Abstract
Background
Matrix metalloproteinases play an important role in extracellular matrix deposition and degradation. Based on previous clinical observations of corneal perforations during topical fluoroquinolone treatment, we decided to evaluate the comparative effects of various fluoroquinolone eye drops on the expression of matrix metalloproteinases (MMPs) in cornea.
Methods
Eighty female Lewis rats were divided into two experimental groups: intact and wounded corneal epithelium. Uniform corneal epithelial defects were created in the right eye with application of 75% alcohol in the center of the tissue for 6 seconds. The treatment groups were tested as follows: 1) Tear drops: carboxymethylcellulose sodium 0.5 % (Refresh, Allergan); 2) Ciprofloxacin 0.3% (Ciloxan, Alcon); 3) Ofloxacin 0.3%(Ocuflox, Allergan); 4) Levofloxacin 0.5%(Quixin, Santen). Eye drops were administered 6 times a day for 48 hours. Rats were sacrificed at 48 hours. Immunohistochemical analysis and zymography were conducted using antibodies specific to MMPs-1, 2, 8 and 9.
Results
MMP-1, MMP-2, MMP-8 and MMP-9 expression were detected at 48 hrs in undebrided corneal epithelium groups treated with the topical fluoroquinolones. No statistical difference was observed in quantitative expression of MMPs among ciprofloxacin 0.3%, ofloxacin 0.3%, levofloxacin 0.5%. When the artificial tear group and the fluoroquinolone groups with corneal epithelial defect were compared, increased expression of MMPs was observed as a result of the wound healing process. However, the fluoroquinolone treated group exhibited high statistically significantly levels of MMPs expression.
Conclusions
Our study provides preliminary evidence that topical application of fluoroquinolone drugs can induce the expression of MMP-1, MMP-2, MMP-8 and MMP-9 in the undebrided corneal epithelium compared to artificial tear eye drops.
Keywords: Fluoroquinolones, Levofloxacin, Ciprofloxacin, Ofloxacin, Matrix metalloproteinases, Cornea
Background
Since their introduction in the United States over a decade ago, the quinolone antibacterial agents have become a mainstay in the treatment of serious bacterial infections. The fluoroquinolones are known for their extremely broad spectrum of antibacterial activity.[1,2] They exert a bactericidal effect by inhibiting bacterial DNA synthesis through interference with the enzymes DNA gyrase and topoisomerase IV. The structural differences of the fluorinated carboxyquinolones commercially available, for topical ophthalmic use, alter their potency as well as their pharmacological profiles. To be clinically useful, antibiotics should be effective with minimal adverse effects. Although fluoroquinolones have been shown to be highly effective antibacterial agents, several clinical studies have reported a fluoroquinolone-induced tendinopathy with bone and articular damage associated with alterations in collagen deposition and chondrocyte function after systematic fluoroquinolone administration. [3-5] Topical fluoroquinolone antibiotics are frequently administered prophylactically in post-surgical care and represent a therapeutic option in the treatment of infectious conjunctivitis and keratitis.[2] These are generally well tolerated and compare favorably with other topical antibiotics in terms of efficacy. However, it is interesting to note that most of the commercial fluoroquinolone ophthalmic solutions contain very low concentrations of the preservatives benzalkonium chloride or edetate disodium (Ciloxan at 0.006%, Ocuflox at 0.05 mg and Quixin at 0.005%) in their formulations. As it is known, much of the reported toxicity has been associated with the presence of certain preservatives in high concentrations (close to 0.1%) which is not the case with the current fluoroquinolone formulations studied. [6]In vivo animal and in vitro studies have also demonstrated that fluoroquinolone agents may adversely affect wound healing by exerting cytotoxic effects on corneal epithelial cells and keratocytes; however, the exact mechanism of fluoroquinolone toxicity is still unknown. [7-10]
A recent study reported an increased incidence of ulcerative keratolysis with corneal perforation after topical fluoroquinolone treatment for microbial keratitis.[11] These findings led us to investigate the role of various commercially available fluoroquinolone eye drop formulations on the physiological remodeling of the corneal extracellular matrix by evaluating matrix metalloproteinase expression in cornea.
