Abstract
Objective
Volume measurements by three-dimensional (3D) ultrasonography are considered more accurate than those performed by two-dimensional (2D) ultrasonography. The purpose of this study was to compare the agreement of three techniques, as well as the inter- and intra-observer agreements for volume measurements of fetal fluid-filled structures.
Methods
Fifty 3D volume datasets of fetal stomachs and bladders were explored. Volume measurements were performed independently by two observers using: 1) Virtual Organ Computer-aided AnaLysis (VOCAL™); 2) inversion mode; and 3) “manual segmentation.” Reliability was evaluated using intraclass correlation coefficient (ICC), and Bland-Altman plots were generated to examine bias and agreement. The time required to complete the measurements was compared using Student’s t-test or the Wilcoxon Signed Rank Test. P-values <0.025 or <0.05 were considered statistically significant wherever appropriate.
Results
All volume datasets could be measured using the three techniques. A high degree of reliability was observed between: 1) VOCAL™ and inversion mode (ICC: 0.995, 95% CI: 0.992–0.997); 2) VOCAL™ and manual segmentation (ICC: 0.997, 95% CI: 0.995–0.998); and 3) inversion mode and manual segmentation (ICC: 0.995, 95% CI: 0.992–0.997). There was good agreement between VOCAL™ and inversion mode (mean: −2.4%, 95% limits of agreement: 15.3% to −20.1%), VOCAL™ and manual segmentation (mean: −8.3%, 95% limits of agreement: 12.2% to −28.8%) as well as between inversion mode and manual segmentation (mean: 5.9%, 95% limits of agreement: −14.3% to 26%). Manual segmentation and inversion mode measurements were obtained significantly faster than those by VOCAL™.
Conclusions
Volume measurements of fetal fluid-filled structures of relatively regular shape with inversion mode and manual segmentation are feasible. Both techniques have good agreement with VOCAL™ and are significantly faster than VOCAL. Inversion mode is a reliable method for volume calculations of fluid-filled organs, whereas manual segmentation can be used when volume measurements by VOCAL™ or inversion mode are technically difficult to obtain, such as solid structures with poorly defined borders as the volume dataset is rotated, like the uterine cervix.
Keywords: 3DUS, fetal bladder, fetal stomach, three, dimensional ultrasonography, volume measurements
INTRODUCTION
The practice of obstetrical ultrasonography frequently involves measurements; these have contributed to a more objective anatomical and functional assessment. Indeed, the biparietal diameter, the abdominal circumference, and the length of the long bones (e.g., femur and humerus) are routinely measured to evaluate fetal size and growth. These parameters require that either one-dimensional (distance) or two-dimensional (2D; circumference and area) measurements be performed. In addition to 2D measurements, volume [a three-dimensional (3D) measurement] traditionally has been calculated by applying the ellipsoid formula (length × depth × width × 0.524) to measurements obtained by 2D ultrasonography (2DUS).1–4 Since this approach assumes that the organ being examined fulfills certain geometric characteristics and has regular contours, it is not surprising that these techniques had poor reproducibility5–8 and inter-observer agreement.5 Indeed, the mean error can be as high as 25% for the measurement of irregularly shaped organs.6,7
Three-dimensional ultrasonography (3DUS) allows examiners to acquire volume datasets and visualize fetal organs and other structures of interest using multiplanar or rendered displays. A unique feature of 3DUS is the possibility to visualize planes that cannot be obtained using 2DUS, making it possible to obtain volumetric measurements without using geometric assumptions. Thus, 3D volumetry has a better validity and reliability than 2DUS volume measurements.7,9–12
Currently, the most frequently used method to obtain volume measurements from 3D volume datasets is VOCAL™ (Virtual Organ Computer-aided AnaLysis, General Electric Medical Systems, Kretztechnik, Zipf, Austria). VOCAL™ has been evaluated both in vitro13 and in vivo,5,14–17 with high reliability, validity,13 and good intra- and inter-observer agreement.5,13–15,17 However, VOCAL™ has some limitations: 1) a tendency to overestimate true volume;13 2) difficulties in identifying the borders of some structures while the volume dataset is rotated (e.g., shadows from ribs, maternal bowel loops, etc.);17 and 3) the length of time needed to perform the measurement can range from 2 to 10 minutes.5,13,17,18
Inversion mode is a relatively new post processing algorithm that has been primarily used to display and reconstruct fluid-filled structures by 3DUS and four-dimensional ultrasonography (4DUS).19 This algorithm inverts the gray scale of the voxels that compose the volume dataset; thus, anechoic structures such as the heart chambers, lumen of the great vessels, stomach and bladder become echogenic.20 This technique has been used for 3D and 4D reconstruction of the embryonic brain, urinary tract, bowel,19 heart chambers,21 and great vessels.20,22 Recently, volumetric measurements of fetal heart chambers using inversion mode have been reported.23,24
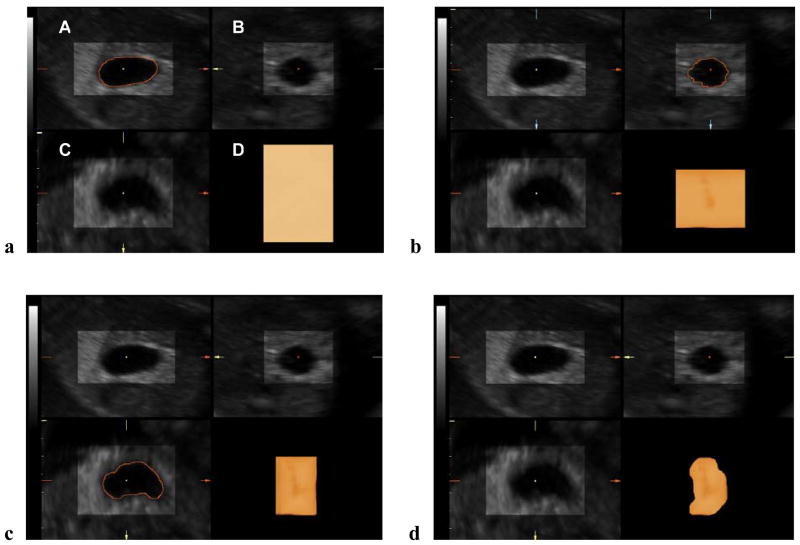
We evaluated manual segmentation, an alternative method for measuring 3D volume datasets because of technical difficulties encountered while attempting to perform VOCAL™ measurements of structures with poorly defined contours during rotation (e.g., the uterine cervix). This method consists of simply ’cutting away’ with the electronic scalpel any structure that lies outside the borders of the structure being measured using the multiplanar display (Figure 1). Our hypothesis is that although manual segmentation may overestimate volume measurements when compared to VOCAL™ and inversion mode, the magnitude of the discrepancies may not be clinically significant. We also hypothesize that this method may have potential advantages over VOCAL™, namely, improved speed and reproducibility.
Figure 1.
Ultrasound images of the fetal stomach at 20 weeks’ gestation showing volume measurement by ‘manual segmentation’. (a) The major axes of the organ of interest were aligned in relationship to each other, and the reference dot was positioned so that the widest diameter of each plane of section was displayed. A rendering box containing the fetal stomach was selected as a region of interest. Render mode was activated and a combination of ‘surface’ and ‘light’ algorithms was applied. Panel D displays a volume-rendered box. The ‘low threshold’ filter was set to 0. Then, the scalpel tool (‘MagiCut’) was activated, the ‘outside contour’ mode was selected, and the organ contour was carefully traced in the sagittal plane. (b, c) The contours of the organ were similarly traced in the transverse and coronal views. (d) Panel D displays the three-dimensional image resulting from manual segmentation.
