–1041. doi: 10.1093/genetics/148.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]