Abstract
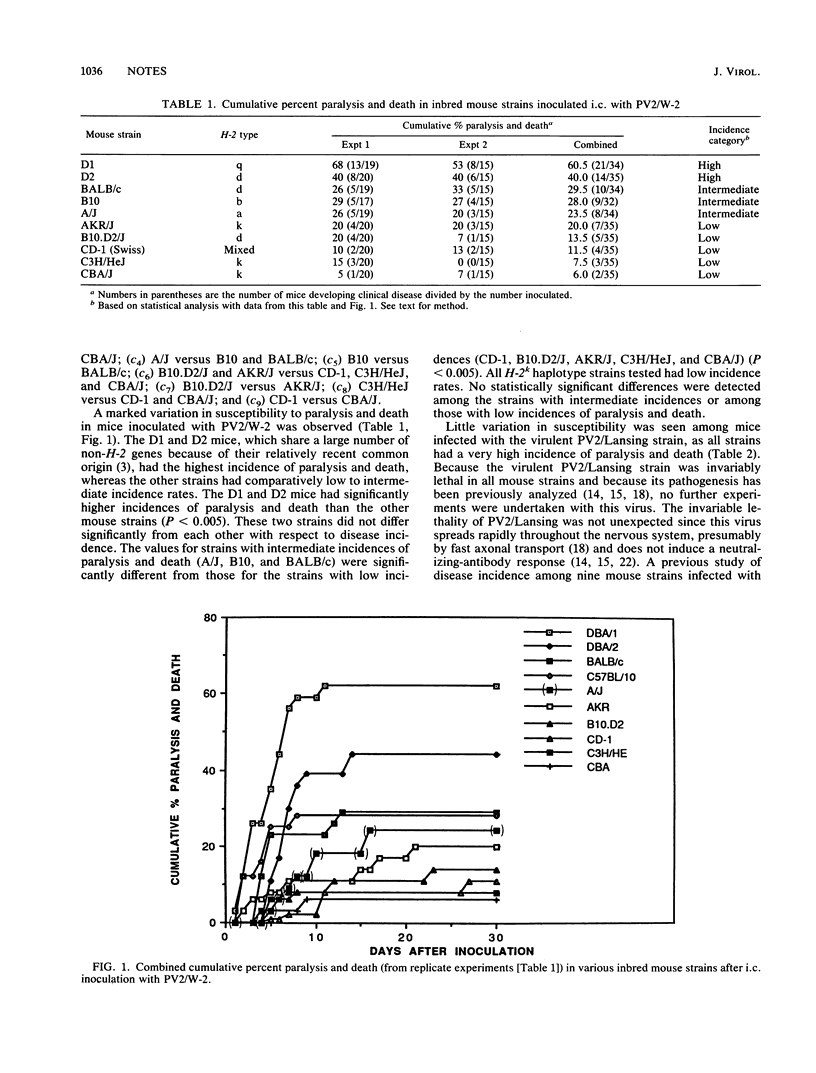
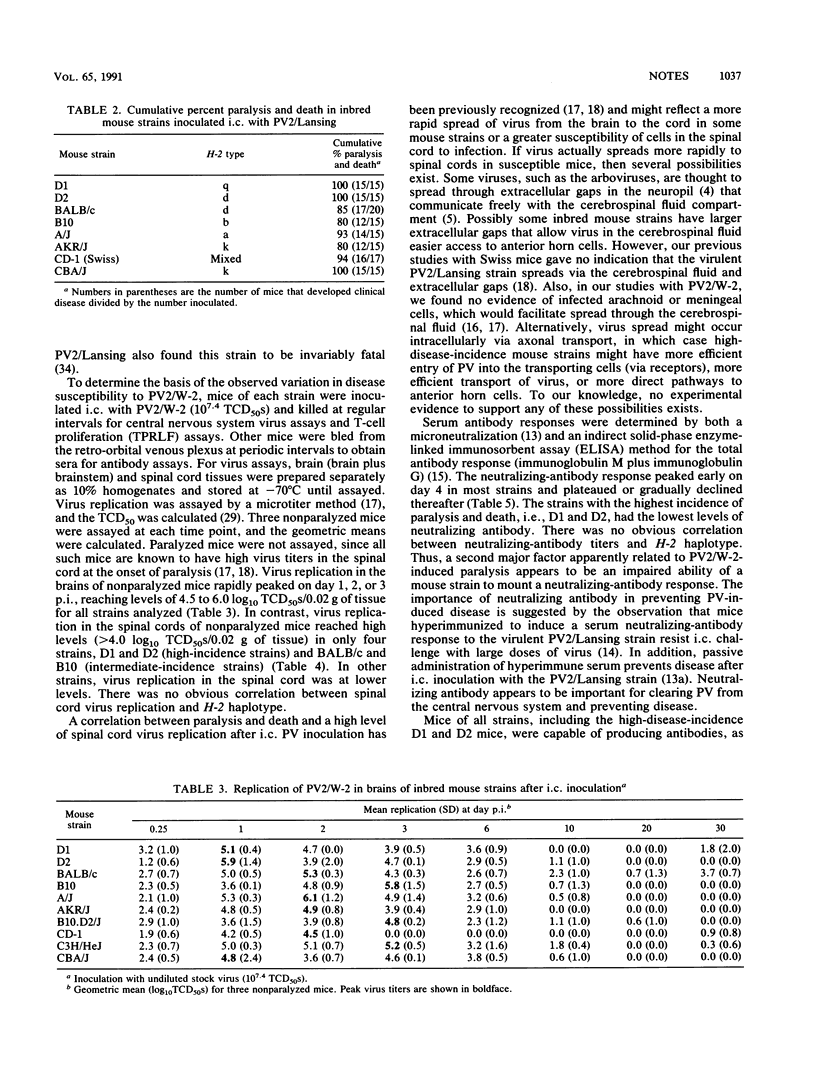
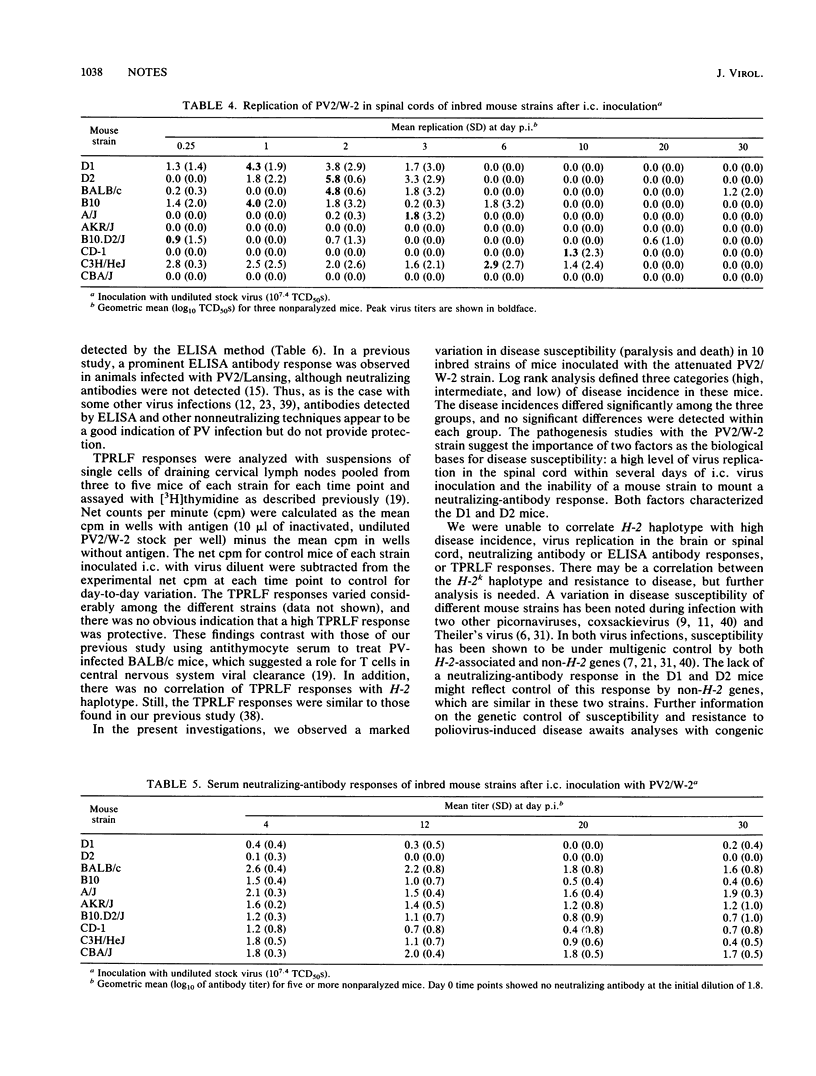
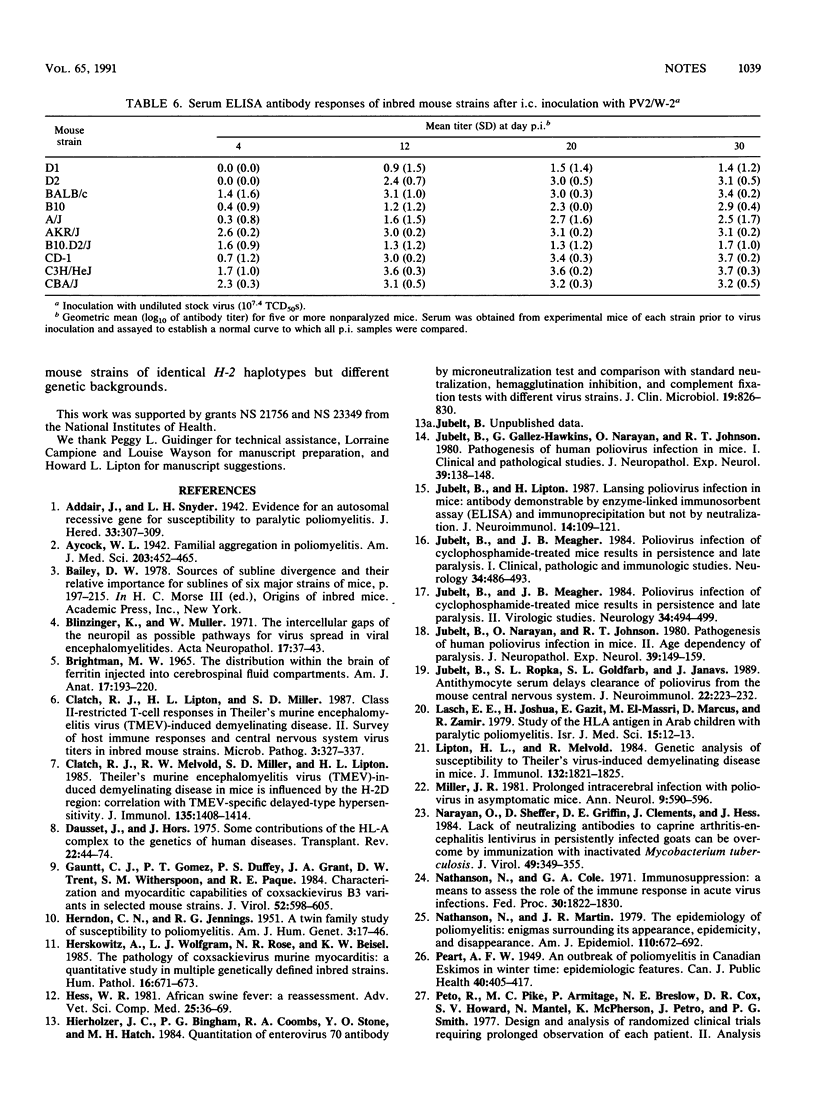
Susceptibility to human poliovirus-induced disease in different inbred mouse strains was analyzed after intracerebral inoculation of two mouse-adapted type 2 polioviruses, the attenuated W-2 strain and the virulent Lansing strain. In contrast to inoculation with the Lansing strain, which was invariably lethal, inoculation with the W-2 strain defined three groups of mice with high, intermediate, or low disease incidence. Those in the high-disease-incidence group, the DBA/1J and DBA/2J mice, exhibited a high level of virus replication in the spinal cord by day 2 postinfection, with no detectable neutralizing-antibody response. Mice in the intermediate- and low-incidence groups had lower levels of virus replication in the spinal cord and/or produced neutralizing antibodies. No correlation was observed between H-2 haplotype and the extent of virus replication, production of neutralizing or enzyme-linked immunosorbent assay-detectable antibodies, or T-cell-proliferative response. However, mice of the H-2k haplotype manifested a low incidence of disease.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blinzinger K., Müller W. The intercellular gaps of the neuropil as possible pathways for virus spread in viral encephalomyelitides. Acta Neuropathol. 1971;17(1):37–43. doi: 10.1007/BF00684739. [DOI] [PubMed] [Google Scholar]
- Brightman M. W. The distribution within the brain of ferritin injected into cerebrospinal fluid compartments. II. Parenchymal distribution. Am J Anat. 1965 Sep;117(2):193–219. doi: 10.1002/aja.1001170204. [DOI] [PubMed] [Google Scholar]
- Clatch R. J., Lipton H. L., Miller S. D. Class II-restricted T cell responses in Theiler's murine encephalomyelitis virus (TMEV)-induced demyelinating disease. II. Survey of host immune responses and central nervous system virus titers in inbred mouse strains. Microb Pathog. 1987 Nov;3(5):327–337. doi: 10.1016/0882-4010(87)90003-9. [DOI] [PubMed] [Google Scholar]