The objectives of this study were: 1) to evaluate the inter-method, and inter- and intra-observer agreements for volumetric measurements of fetal fluid-filled structures performed using VOCAL™, inversion mode and manual segmentation; 2) to propose an alternative, simpler method for obtaining volume measurements from 3D datasets; and 3) tocompare the time required to complete the volume measurements by each method.
METHODS
Volume datasets of the fetal stomach (n=29) and bladder (n=21) acquired by 3DUS between 18.4 – 41.9 weeks’ of gestation were retrieved from our digital library of ultrasonographic images and retrospectively reviewed. 3DUS studies were conducted under protocols approved by the Institutional Review Boards of both Wayne State University and the National Institute of Child Health and Human Development (NICHD/NIH/DHHS). All patients gave written informed consent before participating in the study.
Volume Acquisition
Volume datasets were acquired using 3DUS equipment (Voluson 730 Expert™, GE Healthcare, Milwaukee, WI, US) with a motorized curved array transducer (2–5 or 4–8 MHz). Once a transverse view of the fetal abdomen at the level of the stomach was obtained, volume acquisition was performed using automated sweeps in the absence of fetal movements.
Volume Rendering
All volume datasets were initially explored by one of the investigators using 4D View™ software (version 2.1 Luminary™, GE Healthcare). Briefly, volume datasets were visualized using the multiplanar display. The original plane of acquisition containing the transverse view of the fetal abdomen was displayed in Panel A (upper left panel of the screen). The sagittal orthogonal view was displayed in Panel B (upper right panel) and the coronal orthogonal view was displayed in Panel C (lower left panel). Color filtering, as well as brightness/contrast adjustments, were used to optimize tissue contrast resolution and the volumes were saved on a hard disk prior to volume measurements. Adjustment of the image settings by a single investigator was performed to avoid independent modifications of the optimization parameters by the two observers who performed the measurements, because that could modify the contours of the organs thereby affecting the evaluation of the intertechnique agreement. Both observers began volume manipulation from the initial orientation of the volume at acquisition and were free to rotate the volume every time the measurements were performed.
Volume Measurements
Three different techniques were used to measure organ volumes: VOCAL™; inversion mode; and manual segmentation.
VOCAL™
This software tool allows the performance of volume measurements by rotating the organ or structure of interest around a fixed axis, while 2D contours are manually or automatically delineated on each plane. Different rotation angles (6°, 9°, 15° and 30°) for each contour plane can be selected, which are indirectly related to the number of rotation steps necessary to perform the measurements. For this study, we used a rotation angle of 30° since it allows the quickest possible measurements with no significant compromise in accuracy when compared to lower rotation angles.13
Figure 2 illustrates volume measurement of the fetal stomach by VOCAL™. Briefly, after the reference image was selected in Panel A, the image was manually traced in order to identify and trace any irregularity in the contour of the fetal stomach and bladder. Once the rotation steps had been concluded, contour borders were adjusted in Panel A to ensure that they matched the boundaries of the organ. Finally, the VOCAL™ software automatically calculated the volume of the organ, which was expressed in cubic centimeters.
Figure 2.
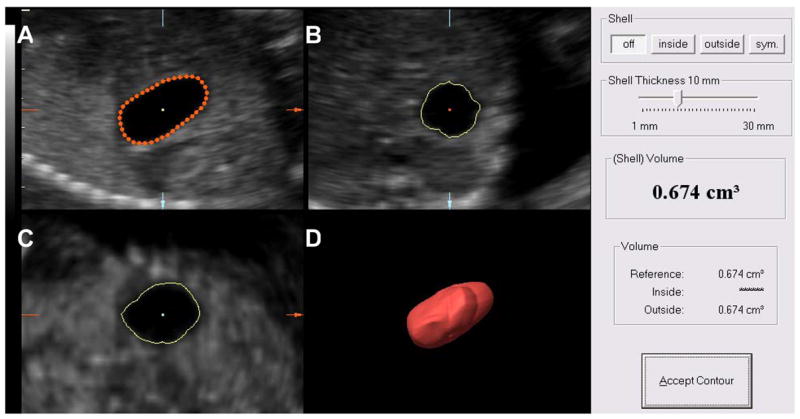
Ultrasound images of the fetal stomach at 20 weeks’ gestation showing volume measurement by Virtual Organ Computer-aided AnaLysis (VOCAL). A 30° rotational angle was used and the organ contours were manually traced (Panel A). Panels B and C show the trace in the transverse and coronal sections. Panel D displays a three-dimensional image of the fetal stomach. The volume is expressed in cubic centimeters.
Inversion mode
Inversion mode is a rendering technique by which the gray-scale voxels of volume datasets are inverted.19,20 Volumetric measurements are possible since the dimension of each voxel contained within the volume dataset is known.19 Volume datasets were rendered using a combination of ‘surface smooth’ and ‘gradient light’ modes. The ‘low threshold’ filter was employed to define the tissue-fluid boundaries instead of relying on the examiner’s ability to manually trace these irregular areas. Thus, the low threshold filter was set to 0 and then adjusted until the magenta color touched the boundaries of the organ being measured. Then, inversion mode was selected, followed by removal of voxels surrounding the structure of interest with the digital scalpel tool ‘MagiCut’ (4D View™ software version 2.1 Luminary™, GE Healthcare). The ‘threshold volume’ function was then selected, displaying the calculated volume (Figure 3).
Figure 3.
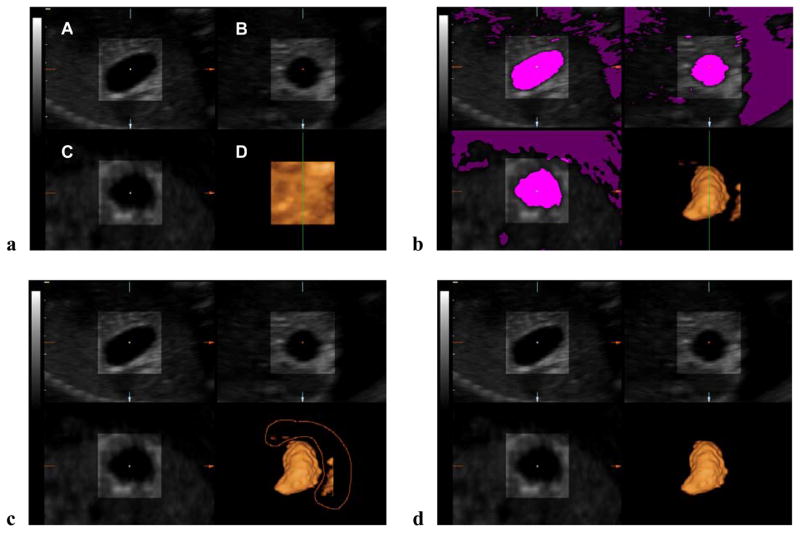
Ultrasound images of the fetal stomach at 20 weeks’ gestation showing volume measurement by inversion mode. Color filtering and brightness/contrast were adjusted to optimize tissue contrast resolution. (a) Render mode was activated and the rendering box containing the fetal stomach was selected as the region of interest. A combination of ‘surface smooth’ and ‘gradient light’ algorithms was applied. Panel D displays a volume-rendered box. The ‘low threshold’ filter was set to 0. (b) The low threshold filter was manually adjusted until the magenta color touched the boundaries of the organ. Then, inversion mode was activated, transforming all transparent voxels included in the magenta color into solid voxels (Panel D). (c) Segmentation tool (‘MagiCut’) was used to trace (red line) and remove voxels surrounding the organ of interest. (d) Panel D shows the volume reconstruction.
