Abstract
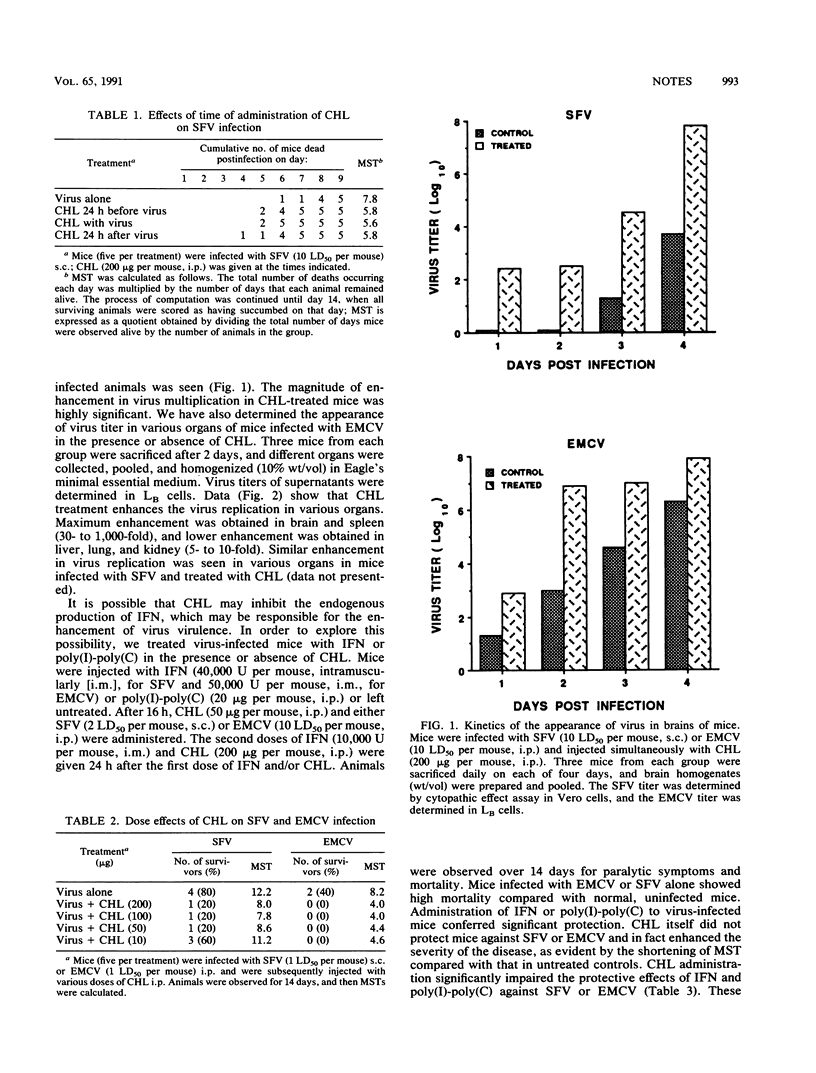
Chloroquine (CHL) has been suggested to play an important role in the development of Burkitt's lymphoma by enhancing Epstein-Barr virus expression. Herpes zoster virus incidence is markedly increased following malaria infection in children being treated with CHL. Recently, CHL has also been shown to dramatically increase the transactivation of Tat protein purified from human immunodeficiency virus. These previous studies indirectly suggest that CHL may be involved in the enhancement of virus replication. This study demonstrates for the first time that CHL indeed enhances Semliki Forest virus and encephalomyocarditis virus replication in mice. These results raise the possible connection between the increased spread of AIDS in endemic malaria areas and the wide use of CHL in those areas for the chemotherapy of malaria.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attallah A. M., Folks T. Interferon enhanced human natural killer and antibody-dependent cell-mediated cytotoxic activity. Int Arch Allergy Appl Immunol. 1979;60(4):377–382. doi: 10.1159/000232367. [DOI] [PubMed] [Google Scholar]
- Bernard K. W., Fishbein D. B., Miller K. D., Parker R. A., Waterman S., Sumner J. W., Reid F. L., Johnson B. K., Rollins A. J., Oster C. N. Pre-exposure rabies immunization with human diploid cell vaccine: decreased antibody responses in persons immunized in developing countries. Am J Trop Med Hyg. 1985 May;34(3):633–647. doi: 10.4269/ajtmh.1985.34.633. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S. K., Pillai C. R., Mathur M., Sen P. Effect of chloroquine and some other antimalarials on the immune mechanism in experimental animals. J Pharm Pharmacol. 1984 Apr;36(4):268–269. doi: 10.1111/j.2042-7158.1984.tb04366.x. [DOI] [PubMed] [Google Scholar]
- Clumeck N., Sonnet J., Taelman H., Mascart-Lemone F., De Bruyere M., Vandeperre P., Dasnoy J., Marcelis L., Lamy M., Jonas C. Acquired immunodeficiency syndrome in African patients. N Engl J Med. 1984 Feb 23;310(8):492–497. doi: 10.1056/NEJM198402233100804. [DOI] [PubMed] [Google Scholar]
- Cook I. F. Herpes zoster in children following malaria. J Trop Med Hyg. 1985 Aug;88(4):261–264. [PubMed] [Google Scholar]