- Clatch R. J., Melvold R. W., Miller S. D., Lipton H. L. Theiler's murine encephalomyelitis virus (TMEV)-induced demyelinating disease in mice is influenced by the H-2D region: correlation with TEMV-specific delayed-type hypersensitivity. J Immunol. 1985 Aug;135(2):1408–1414. [PubMed] [Google Scholar]
- Dausset J., Hors J. Some contributions of the HL-A complex to the genetics of human diseases. Transplant Rev. 1975;22:44–74. doi: 10.1111/j.1600-065x.1975.tb01551.x. [DOI] [PubMed] [Google Scholar]
- Gauntt C. J., Gomez P. T., Duffey P. S., Grant J. A., Trent D. W., Witherspoon S. M., Paque R. E. Characterization and myocarditic capabilities of coxsackievirus B3 variants in selected mouse strains. J Virol. 1984 Nov;52(2):598–605. doi: 10.1128/jvi.52.2.598-605.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERNDON C. N., JENNINGS R. G. A twin-family study of susceptibility to poliomyelitis. Am J Hum Genet. 1951 Mar;3(1):17–46. [PMC free article] [PubMed] [Google Scholar]
- Herskowitz A., Beisel K. W., Wolfgram L. J., Rose N. R. Coxsackievirus B3 murine myocarditis: wide pathologic spectrum in genetically defined inbred strains. Hum Pathol. 1985 Jul;16(7):671–673. doi: 10.1016/s0046-8177(85)80149-0. [DOI] [PubMed] [Google Scholar]
- Hess W. R. African swine fever: a reassessment. Adv Vet Sci Comp Med. 1981;25:39–69. [PubMed] [Google Scholar]
- Hierholzer J. C., Bingham P. G., Coombs R. A., Stone Y. O., Hatch M. H. Quantitation of enterovirus 70 antibody by microneutralization test and comparison with standard neutralization, hemagglutination inhibition, and complement fixation tests with different virus strains. J Clin Microbiol. 1984 Jun;19(6):826–830. doi: 10.1128/jcm.19.6.826-830.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubelt B., Gallez-Hawkins G., Narayan O., Johnson R. T. Pathogenesis of human poliovirus infection in mice. I. Clinical and pathological studies. J Neuropathol Exp Neurol. 1980 Mar;39(2):138–148. doi: 10.1097/00005072-198003000-00003. [DOI] [PubMed] [Google Scholar]