Manual Segmentation
We hypothesize that reasonable volume estimates can be obtained by cutting the boundaries of an organ displayed with multiplanar slicing (manual segmentation). Briefly, the major axes of the organ of interest are displayed in the transverse, sagittal and orthogonal planes and aligned in relationship to each other. The reference dot is positioned so that the widest diameter of each plane of section is displayed. Volume datasets are then rendered using a combination of surface and light algorithms with the low threshold filter set to 0. Then, the scalpel tool is activated and the ‘outside contour’ mode selected. The contour of the organ is carefully traced in each panel and the threshold volume function automatically displays the volume, as described for inversion mode (Figure 1).
In order to test intraobserver agreement, one of the observers measured the volume datasets twice with the three techniques, whereas a second observer measured the volume datasets only once. The interobserver agreement for each technique was calculated considering the first set of measurements of Observer 1 and the set of measurements obtained by Observer 2. All observers were blinded to the other’s measurements. Inversion mode and manual segmentation were compared with VOCAL™ because the latter is a well-established technique considered to be the standard for measuring volume datasets acquired by 3DUS.
Statistical Analysis
Reliability analysis was performed between techniques, and intraclass correlation coefficients (ICC) were calculated. Owing to the presence of heteroskedasticity, the inter-technique agreement, the intra- and interobserver agreements, and the 95% limits of agreement were all calculated using the percentage difference [i.e. 100 (1st measurement - 2nd measurement)/average) vs. the average based on the method proposed by Bland and Altman.25 In addition, the original data as well as the paired differences for the time measurements were first assessed graphically and numerically to determine whether they met the distributional assumptions of the statistical tests being used for analysis. Based on data distribution, the Student’s t-test or the Wilcoxon Signed Rank Test was used to compare the time required for each technique to perform volume measurements. Each pair-wise comparison in Table I was compared to an adjusted P-value of 0.05/2 = 0.025, with 0.05 being the probability of committing a Type I error (i.e. α = 0.05). P-values in Table 2 and all other comparisons in the study were considered statistically significant if <0.05. Statistical analysis was performed with SPSS v. 12.0 (SPSS Inc., Chicago, IL, USA) and MedCalc v.8.1.0.0 (MedCalc Software, Mariakerke, Belgium).
Table I.
Comparison among techniques for the time required for each observer to perform volumetric measurements
| VOCAL™ | Inversion Mode | p-value* | Manual Segmentation | p-value* | |
|---|---|---|---|---|---|
| Observer 1 (1st measurement) | 68.1 ± 11.0 | 54.8 ± 19.5 | <0.0001† | 41.1 ± 10.5 | <0.0001§ |
| Observer 1 (2nd measurement) | 63.9 ± 11.7 | 45.9 ± 13.4 | <0.0001§ | 34.8 ± 8.5 | <0.0001§ |
| Observer 2 | 54.1 ± 12.1 | 45.1 ± 18.0 | 0.001§ | 32.9 ± 9.5 | <0.0001§ |
Values expressed as mean (seconds) ± SD
Based on the distribution of the corresponding paired differences with VOCAL™
Signed Rank Test
Student’s t-test
Table II.
Comparison of the time required by Observer I to perform volumetric measurements between the first and second group of volume data sets by each technique
| Observer 1 1st measurement (n=50) | Observer 1 2nd measurement (n=50) | p-value* | |
|---|---|---|---|
| VOCAL™ | 68.1 ± 11.0 | 63.9 ± 11.7 | 0.002§ |
| Inversion Mode | 54.8 ± 19.5 | 45.9 ± 13.4 | <0.0001† |
| Manual Segmentation | 41.1 ± 10.5 | 34.8 ± 8.5 | <0.0001† |
Values expressed as mean (seconds) ± SD
Based on the distribution of the corresponding paired differences
Signed Rank Test
Student’s t-test
RESULTS
Both operators were able to obtain volume measurements for each organ using the three techniques. Overall, the first and second observer measured 300 and 150 volumes, respectively. Volume measurements of the fetal stomach and bladder ranged from 0.05 to 36.37 cm3.
Inversion mode and manual segmentation measurements were slightly larger than VOCAL™ measurements by a mean (± standard error of the mean) of 2.4 (± 1.3) and 8.3 (± 1.5), respectively. Figure 4 shows the volumetric measurement of a fetal stomach at 20 weeks’ of gestation performed by the three techniques.
Figure 4.
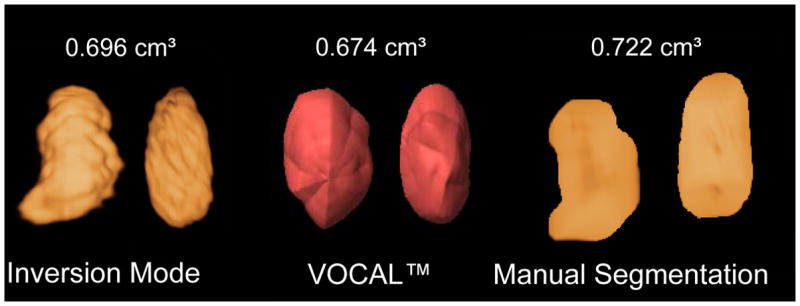
Fetal stomach volume at 20 weeks’ gestation rendered and measured by the three techniques. Inversion mode and manual segmentation measurements were slightly larger than those performed by Virtual Organ Computer-aided AnaLysis (VOCAL).
Inter-method agreement
VOCAL™ vs. inversion mode
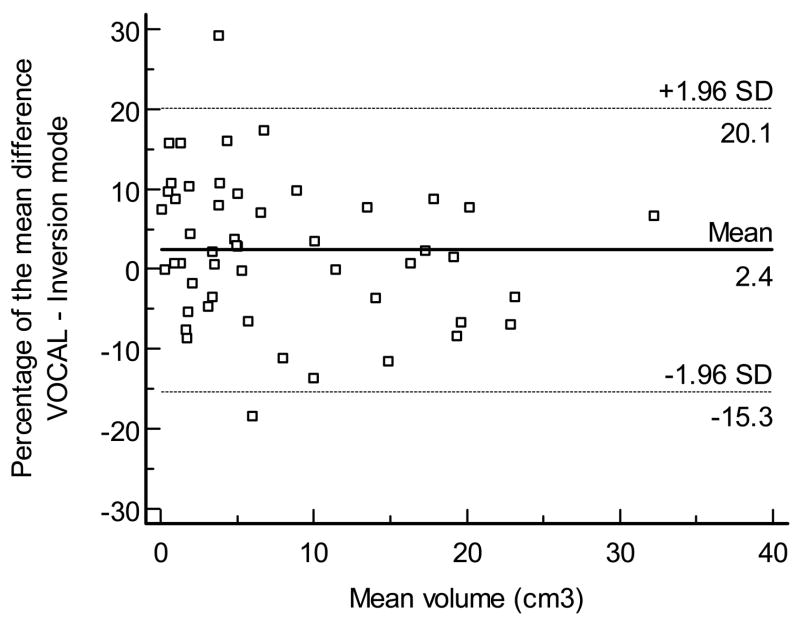
A high degree of reliability for volume measurements was observed between VOCAL™ and inversion mode (ICC, 0.995, 95% CI, 0.992–0.997). Figure 5 displays the Bland - Altman plot for the percentage of the mean difference and 95% limits of agreement between VOCAL™ and inversion mode (mean: −2.4%; 95% limits of agreement, −20.1 to 15.3%).