Matrix metalloproteinases (MMPs) are involved in numerous physiological and pathological processes of corneal extracellular matrix remodeling and degradation. [12-14] The proteolytic properties of these enzymes may play an important role in the unidentified mechanism of ulcerative keratolysis associated with the topical use of fluoroquinolone eye drops. The MMPs can be grouped into different subclasses according to their natural substrates. We focused on two specific groups: collagenases and gelatinases; evaluating the expression of interstitial collagenase (MMP-1) and PMN collagenase (MMP-8), both of which can be secreted by corneal cells and are able to cleave corneal stromal collagens. We assessed the expression of 72-kDa gelatinase A (MMP-2) and 92-kDa gelatinase B (MMP-9) as well. These gelatinases help to degrade the basement membrane and act cooperatively with the collagenases to completely degrade interstitial corneal collagens.
Methods
Eighty female Lewis rats each weighing 250 g were first divided into two experimental groups. Each rat was prepared to produce a debrided corneal epithelium in the right eye and intact corneal epithelium in the left eye. The animals were then randomly divided into four experimental test groups of 20 rats each, as follows: Group 1 received tear drops consisting of carboxymethylcellulose sodium 0.5% (Refresh, Allergan, Irvine, CA); Group 2 received ciprofloxacin 0.3% (Ciloxan, Alcon, Fort Worth, TX); Group 3 was instilled ofloxacin 0.3% (Ocuflox, Allergan, Irvine, CA); finally, group 4 received levofloxacin 0.5%(Quixin, Santen, Napa, CA).
All of the animals were treated in accordance with the tenets of the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of animals in Ophthalmic and Vision Research.
Anesthesia was achieved by intramuscular injection of 0.5 ml/kg body weight of a mixture 1:1 of 100 mg/ml ketamine and 20 mg/ml xylazine. Uniform corneal epithelial defects were created in the right eyes with the application of a filter paper disk soaked in 75% ethanol solution in the center of the tissue for 60 seconds. Immediately after the procedure, treated eyes were thoroughly irrigated with balanced salt solution. The left eye served as an intact epithelium control in each rat. Eye drops were instilled six times/day for 48 hours. Rats were sacrificed at 48 hours after initiation of treatment. The corneas from fifty six rats were removed at the scleral ring, embedded in ornithine carbamoyltransferase compound (OCT Tissue-Tek, Miles Inc., Elkhart, USA), and frozen at -80°C. Cryostat sections of 8 μm from all corneas were stained with hematoxylin and eosin, or immunohistochemically using antibodies specific for MMP-1, MMP-2, MMP-8 and MMP-9. We used six rats from each treatment group (n = 24) to perform immunoblotting and zymography studies.
Immunohistochemistry
Immunohistochemical staining was performed using an avidin-biotin-peroxidase complex technique. Primary antibodies consisted of polyclonal antibodies recognizing epitopes of both the pro- and active forms of MMP-1 and MMP-8 (Chemicon, Temecula, CA) as well as monoclonal antibodies against MMP-2 and MMP-9 (Oncogene, Cambridge, MA and Chemicon, Temecula, CA respectively). The primary antibodies were applied to the corneal sections and incubated at room temperature for 1 hour. After washing with TBS (20 mM Tris-HCl pH 7.5, 150 mM NaCL), a biotin-labeled secondary antibody, either goat anti-rabbit or horse anti-mouse IgG (Vector Laboratories, Burlingame, CA) was used. Finally, after incubation with the avidin-biotin-peroxidase complex (Vector Laboratories, Burlingame, CA), slides were developed in 3,3' diaminobenzidine and counterstained with 1 % methyl green in methanol. Analysis of immunostaining was performed to determine possible statistical significance between the artificial tear group and the fluoroquinolone treated groups. All samples were stained in parallel to minimize specimen variation and cell staining intensity was graded by a masked observer. A statistical calculation and significant differences in immunostaining between the treated groups and the artificial tear control group were defined as p < 0.05 by Fisher's exact test.
To quantify the metalloproteinase expression after the application of fluoroquinolone eye drops, the four test groups were analyzed by western-blot and zymography.