- Frankel A. D., Pabo C. O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988 Dec 23;55(6):1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Vogel S. N. Interferons with special emphasis on the immune system. Adv Immunol. 1983;34:97–140. doi: 10.1016/s0065-2776(08)60378-8. [DOI] [PubMed] [Google Scholar]
- Greenberg A. E., Nguyen-Dinh P., Mann J. M., Kabote N., Colebunders R. L., Francis H., Quinn T. C., Baudoux P., Lyamba B., Davachi F. The association between malaria, blood transfusions, and HIV seropositivity in a pediatric population in Kinshasa, Zaire. JAMA. 1988 Jan 22;259(4):545–549. [PubMed] [Google Scholar]
- Greenberg A. E., Schable C. A., Sulzer A. J., Collins W. E., Phuc Nguyen-Dinh Evaluation of serological cross-reactivity between antibodies to Plasmodium and HTLV-III/LAV. Lancet. 1986 Aug 2;2(8501):247–249. doi: 10.1016/s0140-6736(86)92071-4. [DOI] [PubMed] [Google Scholar]
- Henle G., Henle W., Clifford P., Diehl V., Kafuko G. W., Kirya B. G., Klein G., Morrow R. H., Munube G. M., Pike P. Antibodies to Epstein-Barr virus in Burkitt's lymphoma and control groups. J Natl Cancer Inst. 1969 Nov;43(5):1147–1157. [PubMed] [Google Scholar]
- Karmali R. A., Horrobin D. F., Menezes J., Patel P., Musto J. Chloroquine enhances Epstein-Barr virus expression. Nature. 1978 Oct 5;275(5679):444–445. doi: 10.1038/275444a0. [DOI] [PubMed] [Google Scholar]
- Klein G. The Epstein-Barr virus and neoplasia. N Engl J Med. 1975 Dec 25;293(26):1353–1357. doi: 10.1056/NEJM197512252932607. [DOI] [PubMed] [Google Scholar]
- Olweny C. L., Atine I., Kaddu-Mukasa A., Owor R., Andersson-Anvret M., Klein G., Henle W., de-Thé G. Epstein-Barr virus genome studies in Burkitt's and non-Burkitt's lymphomas in Uganda. J Natl Cancer Inst. 1977 May;58(5):1191–1196. doi: 10.1093/jnci/58.5.1191. [DOI] [PubMed] [Google Scholar]
- Pedersen B. K., Bygbjerg I. C., Theander T. G., Andersen B. J. Effects of chloroquine, mefloquine and quinine on natural killer cell activity in vitro. An analysis of the inhibitory mechanism. Allergy. 1986 Sep;41(7):537–542. doi: 10.1111/j.1398-9995.1986.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Titus E. O. Recent developments in the understanding of the pharmacokinetics and mechanism of action of chloroquine. Ther Drug Monit. 1989;11(4):369–379. [PubMed] [Google Scholar]