Figure 5.
Bland and Altman plot for the percentage of the mean difference and 95% limits of agreement between VOCAL™ and inversion mode.
VOCAL™ vs. manual segmentation
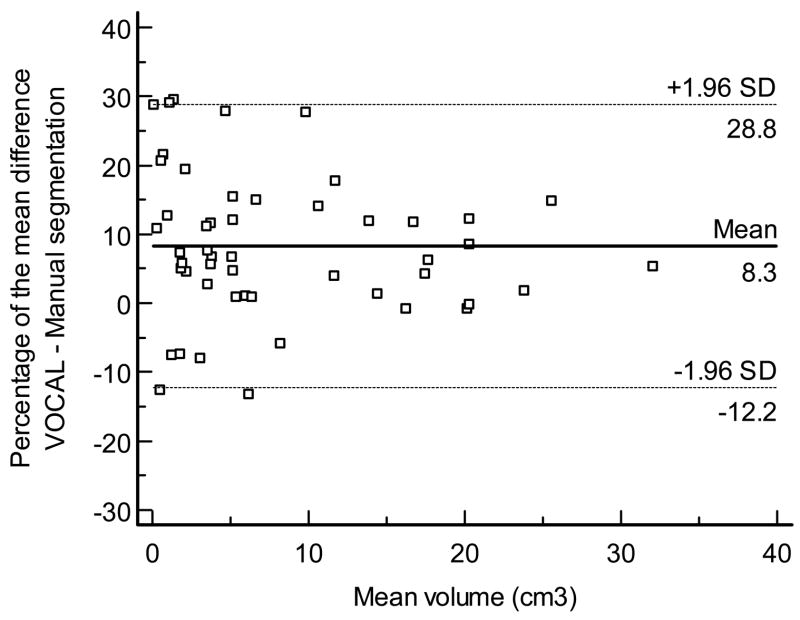
Volume measurements performed by VOCAL™ and manual segmentation demonstrated a high degree of reliability (ICC, 0.997, 95% CI, 0.995–0.998). Figure 6 displays the Bland - Altman plot for the percentage of the mean difference and 95% limits of agreement between VOCALTM and manual segmentation. In accordance with our hypothesis, volume measurements performed with VOCAL™ were slightly smaller than those performed with manual segmentation (mean: −8.3%, 95% limits of agreement: 12.2% to −28.8%).
Figure 6.
Bland and Altman plot for the percentage of the mean difference and 95% limits of agreement between VOCAL™ and manual segmentation.
Inversion mode vs. manual segmentation
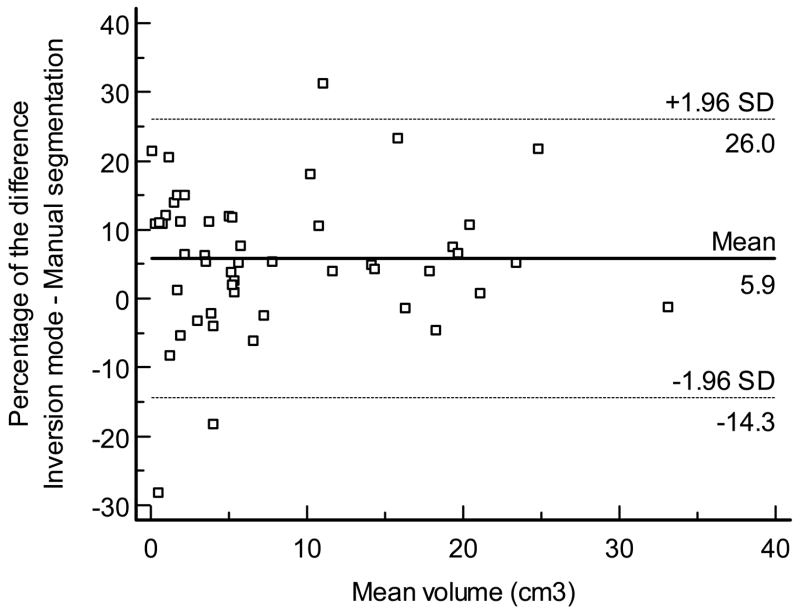
Figure 7 displays the Bland - Altman plot for the percentage of the mean difference and 95% limits of agreement between inversion mode and manual segmentation (mean, 5.9%; 95% limits of agreement: −14.3% to 26%). A high degree of reliability for volume measurements was observed between inversion mode and manual segmentation (ICC, 0.995, 95% CI, 0.992–0.997).
Figure 7.
Bland and Altman plot for the percentage of the mean difference and 95% limits of agreement between inversion mode and manual segmentation.