Zymography
MMP-2 and MMP-9 levels were also assayed by zymography. Debrided (n = 24) and intact rat corneas (n = 24) from the four treatment groups were dissected and trephined (3 mm) at 48 hours and immediately cultivated in a 24-well culture dish with 500 μl of serum-free medium (MEM + minimal essential amino acid + antibiotic/antimycotic) at 37°C, 5% CO2 atmosphere for 72 hours. Each and every conditioned media aliquot was subjected to gel electrophoresis (10% SDS polyacrylamide gel containing 1 mg/ml gelatin) under nonreducing conditions. Fifteen microliters of each conditioned medium and zymography sample buffer were combined and incubated at room temperature for 5 minutes. Electrophoresis was carried out at 100 to 150 volts for 2–4 hours. The gels were washed in renaturing buffer (Bio-Rad Laboratories, Hercules, CA) for 30 minutes at room temperature, incubated with development buffer (Bio-Rad) at 37°C for 16 hours, stained for 3 hours with Coomassie Brillant Blue R-250, and destained with three changes (15, 30, 60 minutes) of destain solution (Bio-Rad Laboratories).
Immunoblot Assays
The levels of MMP-1 and MMP-8 in the cornea-conditioned culture medium were assessed by Western blot. The blot was probed with polyclonal antibodies against MMP-1 or MMP-8, (Chemicon, CA). Fifteen microliters of conditioned medium was mixed with electrophoresis Laemmli buffer and heated at 90°C for 5 minutes, and then kept on ice. The samples were run on 10% polyacrylamide SDS gel at 100 volts for 2 hours, and transferred to nitrocellulose membranes at 120 volts for 2 hours (Bio-Rad Richmond, CA). The nitrocellulose paper was agitated in blocking buffer at room temperature (PBS, 0.05% Tween 20, 0.5% non-fat dry milk) for 1 hour. The primary antibody (1:5000 dilution) and secondary antibody (1:2000 dilution) incubations were carried out for 1 hour respectively. After three washes with TBS-T (TBS, 0.05% Tween-20), the membranes were incubated in enhanced chemiluminescence solution (Amersham Life Science, Arlington Heights, IL) followed by exposure to chemiluminescence film for 15 seconds.
Statistical Analysis
The western blots and zymograms from all treated eyes, each one in duplicate were subjected to densitometry analysis. The statistical analysis was done using one-way ANOVA test, Dunnett's multiple comparison test and Bonferroni test (SPSS, ver. 10.0 for Windows; SPSS Inc., Chicago, IL) and Microstat-Ecosoft Inc. (Indianapolis, Inc.), the values lower than 0.05 was considered statistically significant (fluoroquinolone treated groups compared to artificial tear treated group as control). The quantification of metalloproteinase values of each X-ray film and gel zymograms from all treated eyes were performed by scanning densitometry (Molecular Dynamics, Sunnyvale, CA).
Results
Using immunohistochemical staining we found positive collagenases MMP-1 and MMP-8 staining in the wounded corneal epithelium of the artificial tear group and the absence of MMP staining in the unwounded eyes of the same group (Table 1). These results are consistent with an established correlation between the wound healing and extracellular matrix remodeling process and the up-regulation of MMPs. The current artificial tear solution was demonstrated to be innocuous to the corneal tissue and did not have any effect on the expression of corneal metalloproteinases in unwounded corneal epithelium.
Table 1.
Effects of Topical fluoroquinolones on Collagenase-type Metalloproteinases Immunohistochemical detection at 48 Hours
| Type of eye drops | MMP-1 | MMP-8 | |
| Applied q.i.d. | Epithelium | ||
| Artificial Tear | Intact | 0/14 | 0/14 |
| Debrided | 14/14 | 13/14 | |
| Ciloxan | Intact | 14/14 ** | 13/14 ** |
| Debrided | 14/14 | 14/14 | |
| Ocuflox | Intact | 13/14** | 12/14** |
| Debrided | 14/14 | 14/14 | |
| Quixin | Intact | 13/14** | 12/14** |
| Debrided | 14/14 | 14/14 |
Double masked observer determined the total positive immunostained eyes for MMP-1 and MMP-8 in each test group. Immunoreactivity is reported as number of positively immunostained corneas / total number of corneas examined. ** Statistically different when comparing the corresponding fluoroquinolone group to artificial tear. (P < 0.05 using Fisher's exact test analysis).