- Jubelt B., Lipton H. Lansing poliovirus infection in mice: antibody demonstrable by enzyme-linked immunosorbent assay (ELISA) and immunoprecipitation but not by neutralization. J Neuroimmunol. 1987 Feb;14(1):109–121. doi: 10.1016/0165-5728(87)90105-6. [DOI] [PubMed] [Google Scholar]
- Jubelt B., Meagher J. B. Poliovirus infection of cyclophosphamide-treated mice results in persistence and late paralysis: I. Clinical, pathologic, and immunologic studies. Neurology. 1984 Apr;34(4):486–493. doi: 10.1212/wnl.34.4.486. [DOI] [PubMed] [Google Scholar]
- Jubelt B., Meagher J. B. Poliovirus infection of cyclophosphamide-treated mice results in persistence and late paralysis: II. Virologic studies. Neurology. 1984 Apr;34(4):494–499. doi: 10.1212/wnl.34.4.494. [DOI] [PubMed] [Google Scholar]
- Jubelt B., Narayan O., Johnson R. T. Pathogenesis of human poliovirus infection in mice. II. Age-dependency of paralysis. J Neuropathol Exp Neurol. 1980 Mar;39(2):149–159. doi: 10.1097/00005072-198003000-00004. [DOI] [PubMed] [Google Scholar]
- Jubelt B., Ropka S. L., Goldfarb S. J., Janavs J. L. Anti-thymocyte serum delays clearance of poliovirus from the mouse central nervous system. J Neuroimmunol. 1989 May;22(3):223–232. doi: 10.1016/0165-5728(89)90020-9. [DOI] [PubMed] [Google Scholar]
- Lasch E. E., Joshua H., Gazit E., El-Massri M., Marcus O., Zamir R. Study of the HLA antigen in Arab children with paralytic poliomyelitis. Isr J Med Sci. 1979 Jan;15(1):12–13. [PubMed] [Google Scholar]
- Lipton H. L., Melvold R. Genetic analysis of susceptibility to Theiler's virus-induced demyelinating disease in mice. J Immunol. 1984 Apr;132(4):1821–1825. [PubMed] [Google Scholar]