Intraobserver agreement
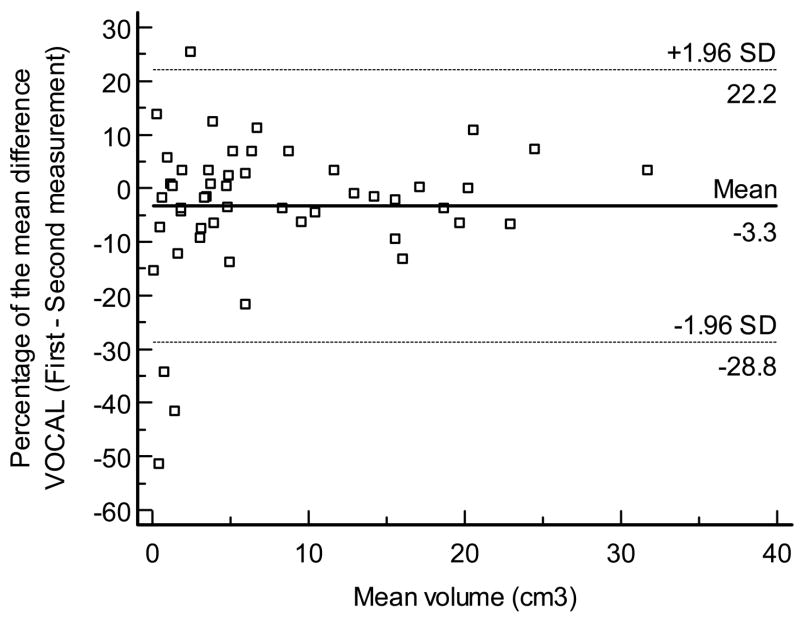
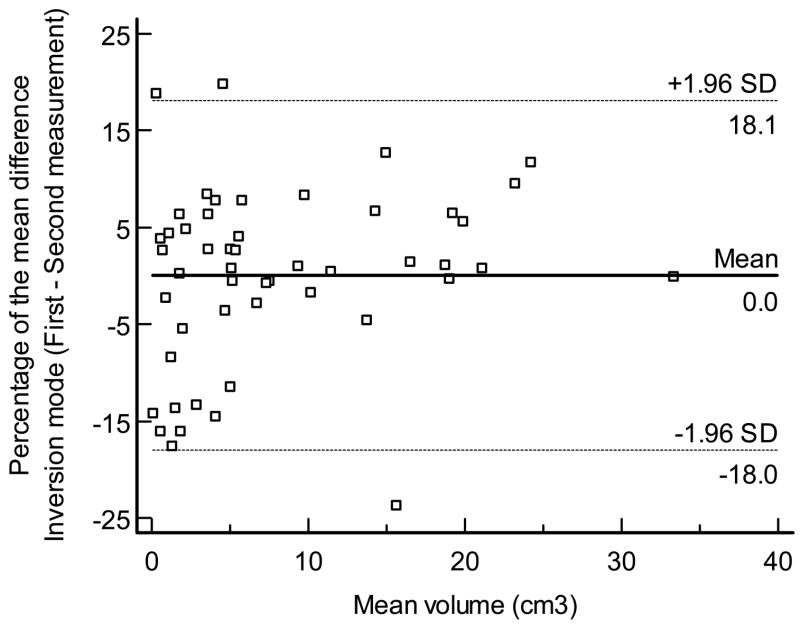
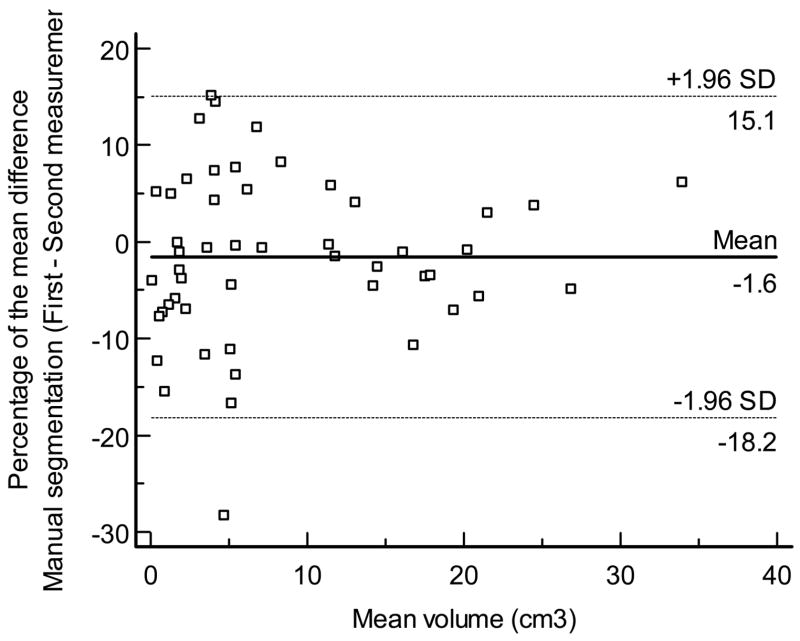
Figures 8 displays the Bland - Altman plots for the percentage of the mean difference and 95% limits of agreement for the intraobserver measurements performed by VOCAL™ (mean, −3.3%; 95% limits of agreement, −18.8% to 22.2% Figure 8a), inversion mode (mean: 0.0%; 95% limits of agreement, −18% to 18.1% Figure 8b), and manual segmentation (mean, −1.6%; 95% limits of agreement, −18.2% to 15.1% Figure 8c), respectively. A high degree of reliability was observed for the intraobserver volume measurements obtained by each technique, as follows: 1) VOCAL™ ICC: 0.996, 95%, CI, 0.993–0.997; 2) inversion mode ICC, 0.995, 95% CI, 0.992–0.997; and 3) manual segmentation ICC: 0.996, 95% CI, 0.993–0.997.
Figure 8.
Figure 8a. Bland and Altman plot for the percentage of the mean difference and 95% limits of agreement for intra-observer measurements performed by VOCAL™
Figure 8b. Bland and Altman plot for the percentage of the mean difference and 95% limits of agreement for intra-observer measurements performed by inversion mode.
Figure 8c. Bland and Altman plot for the percentage of the mean difference and 95% limits of agreement for intra-observer measurements performed by manual segmentation.
Interobserver agreement
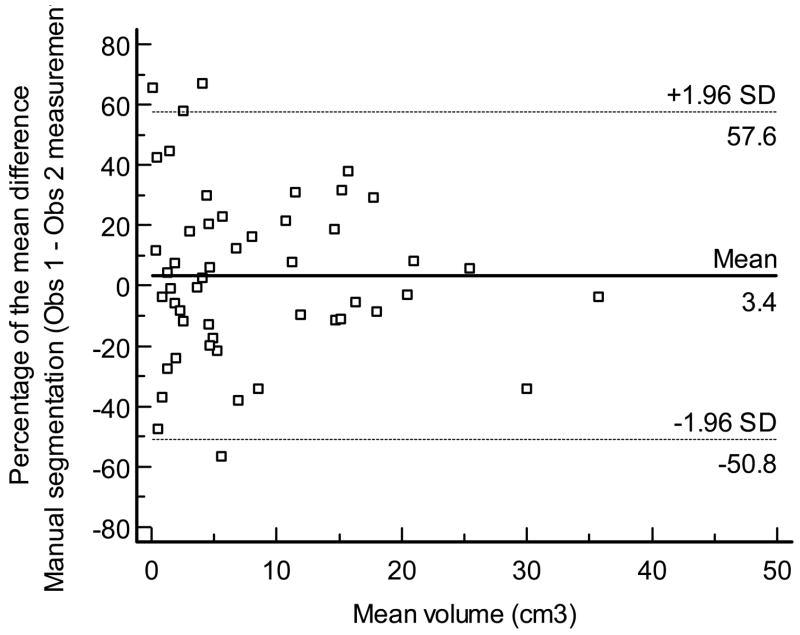
Figures displays the Bland - Altman plot for the percentage of the mean difference and 95% limits of agreement for the interobserver measurements performed by VOCAL™ (mean, 1.3%; 95% limits of agreement,: −52.1% to 54.7%), inversion mode (mean: 12.3%, 95% limits of agreement: −26.7% to 51.4% Figure 9b), and manual segmentation (mean, 3.4%, 95% limits of agreement, −50.8% to 57.6% Figure 9c), respectively.
Figure 9.