We also examined the MMP expression after the application of commercially available fluoroquinolone eye drops. Analysis of immunohistochemical staining disclosed a positive staining for metalloproteinase at corneal epithelium and superficial stroma. Interestingly, the percentage of eyes positively staining for MMP-1 and MMP-8 was not found to be significantly different between unwounded groups treated with each of the commercially available fluoroquinolone eye drops. This result indicates that the fluoroquinolone ophthalmic solutions are the major trigger for the increased expression of these MMPs in the normal corneal epithelium. In previous studies, we also observed MMP-8 expression at the level of the corneal epithelium associated with ulcerative keratolysis. These findings identify a novel source of MMP-8 expression at the corneal epithelial cells as a non-PMN cellular source of this collagenase. Using hematoxylin-eosin stain, the polymorpho nuclear neutrophils (PMNs) were identified on the basis of their characteristic multilobed nucleus and staining pattern. In the current study, the fluoroquinolone treatment causes a non significant increase of PMNs in unwounded corneas compared to the artificial tear group at 48 hours. Figure 1 illustrates MMP-1 immunostaining primarily in the epithelial cells and occasionally in the superficial stromal keratocytes of intact corneas of the fluoroquinolone treated groups.
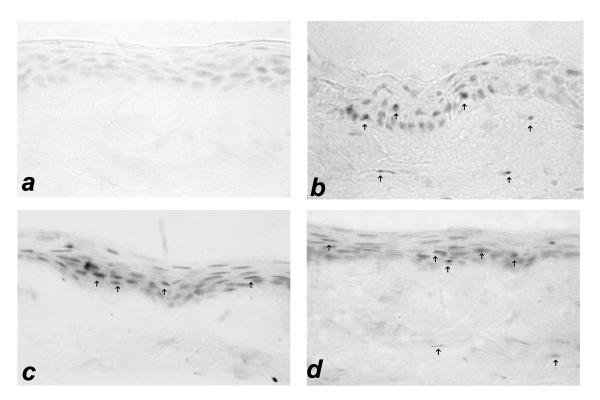
Figure 1.
Immunolocalization of MMP-1 in intact corneal epithelium at 48 hours is shown for each treatment group. Panel a: artificial tears; panel b: Ciprofloxacin 0.3%; panel c: Ofloxacin 0.3%; panel d: Levofloxacin 0.5%. A photomicrograph of the artificial tear group shows negative corneal staining for MMP-1. However, intense corneal epithelial and superficial stromal expression of MMP-1 was identified in intact corneal epithelium groups treated with fluoroquinolone eye drops (panel's b-d). The presence of MMP-1 was detected mostly in the corneal epithelial cells (arrows). (Immunohistochemistry, ×400 magnification).
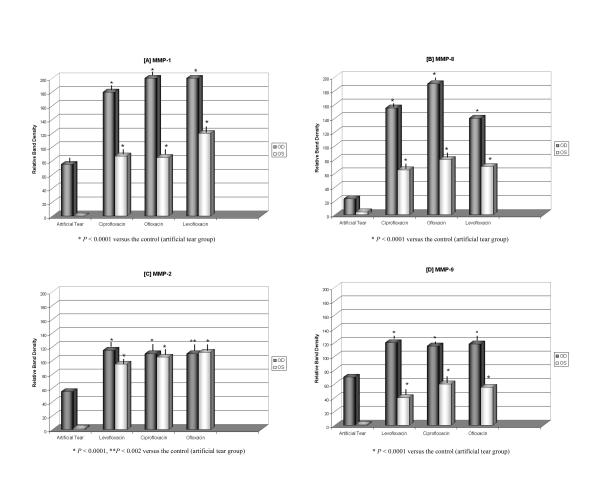
To confirm the results illustrated by immunohistochemical staining and to provide a more accurate quantitative analysis, we used western blotting and zymography techniques to study the early MMP expression after fluoroquinolone eye drop treatment. The values of each MMP bands from western blots and zymography studies made in all treated eyes were quantified by densitometry analysis and also studied statistically to indicate any significance between each fluoroquinolone treated groups and the artificial tear group (Figure 4A through 4D).