- Miller J. R. Prolonged intracerebral infection with poliovirus in asymptomatic mice. Ann Neurol. 1981 Jun;9(6):590–596. doi: 10.1002/ana.410090613. [DOI] [PubMed] [Google Scholar]
- Narayan O., Sheffer D., Griffin D. E., Clements J., Hess J. Lack of neutralizing antibodies to caprine arthritis-encephalitis lentivirus in persistently infected goats can be overcome by immunization with inactivated Mycobacterium tuberculosis. J Virol. 1984 Feb;49(2):349–355. doi: 10.1128/jvi.49.2.349-355.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson N., Cole G. A. Immunosuppression: a means to assess the role of the immune response in acute virus infections. Fed Proc. 1971 Nov-Dec;30(6):1822–1830. [PubMed] [Google Scholar]
- Nathanson N., Martin J. R. The epidemiology of poliomyelitis: enigmas surrounding its appearance, epidemicity, and disappearance. Am J Epidemiol. 1979 Dec;110(6):672–692. doi: 10.1093/oxfordjournals.aje.a112848. [DOI] [PubMed] [Google Scholar]
- PEART A. F. W., RHODES A. J. An outbreak of poliomyelitis in Canadian Eskimos in wintertime. Can J Public Health. 1949 Oct;40(10):405–419. [PubMed] [Google Scholar]
- Pietsch M. C., Morris P. J. An association of HL-A3 and HL-A7 with paralytic poliomyelitis. Tissue Antigens. 1974;4(1):50–55. doi: 10.1111/j.1399-0039.1974.tb00224.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., David C. S. Demyelination induced by Theiler's virus: influence of the H-2 haplotype. J Immunol. 1985 Sep;135(3):2145–2148. [PubMed] [Google Scholar]
- Roos R. P. Genetically controlled resistance to virus infections of the central nervous system. Prog Med Genet. 1985;6:241–276. [PubMed] [Google Scholar]