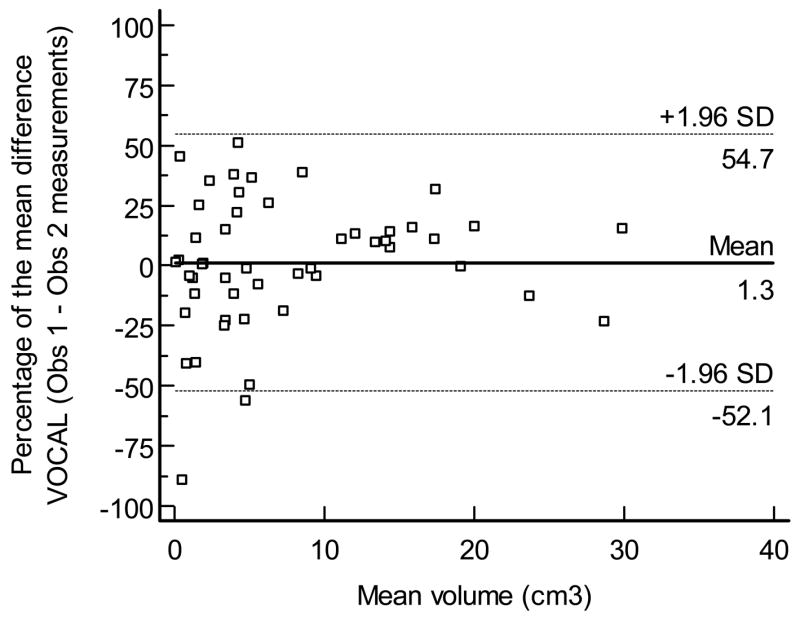
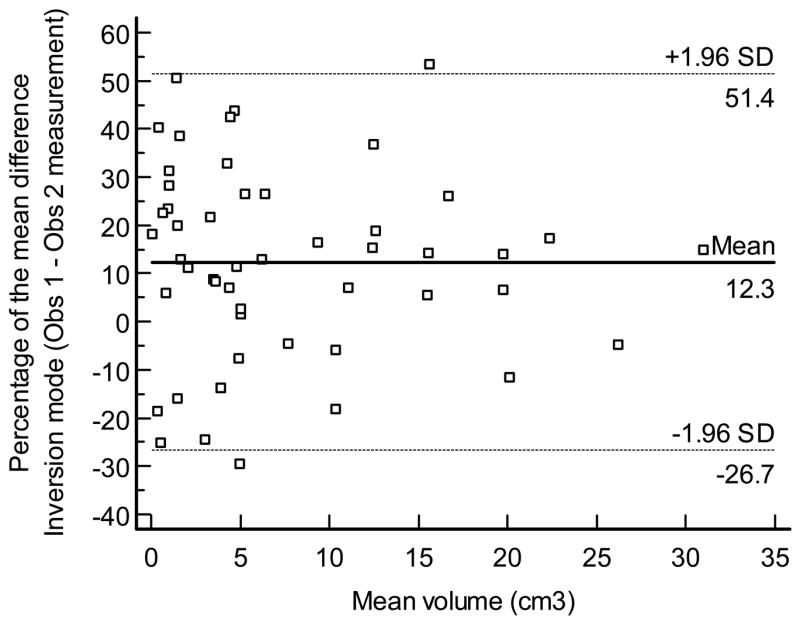
Figure 9a. Bland and Altman plot for the percentage of the mean difference and 95% limits of agreement for inter-observer measurements performed by VOCAL™.
Figure 9b. Bland and Altman plot for the percentage of the mean difference and 95% limits of agreement for inter-observer measurements performed by inversion mode.
Figure 9c. Bland and Altman plot for the percentage of the mean difference and 95% limits for inter-observer measurements performed by manual segmentation.
Time required for performing volume measurements
Table 1 shows the time required to perform the volumetric measurements by each method. Inversion mode and manual segmentation were significantly faster than VOCAL™ (P<0.01). Table 2 displays the comparison of the time required by Observer 1 to perform volumetric measurements between the first and second group of volume datasets by each technique. The second group of measurements was performed significantly faster than the first one, regardless of the technique, suggesting a learning curve.
DISCUSSION
Principal findings of the study
1) Similar volume measurements of fetal fluid-filled structures using VOCAL™, inversion mode, and manual segmentation are feasible; 2) Volume calculations were performed by the three techniques with a high degree of reliability, as demonstrated by excellent intraclass correlation coefficients, as well as good intermethod, intra- and interobserver agreements; 3) Inversion mode and manual segmentation had slightly larger measurements than VOCAL™, with a mean error of 2% and 8%, respectively; and 4) Inversion mode and manual segmentation were significantly faster than VOCAL™, even when the measurements were performed using a 30° rotation angle.
Techniques used to measure volume datasets by 3DUS
Manual planimetry
The introduction of 3DUS has made volume measurements more feasible because it allows the visualization of the coronal plane, correcting for contour irregularities. One of the most common 3DUS techniques for volume measurements was manual planimetry, which consists of slicing a 3D volume dataset into a series of equally spaced parallel 2D images, then manually tracing each image. Once these steps are completed, the cross-sectional area of the slices are summed and multiplied by the interslice distance to calculate volume.26 There is evidence that volumetric calculations obtained by 3DUS using the manual planimetry technique are more accurate than volume estimates derived from 2DUS measurements by the ellipsoid formula. Riccabona et al.12 measured 21 balloons of various shapes and volumes (range, 20 to 490 mL) by 2DUS and 3DUS. Conventional 2DUS volume estimates had a mean absolute error of 12.6 ± 8.7% (range, −27.5% to 39.2%), compared to a mean absolute error of 6.4% ± 4.4% (range, −6% to 15.5%) for volume measurements by 3DUS. The difference was even more pronounced for irregularly shaped objects (2DUS: 17.3% ± 12.1% vs. 3DUS: 7.1% ± 4.6%). However, this technique requires the manual tracing of 5 to 35 slices,10,27 a process that – as it takes from 3 to 30 minutes - may be considered tedious and time consuming.13,27–29
VOCAL™
The development of VOCAL™ has been considered an important progress in volume measurements. VOCAL™ has been: 1) tested in vitro by Raine-Fenning et al.;13 2) shown to be more reliable and significantly more valid in calculating volumes than manual planimetry;13 and 3) used in vivo to measure the volume of solid organs and structures like the ovaries,5,15 uterus,17 endometrium,14 and fetal lungs.16,18,30 It is important to consider that although VOCAL™ tends to overestimate true volumes, the magnitude of the discrepancy (only 1.4% using a 6° rotation angle and approximately 3% using a 30° rotation angle)13 has been reported to be not clinically significant. However, in some instances, it may be difficult to determine the boundaries of some structures while the volume dataset is rotating, which could lead to incorrect estimations of the margins of these structures and inaccurate volume calculations. Indeed, Yaman et al.17 performed transvaginal ultrasonography examinations in 48 consecutive patients before hysterectomy to evaluate the accuracy of uterine volume measurements by VOCAL™ when compared to 2DUS measurements. Eight patients had to be excluded due to large fibroids and five because of difficulty of identifying uterine borders. Another important limitation of VOCAL™ technique is the time required to trace the contours of the organ. Most of the studies that have used VOCAL™ arbitrarily selected the 30° rotation angle, because this requires only six steps to trace organ boundaries, compared to 30 steps if a rotation angle of 6° is selected. An in vitro study that performed volume calculations in three water-filled phantoms using four different rotation angles (6°, 9°, 15° and 30°) described a range of two to more than seven minutes to perform volume calculations with a rotation angle of 30° to 6°, respectively. These results demonstrated that using a 30° rotation angle was the fastest choice.13 Moreover, it has been reported that volume measurements of solid organs may take from five to ten minutes using a 30° rotation angle.16–18 We did not encounter these limitations, probably because the boundaries of the fetal stomach and bladder were easily distinguished from the surrounding tissues. Moreover, this may have accounted for the shorter times required to perform volume measurements with VOCAL™ when compared to previous studies.