Figure 4.
Effects of fluoroquinolone eye drops on the corneal rat's metalloproteinases expression. The bands densities of western immunoblots for MMP-1 (A) and MMP-8 (B) and zymograms for MMP-2 (C), MMP-9 (D) were subjected to densitometry analysis, the statistical significance was determined by one-way ANOVA test (values are means, error bars). P < 0.05 was considered statistically significant when compared fluoroquinolones groups vs. artificial tear group. Note the highest values of MMPs levels from topical fluoroquinolone groups compared to artificial tear group in unwounded corneas.
A comparison of topical artificial tear group as control versus groups treated with ciprofloxacin, ofloxacin and levofloxacin on the expression of corneal metalloproteinases was conducted. These studies also disclosed that the application of the present ophthalmic fluoroquinolone solutions (Ciloxan, Ocuflox and Quixin) increases MMP-1, MMP-8 (immunoblots, figure 2) and MMP-2, MMP-9 (zymography, figure 3) expression in both intact and wounded corneas although the levels of expression may be slightly different among various groups. In accordance with our previous findings, treatment with the artificial tear does not appreciably induce the expression of MMPs in intact corneal epithelium. The MMP up-regulation detected in all groups of wounded eyes presumably resulted from the wound healing process and extracellular matrix remodeling. However, the expression of MMPs in wounded eyes was up-regulated by the topical application of the fluoroquinolone drugs when compared to the artificial tear treated group. The results presented in this study demonstrated a statistically significant difference between fluoroquinolone treated groups (P < 0.0001) versus artificial tear treated group as control in the expression of corneal metalloproteinases.
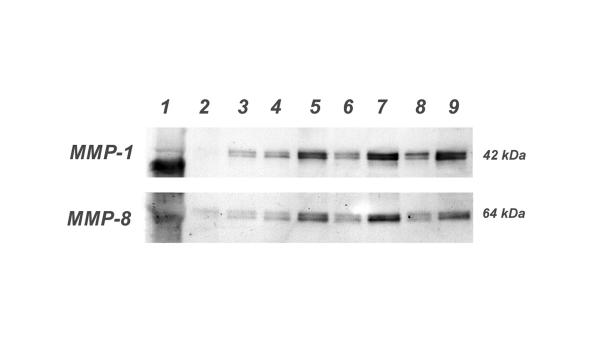
Figure 2.
A representative western blot of MMP-1 and MMP-8 expression of supernatant samples from control (artificial tear) and the fluoroquinolone test groups. Corneas with debrided (OD) and intact (OS) epithelium were analyzed. 1. MW; 2. Tears OS; 3. Tears OD; 4. Ciprofloxacin OS; 5. Ciprofloxacin OD; 6. Ofloxacin OS; 7. Ofloxacin OD; 8. Levofloxacin OS; 9. Levofloxacin OD The molecular sizes of both MMPs were calculated based on a molecular weight standard and recorded in kilodaltons (kDa). The fluoroquinolone eye drops increased the expression of both metalloproteinases, MMP-1 (42 kDa) and of MMP-8 (64 kDa) in intact corneas; whereas, the expression of these MMPs was negative in the controls treated with artificial tears.
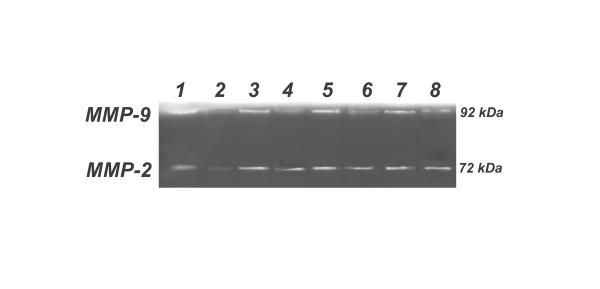
Figure 3.