- SABIN A. B. Paralytic consequences of poliomyelitis infection in different parts of the world and in different population groups. Am J Public Health Nations Health. 1951 Oct;41(10):1215–1230. doi: 10.2105/ajph.41.10.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. G., Sun L. Z., Jubelt B., Waltenbaugh C. Cell-mediated immune responses to poliovirus. I. Conditions for induction, characterization of effector cells, and cross-reactivity between serotypes for delayed hypersensitivity and T cell proliferative responses. Cell Immunol. 1989 Apr 1;119(2):252–262. doi: 10.1016/0008-8749(89)90242-6. [DOI] [PubMed] [Google Scholar]
- Wang K., Sun L., Jubelt B., Waltenbaugh C. Cell-mediated immune responses to poliovirus II. Survey of delayed hypersensitivity and T-cell proliferative responses in inbred mouse strains. Viral Immunol. 1990 Summer;3(2):111–117. doi: 10.1089/vim.1990.3.111. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Clapham P. R., Cheingsong-Popov R., Dalgleish A. G., Carne C. A., Weller I. V., Tedder R. S. Neutralization of human T-lymphotropic virus type III by sera of AIDS and AIDS-risk patients. Nature. 1985 Jul 4;316(6023):69–72. doi: 10.1038/316069a0. [DOI] [PubMed] [Google Scholar]
- Wolfgram L. J., Beisel K. W., Herskowitz A., Rose N. R. Variations in the susceptibility to Coxsackievirus B3-induced myocarditis among different strains of mice. J Immunol. 1986 Mar 1;136(5):1846–1852. [PubMed] [Google Scholar]
- Wyatt H. V. Abortive poliomyelitis or minor illness as a clue to genetic susceptibility. Med Microbiol Immunol. 1978 Nov 17;166(1-4):29–36. doi: 10.1007/BF02121131. [DOI] [PubMed] [Google Scholar]
- Zander H., Grosse-Wilde H., Kuntz B., Scholz S., Albert E. D. HLA-A, -B, and -D antigens in paralytic poliomyelitis. Tissue Antigens. 1979 Apr;13(4):310–313. doi: 10.1111/j.1399-0039.1979.tb00800.x. [DOI] [PubMed] [Google Scholar]
- van Eden W., Persijn G. G., Bijkerk H., de Vries R. R., Schuurman R. K., van Rood J. J. Differential resistance to paralytic poliomyelitis controlled by histocompatibility leukocyte antigens. J Infect Dis. 1983 Mar;147(3):422–426. doi: 10.1093/infdis/147.3.422. [DOI] [PubMed] [Google Scholar]