Inversion mode
Inversion mode is a new postprocessing algorithm that has been used to evaluate fluid-filled structures, with potential applications for 3D reconstruction of fetal anechoic structures such as the urinary tract,19 heart chambers,21 and great vessels.20,22 Recently, volume measurement of fetal heart chambers using VOCAL™ and inversion mode in volume datasets acquired with spatio temporal image correlation (STIC) has been proposed by both Larsen et al.23 and Messing et al.,24 However, Nelson31 suggested more than ten years ago the feasibility of volumetric measurements of the cardiac chambers. A limitation of this technique is that it can not be used to measure solid organs. In the current study, volume calculations with inversion mode were faster than those performed with VOCAL™. Although border detection with inversion mode has the potential to be more subjective than tracing well-contrasted borders using VOCAL™, the good interobserver agreement as well as excellent intraobserver and intermethod agreements between volume measurements performed by inversion mode and VOCAL™ suggest that inversion mode can be reliably used for volume calculations of fluid-filled structures.
In this study, volume measurements performed by inversion mode were standardized beginning with the low threshold filter set to 0, and then it was adjusted until the magenta color touched the internal boundaries of the organ being measured. We have observed that adjusting the threshold level until the speckle within the volume disappears may lead to an overestimation of the volume. Thus, investigators may be tempted to change the threshold level to eliminate the speckle within the volume; however, there is no evidence that speckle may disappear at the same threshold in different volume datasets.
Manual segmentation
In this study, we propose a simple method for 3D volume dataset measurements to overcome some of the limitations of both VOCAL™ and inversion mode, especially for the volume calculation of solid structures. manual segmentation showed good intra- and interobserver agreement and was faster than both VOCAL™ and inversion mode, demonstrating that reasonable volume measurements could be obtained by tracing the boundaries and cutting away with an electronic scapel any structure that lies outside the borders of an organ displayed in each of the three orthogonal planes, while avoiding technical difficulties in measuring structures with poorly defined contours during rotation as occur with VOCAL™. An inherent limitation of this technique is that the use of only three planes of section to determine the limits of an organ results in less detailed contouring, leading to marginally larger volume measurements. Although our study demonstrated that this overestimation was minimal, the results may be explained by the relatively regular shape of the fetal stomach and bladder. We urge caution in extrapolating these results for volume measurement of irregular structures, such as cerebral ventricles. Thus, it is possible that manual segmentation may not be an optimal method for volume measurements of irregularly shaped objects. However, it may be applicable to regularly shaped structures such as the uterine cervix, because inversion mode cannot be applied and VOCAL™ has limitations recognizing the cervical contour during rotation. In this context, the use of manual segmentation may have a practical value. Owing to its limitations, we propose that this technique should be used when volume measurements by either VOCAL™ or inversion mode are not technically possible.
Limitations
This study has some limitations. First, the validity of the measurements performed for each method cannot be evaluated, because is not possible to calculate the true volume of the fetal fluid-filled organs in vivo. However, VOCAL™ has been extensively validated and is considered the gold standard 3DUS method for performing volumetric measurements, against which inversion mode and manual segmentation were tested. Second, volume datasets were manipulated prior to volume measurements to optimize tissue contrast resolution, and these modifications could affect the intra- and interobserver agreements for ultrasonographic measurements. However, this was done to avoid independent corrections by the investigators that could alter the identification of the organ boundaries, which is important to consider when evaluating the inter-technique agreement, because the object of interest should be the same for each technique. Third, the applicability of the limits of agreement for the different techniques is restricted to the range of volumes from 0.05 to 36 cm3. Most of the dispersion observed remained between the limits of agreement, being close to the mean for volumes smaller than 10 cm3 and higher in volumes ranging from 10 to 36 cm3. Therefore, we caution against extrapolating the results of this study to larger volumes. Fourth, we evaluated only the 30° rotation angle in VOCAL™, as it allows for the fastest measurements with no significant compromise in accuracy when compared with lower rotation angles.13
CONCLUSION
The results of this study demonstrated that VOCAL™, inversion mode and manual segmentation can be used to perform volume measurements in fetal fluid-filled structures of relatively regular shape. Owing to the limitations of VOCAL™ related to time and identification of borders, inversion mode offers a more efficient method for volume calculations of fluid-filled organs (e.g., fetal stomach, bladder, gallbladder, heart chambers). Alternatively, manual segmentation can be particularly useful for volume measurements of regularly shaped solid structures with poorly defined borders, such as the uterine cervix.
Acknowledgments
This research was supported, in part, by the Intramural Research Program of the National Institute of Child Health and Human Development, NIH, DHHS. The authors wish to acknowledge Mamtha Balasubramaniam, biostatistician, for her contribution to the statistical analysis.
Reference List
- 1.Griffin IJ, Cole TJ, Duncan KA, Hollman AS, Donaldson MD. Pelvic ultrasound measurements in normal girls. Acta Paediatr. 1995;84:536–543. doi: 10.1111/j.1651-2227.1995.tb13689.x. [DOI] [PubMed] [Google Scholar]
- 2.Higgins RV, van NJ, Jr, Woods CH, Thompson EA, Kryscio RJ. Interobserver variation in ovarian measurements using transvaginal sonography. Gynecol Oncol. 1990;39:69–71. doi: 10.1016/0090-8258(90)90401-6. [DOI] [PubMed] [Google Scholar]
- 3.Pavlik EJ, DePriest PD, Gallion HH, Ueland FR, Reedy MB, Kryscio RJ, van NJ., Jr Ovarian volume related to age. Gynecol Oncol. 2000;77:410–412. doi: 10.1006/gyno.2000.5783. [DOI] [PubMed] [Google Scholar]
- 4.Syrop CH, Willhoite A, Van Voorhis BJ. Ovarian volume: a novel outcome predictor for assisted reproduction. Fertil Steril. 1995;64:1167–1171. doi: 10.1016/s0015-0282(16)57979-5. [DOI] [PubMed] [Google Scholar]