Typical gelatin zymography analyses of gelatinase A (MMP-2) and gelatinase B (MMP-9) expression in artificial tear group rat and in the test groups were performed. Corneal conditioned medium samples from debrided (OD) and intact (OS) corneal epithelium of each group were analyzed. 1. Tears OD; 2. Tears OS; 3. Levofloxacin 0.5% OD; 4. Levofloxacin 0.5% OS; 5. Ciprofloxacin OD; 6. Ciprofloxacin OS; 7. Ofloxacin OD; 8. Ofloxacin OS. MMP-9 (92 kDa) and MMP-2 (72 kDa) expression were up regulated in fluoroquinolone treated corneas and were present at basal level in unwounded corneas receiving artificial tears. Corneal epithelium debridement up regulated the expression of MMP-9 and MMP-2 in all test groups.
Conclusions
Fluoroquinolones are one class of synthetic antibacterial agents, which inhibit the bacterial topoisomerases involved in bacterial DNA replication. Since the 1980s, the U.S. Food and Drug Administration has approved several generations of fluoroquinolones. Recently, levofloxacin 0.5% solution, a third generation fluoroquinolone, was introduced as a commercial eye drop for clinical use in therapy of acute bacterial conjunctivitis. The unique structure of this drug differs from other fluoroquinolones conferring high solubility to potentially allow formulation at higher concentrations. It also extends the Gram-positive spectrum; and enhances the activity against anaerobic microbes while maintaining Gram-negative activity.[1] Fluoroquinolones are generally well tolerated with minimal side effects when used appropriately.[15] However, several in vitro and in vivo studies have demonstrated that both ciprofloxacin and ofloxacin eye drops significantly delay corneal wound healing.[7,8,16] In addition, ciprofloxacin hydrochloride 0.3% solution has demonstrated a tendency for precipitation with corneal crystal deposition, mainly in association with the pH dependent solubility of the eye drop formulation.[17,18] The above mentioned studies suggest that the current commercially available topical fluoroquinolone agents, and their accompanying preservatives, have cytotoxic effects on corneal cells.
The metalloproteinases are potent enzymes that are capable of degrading a wide range of extracellular matrix components. Numerous studies have documented the important role of matrix metalloproteinases in the corneal wound healing process.[19,20] The perturbation of the normal regulation of degradative processes may result in the accelerated breakdown of connective tissues and may lead to conditions such as ulcerative keratolysis. There is increasing experimental and clinical evidence that during corneal injury and inflammation, MMPs play a major role in the proteolytic processes, which can predispose the cornea to perforation.[21] Thus, this study focuses on evaluating the expression of MMPs after the use of commercially available fluoroquinolone eye drops in order to elucidate a relationship between keratolysis, corneal perforation and the use of ophthalmic fluoroquinolone drugs as reported in previous clinical studies.[11] The findings in the current study provide the first evidence that some undesirable effects on corneal wound healing from the use of specific fluoroquinolone eye drops (or their use in vulnerable corneal epithelium) may be secondary to the over-expression of the metalloproteinases. Furthermore, the results of our study clearly demonstrate that the commercial fluoroquinolone ophthalmic agents tested stimulate expression of corneal collagenases (MMP-1 and MMP-8) and gelatinases (MMP-2 and MMP-9) in normal eyes compared to a negative expression of MMPs in the artificial tear group. Additional studies are required to confirm the exact cellular origin of MMP-8 expressed in corneal tissue, although it is now recognized that cell types other than PMNs can express this collagenase.
Recent in vivo and in vitro studies indicate that the pathologic mechanism with fluoroquinolones may be related to significant increases of matrix-degrading proteolytic activity, inhibitory effects on cell metabolism, as well as the degenerative and ultrastructural cell changes with increased levels and interferences in the regulative pathways of several cytokines. [22-26] The clinical significance of these findings revolve around two main questions: how is the expression of these matrix metalloproteinase increased after the application of topical fluoroquinolone eye drops? and how significant are the eye drop preservatives at low concentration in stimulating MMP expression in the cornea? A new generation fluoroquinolone agent, moxifloxacin 0.5% (Vigamox, Alcon Labs, Fort Worth, TX) is commercially formulated without preservatives. Further studies addressing these important questions and explaining the cascade of factors involved in producing this increased expression of MMPs seem warranted. Physicians should be aware of the possible consequences of using fluoroquinolones for both normal and impaired corneal tissue especially with a prophylactic aim. Clinical decisions should balance safety with efficacy especially in the light of studies, such as this one, documenting the potential for corneal cytotoxicity and wound healing impairment after the topical application of commercially available fluoroquinolone drugs.
Competing Interests
None declared.
Authors' Contributions
Victor E. Reviglio, MD: AB, JY
Melinda A. Hakim, MD: JY
Jae K. Song, MD: JY, ES
Terrence P. O'Brien, MD: FG
Victor E. Reviglio, Melinda A. Hakim and Jae K. Song carried out animal model, sample preparation, microscopy, immunostaining, zymography and immunoblotting.
Victor E. Reviglio and Terrence P. O'Brien were both involved in the experimental design, data analysis and preparation of manuscript.
All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
The authors would like to thanks Dr. Juan C. Muiño for providing the statistical assistance.
Contributor Information
Victor E Reviglio, Email: victorwilmer@aol.com.
Melinda A Hakim, Email: mhakim@jhmi.edu.
Jae K Song, Email: navymed@hanmail.net.
Terrence P O'Brien, Email: tobrien@jhmi.edu.
References
- Graves A, Henry M, O'Brien TP, Hwang DG, Van Buskirk A, Trousdale MD. In Vitro Susceptibilities of Bacterial Ocular Isolates to Fluoroquinolones. CORNEA. 2001;20:301–305. doi: 10.1097/00003226-200104000-00012. [DOI] [PubMed] [Google Scholar]
- O'Brien TP, Maguire MG, Fink NE, Alfonso E, McDonnell P. Efficacy of ofloxacin vs cefazolin and tobramycin in the therapy of bacterial keratitis. Arch Ophthalmol. 1995;113:1257–1265. doi: 10.1001/archopht.1995.01100100045026. [DOI] [PubMed] [Google Scholar]
- Harrell RM. Fluoroquinolone-induced tendinopathy. What do we know? South Med J. 1999;92:622–625. doi: 10.1097/00007611-199906000-00014. [DOI] [PubMed] [Google Scholar]
- Huston KA. Achilles tendonitis and tendon rupture due to fluoroquinolone antibiotics (Letter) N Eng J Med. 1994;331:748. doi: 10.1056/NEJM199409153311116. [DOI] [PubMed] [Google Scholar]
- Redmond AO. Risk-benefit experience of ciprofloxacin use in pediatric patients in the United Kingdom. Pediatr Infect Dis J. 1997;16:147–9. doi: 10.1097/00006454-199701000-00040. discussion 160–2. [DOI] [PubMed] [Google Scholar]
- Burstein NL. Corneal cytotoxicity of topically applied drugs, vehicles and preservatives. Surv Ophthalmol. 1980;25:15–30. doi: 10.1016/0039-6257(80)90072-7. [DOI] [PubMed] [Google Scholar]
- Cutarelli PE, Lass JH, Lazarus HM, Putman SC, Jacobs R. Topical fluoroquinolones: antimicrobial activity and in vitro corneal epithelial toxicity. Curr Eye Res. 1991;10:557–563. doi: 10.3109/02713689109001764. [DOI] [PubMed] [Google Scholar]
- Seitz B, Hayashi S, Wee WR, LaBree L, McDonnell PJ. In vitro effects of aminoglycosides and fluoroquinolones on keratocytes. Invest Ophthal Vis Sci. 1996;37:656–665. [PubMed] [Google Scholar]
- Morlet N, Minassian D, Butcher J. Ofloxacin Study Groups. Risk factors for treatment of suspected microbial keratitis. Br J Ophthalmol. 1999;83:1027–1031. doi: 10.1136/bjo.83.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekoe NA, Li Q, Ashraf F, O'Brien TP. Effects of ciprofloxacin, ofloxacin and gentamicin on corneal cells and wound healing. Invest Ophthalmol Vis Sci. 1999;40:S548. [Google Scholar]
- Mallari PLT, McCarty DJ, Daniell M, Taylor H. Increased incidence of corneal perforation after topical fluoroquinolone treatment for microbial keratitis. Am J Ophthalmol. 2001;131:131–133. doi: 10.1016/S0002-9394(00)00642-5. [DOI] [PubMed] [Google Scholar]