- 5.Raine-Fenning NJ, Campbell BK, Clewes JS, Johnson IR. The interobserver reliability of ovarian volume measurement is improved with three-dimensional ultrasound, but dependent upon technique. Ultrasound Med Biol. 2003;29:1685–1690. doi: 10.1016/s0301-5629(03)01068-8. [DOI] [PubMed] [Google Scholar]
- 6.Riccabona M, Nelson TR, Pretorius DH, Davidson TE. Distance and volume measurement using three-dimensional ultrasonography. J Ultrasound Med. 1995;14:881–886. doi: 10.7863/jum.1995.14.12.881. [DOI] [PubMed] [Google Scholar]
- 7.Riccabona M, Nelson TR, Pretorius DH, Davidson TE. In vivo three-dimensional sonographic measurement of organ volume: validation in the urinary bladder. J Ultrasound Med. 1996;15:627–632. doi: 10.7863/jum.1996.15.9.627. [DOI] [PubMed] [Google Scholar]
- 8.Hwang JY, Byun SS, Oh SJ, Kim HC. Novel algorithm for improving accuracy of ultrasound measurement of residual urine volume according to bladder shape. Urology. 2004;64:887–891. doi: 10.1016/j.urology.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 9.Chou CY, Hsu KF, Wang ST, Huang SC, Tzeng CC, Huang KE. Accuracy of three-dimensional ultrasonography in volume estimation of cervical carcinoma. Gynecol Oncol. 1997;66:89–93. doi: 10.1006/gyno.1997.4714. [DOI] [PubMed] [Google Scholar]
- 10.Farrell T, Leslie JR, Chien PF, Agustsson P. The reliability and validity of three dimensional ultrasound volumetric measurements using an in vitro balloon and in vivo uterine model. BJOG. 2001;108:573–582. doi: 10.1111/j.1471-0528.2001.00148.x. [DOI] [PubMed] [Google Scholar]
- 11.Kyei-Mensah A, Zaidi J, Pittrof R, Shaker A, Campbell S, Tan SL. Transvaginal three-dimensional ultrasound: accuracy of follicular volume measurements. Fertil Steril. 1996;65:371–376. [PubMed] [Google Scholar]
- 12.Riccabona M, Nelson TR, Pretorius DH. Three-dimensional ultrasound: accuracy of distance and volume measurements. Ultrasound Obstet Gynecol. 1996;7:429–434. doi: 10.1046/j.1469-0705.1996.07060429.x. [DOI] [PubMed] [Google Scholar]
- 13.Raine-Fenning NJ, Clewes JS, Kendall NR, Bunkheila AK, Campbell BK, Johnson IR. The interobserver reliability and validity of volume calculation from three-dimensional ultrasound datasets in the in vitro setting. Ultrasound Obstet Gynecol. 2003;21:283–291. doi: 10.1002/uog.61. [DOI] [PubMed] [Google Scholar]
- 14.Bordes A, Bory AM, Benchaib M, Rudigoz RC, Salle B. Reproducibility of transvaginal three-dimensional endometrial volume measurements with virtual organ computer-aided analysis (VOCAL) during ovarian stimulation. Ultrasound Obstet Gynecol. 2002;19:76–80. doi: 10.1046/j.0960-7692.2001.00550.x. [DOI] [PubMed] [Google Scholar]
- 15.Jarvela IY, Sladkevicius P, Tekay AH, Campbell S, Nargund G. Intraobserver and interobserver variability of ovarian volume, gray-scale and color flow indices obtained using transvaginal three-dimensional power Doppler ultrasonography. Ultrasound Obstet Gynecol. 2003;21:277–282. doi: 10.1002/uog.62. [DOI] [PubMed] [Google Scholar]
- 16.Moeglin D, Talmant C, Duyme M, Lopez AC. Fetal lung volumetry using two- and three-dimensional ultrasound. Ultrasound Obstet Gynecol. 2005;25:119–127. doi: 10.1002/uog.1799. [DOI] [PubMed] [Google Scholar]
- 17.Yaman C, Jesacher K, Polz W. Accuracy of three-dimensional transvaginal ultrasound in uterus volume measurements; comparison with two-dimensional ultrasound. Ultrasound Med Biol. 2003;29:1681–1684. doi: 10.1016/s0301-5629(03)01070-6. [DOI] [PubMed] [Google Scholar]
- 18.Kalache KD, Espinoza J, Chaiworapongsa T, Londono J, Schoen ML, Treadwell MC, Lee W, Romero R. Three-dimensional ultrasound fetal lung volume measurement: a systematic study comparing the multiplanar method with the rotational (VOCAL) technique. Ultrasound Obstet Gynecol. 2003;21:111–118. doi: 10.1002/uog.39. [DOI] [PubMed] [Google Scholar]
- 19.Lee W, Goncalves LF, Espinoza J, Romero R. Inversion mode: a new volume analysis tool for 3-dimensional ultrasonography. J Ultrasound Med. 2005;24:201–207. doi: 10.7863/jum.2005.24.2.201. [DOI] [PubMed] [Google Scholar]
- 20.Goncalves LF, Espinoza J, Lee W, Mazor M, Romero R. Three- and four-dimensional reconstruction of the aortic and ductal arches using inversion mode: a new rendering algorithm for visualization of fluid-filled anatomical structures. Ultrasound Obstet Gynecol. 2004;24:696–698. doi: 10.1002/uog.1754. [DOI] [PubMed] [Google Scholar]
- 21.Goncalves LF, Espinoza J, Lee W, Nien JK, Hong JS, Santolaya-Forgas J, Mazor M, Romero R. A new approach to fetal echocardiography: digital casts of the fetal cardiac chambers and great vessels for detection of congenital heart disease. J Ultrasound Med. 2005;24:415–424. doi: 10.7863/jum.2005.24.4.415. [DOI] [PubMed] [Google Scholar]
- 22.Espinoza J, Goncalves LF, Lee W, Mazor M, Romero R. A novel method to improve prenatal diagnosis of abnormal systemic venous connections using three- and four-dimensional ultrasonography and ‘inversion mode’. Ultrasound Obstet Gynecol. 2005;25:428–434. doi: 10.1002/uog.1877. [DOI] [PubMed] [Google Scholar]
- 23.Larsen LU, Uldbjerg N, Sloth E, Sorensen K, Peterson OB. Fetal cardiac ejection fraction assessed from 4D ultrasound: spatio temporal image correlation and volume calculation. Ultrasound Obstet Gynecol. 2005;26:365–366. (abstract) [Google Scholar]
- 24.Messing B, Valsky DV, Hochner-Celnikier D, Savchev S, Cohen SM, Yagel SY. 3D inversion mode combined with STIC: a novel technique for fetal heart ventricle volume quantification. Ultrasound Obstet Gynecol. 2005;26:469. (abstract) [Google Scholar]
- 25.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 26.Nelson TR, Downey DB, Pretorius DH, Fenster A. Three-Dimensional Ultrasound. Lippincott Williams & Wilkins; Philadelphia, PA: 1999. Quantitative Analysis Methods; pp. 55–70. [Google Scholar]
- 27.Chang FM, Hsu KF, Ko HC, Yao BL, Chang CH, Yu CH, Liang RI, Chen HY. Fetal heart volume assessment by three-dimensional ultrasound. Ultrasound Obstet Gynecol. 1997;9:42–48. doi: 10.1046/j.1469-0705.1997.09010042.x. [DOI] [PubMed] [Google Scholar]
- 28.Chang FM, Liang RI, Ko HC, Yao BL, Chang CH, Yu CH. Three-dimensional ultrasound-assessed fetal thigh volumetry in predicting birth weight. Obstet Gynecol. 1997;90:331–339. doi: 10.1016/s0029-7844(97)00280-9. [DOI] [PubMed] [Google Scholar]
- 29.Lee A, Sator M, Kratochwil A, Deutinger J, Vytiska-Binsdorfer E, Bernaschek G. Endometrial volume change during spontaneous menstrual cycles: volumetry by transvaginal three-dimensional ultrasound. Fertil Steril. 1997;68:831–835. doi: 10.1016/s0015-0282(97)00362-2. [DOI] [PubMed] [Google Scholar]
- 30.Ruano R, Joubin L, Sonigo P, Benachi A, Aubry MC, Thalabard JC, Brunelle F, Dumez Y, Dommergues M. Fetal lung volume estimated by 3-dimensional ultrasonography and magnetic resonance imaging in cases with isolated congenital diaphragmatic hernia. J Ultrasound Med. 2004;23:353–358. doi: 10.7863/jum.2004.23.3.353. [DOI] [PubMed] [Google Scholar]
- 31.Nelson TR. Three-dimensional fetal echocardiography. Prog Biophys Mol Biol. 1998;69:257–272. doi: 10.1016/s0079-6107(98)00011-x. [DOI] [PubMed] [Google Scholar]