- Massova I, Kotra LP, Fridman R, et al. Matrix metalloproteinases: structures, evolution and diversification. FASEB J. 1998;12:1075–1095. [PubMed] [Google Scholar]
- Fini ME, Parks WC, Rinehart WB, Girard MT, et al. Role of matrix metalloproteinases in failure to re-epithelialize after corneal injury. Am J Pathol. 1996;149:1287–302. [PMC free article] [PubMed] [Google Scholar]
- Ye HQ, Azar DT. Statement of gelatinases A and B, and TIMPs 1 and 2 during wound healing. Invest Ophthalmol Vis Sci. 1998;39:913–21. [PubMed] [Google Scholar]
- Leibowitz HM. Antibacterial effectiveness of ciprofloxacin 3% ophthalmic solution in the treatment of bacterial conjunctivitis. Am J Ophthalmol. 1991;112:29S–33S. [PubMed] [Google Scholar]
- Moreira LB, Lee RF, Oliveira C, LaBree L, McDonnell PJ. Effect of topical fluoroquinolones on corneal re-epithelialization after excimer laser keratectomy. J Cataract Refract Surg. 1997;23:845–848. doi: 10.1016/s0886-3350(97)80241-6. [DOI] [PubMed] [Google Scholar]
- Essepian JP, Rajpal R, O'Brien TP. Tandem scanning confocal microscopic analysis of ciprofloxacin corneal deposits in vivo. Cornea. 1995;14:402–407. doi: 10.1097/00003226-199507000-00009. [DOI] [PubMed] [Google Scholar]
- Kanellopoulos AJ, Miller F, Wittpenn JR. Deposition of topical ciprofloxacin to prevent re-epithelialization of corneal defect (Letter) Am J Ophthalmol. 1994;117:258–259. doi: 10.1016/s0002-9394(14)73086-7. [DOI] [PubMed] [Google Scholar]
- Fini ME, Yue YJT, Sugar J. Collagenolytic / gelatinolytic metalloproteinases in normal and keratoconus corneas. Curr Eye Res. 1992;11:849–62. doi: 10.3109/02713689209033483. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H, Moore WG, Bodden MK, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- O'Brien TP, Li QJ, Sauerburger F, Reviglio VE, et al. The role of matrix metalloproteinases in ulcerative keratolysis associated with perioperative diclofenac use. Ophthalmology. 2001;108:656–659. doi: 10.1016/S0161-6420(00)00590-X. [DOI] [PubMed] [Google Scholar]
- Riesbeck K, Sigvardsson M, Leanderson T, Forsgren A. Superinduction of cytokine gene transcription by ciprofloxacin. J Immunol. pp. 343–52. 1994 Jul 1. [PubMed]
- Williams RJ, III, Attia E, Wickiewicz TL, Hannafin JA. The effect of ciprofloxacin on tendon, paratenon, and capsular fibroblast metabolism. Am J Sports Med. 2000;28:364–9. doi: 10.1177/03635465000280031401. [DOI] [PubMed] [Google Scholar]
- Shakibaei M, Stahlmann R. Ultrastructure of Achilles tendon from rats after treatment with fleroxacin. Arch Toxicol. 2001;75:97–102. doi: 10.1007/s002040000203. [DOI] [PubMed] [Google Scholar]
- Egerbacher M, Seiberl G, Wolfesberger B, Walter I. Ciprofloxacin causes Cytoskeletal changes and detachment of human and rat chondrocytes in vitro. Arch Toxicol. 2000;73:557–63. doi: 10.1007/s002040050008. [DOI] [PubMed] [Google Scholar]
- Shakibaei M, Stahlmann R. Ultrastructural changes induced by the des-F(6)-quinolone garenoxacin (BMS-284756) and two fluoroquinolones in Achilles tendon from immature rats. Arch Toxicol. 2003. [DOI] [PubMed]