Abstract
Summary
Lifespan in individually housed medflies (virgins of both sexes) and daily reproduction for females were studied following one of 12 dietary restriction (DR) treatments in which the availability of high-quality food (yeast–sugar mixture) for each fly was based on a Markov chain feeding scheme – a stochastic dietary regime which specifies that the future dietary state depends only on the present dietary state and not on the path by which the present state was achieved. The stochastic treatments consisted of a combination of one of four values of a ‘discovery’ parameter and one of three values of a ‘persistence’ parameter. The results supported the hypotheses that: (i) longevity is extended in most medfly cohorts subject to stochastic DR; and (ii) longevity is more affected by the patch discovery than the patch persistence parameter. One of the main conclusions of the study is that, in combination with the results of earlier dietary restriction studies on the medfly, the results reinforce the concept that the details of the dietary restriction protocols have a profound impact on the sign and magnitude of the longevity extension relative to ad libitum cohorts and that a deeper understanding of the effect of food restriction on longevity is not possible without an understanding of its effect on reproduction.
Keywords: ad libitum feeding; birth and death rates; lifespan; life tables; longevity; mortality response; reproduction; sex differentials, Tephritidae; unpredictable environments
Introduction
Although it is well known that dietary restriction (DR) extends longevity in a wide range of organisms, including yeast (Guarente & Kenyon, 2000; Lin et al., 2002), nematodes (Kenyon, 1997), Drosophila (Chapman & Partridge, 1996; Clancy et al., 2002; Mair et al., 2003; Tu & Tatar, 2003; Mair et al., 2004; Rauser et al., 2004) and rodents (McCay et al., 1935; Masoro, 1988; Weindruch & Walford, 1988; Austad & Kristan, 2003) as well as in several species that are not traditionally used in aging research, including spiders (Austad, 1989) and rotifers (Kirk, 2001), nearly all DR studies have been based on feeding protocols in which food quality, quantity and access are both constant and predictable (Weindruch & Walford, 1988; Bertarand et al., 1999). Despite the obvious advantages of using standard DR protocols, including technical simplicity, daily predictability and nutritional uniformity, one of the shortcomings of DR studies based on these standard protocols is that they create conditions unlike those found in the wild – conditions in which virtually all animals are subject to large variations in the quality and quantity of food available to them (Andrewartha & Birch, 1974; White, 1976, 1978; Begon et al., 1996).
Because of this difference between the food restriction patterns used in laboratory studies and the realities of food availability in the wild, and because the DR response of individuals studied under laboratory conditions serves as the evolutionary explanation for the longevity extension response (Holliday, 1989; Shanley & Kirkwood, 2000), it follows that the results of DR studies which incorporate stochasticity into a DR experimental protocol may shed important new light on the longevity response in model organisms. For example, virtually no information exists on questions such as: what is the life history response of animals subject to a feeding regime in which ‘feast’ and ‘famine’ days occur at random? Does a short period of high-quality food availability that occurs within a longer period of food restriction have an offsetting effect on the longevity extension response to DR?
An experimental protocol that can be brought to bear on questions involving the DR response of animals subject to dietary stochasticity is one involving a Markov feeding scheme, which specifies that the future dietary state (e.g. high- or low-quality food) depends only on the present dietary state and not on the path by which the present state was achieved. This is the conceptual basis for the current study, which we refer to as stochastic DR. Our broad goal in the current study was to determine the life history response of medflies subject to a stochastic DR protocol in order to assess the generality of the longevity extension response to DR. Our specific objectives in this study were to test two hypotheses regarding the overall life history response to stochastic food availability: (1) longevity will be extended in individuals in which daily access to either high- or low-quality food is stochastic. This hypothesis is based on the concept derived from previous DR research that any level of DR will extend longevity in animals and that the details of the restriction pattern will not have an effect on the qualitative outcome (i.e. longevity extension). (2) Improvement in longevity due to restriction will be more affected by the frequency of access to high-quality food (discovery probability) than by the length of its availability (persistence probability). This hypothesis is based on the medfly’s natural history (see short review in Experimental procedures), physiological ecology (McNab, 2002; Costa & Sinervo, 2004) and life history traits (Hagen et al., 1981), all of which point towards a species that would be classified as opportunistic (Begon et al., 1996) – weedy plants and animal pests whose populations are capable of growing in large bursts in disturbed environmental conditions. Unlike so-called equilibrium species such as turtles and long-lived beetles whose populations fluctuate less, most opportunistic species such as the medfly have limited storage capability of high-quality food for both maintenance and offspring production (Warburg & Yuval, 1996). Thus individuals will require frequent access to high-quality food for optimizing their demographic response (net reproduction and survival).
We believe that the outcomes of experiments designed to test these hypotheses are important because not only will the results shed light on the broad question concerning the actuarial response of medflies to other restriction techniques, but the results will provide deeper insights into longevity concepts related to the evolutionary and field ecology of food foraging by medflies (Prokopy & Roitberg, 1984; Hendrichs et al., 1993) as well as into the relative effect on longevity extension of details of food restriction protocols including both food type and timing.
Treatment differences
Life expectancy
Life expectancy across the 12 treatment cohorts ranges from 30.5 to 43.0 days (12.5-day difference) in females and from 35.4 to 55.7 days (20.3-day difference) in males, with greater longevity corresponding to higher values of both the discovery (p) and persistence (q) probabilities for females but with only the discovery parameter (p) for males (Table 1). The mean life expectancy of males exceeds that for females across all treatments by an average of 11.5 days – males lived an average of 47.2 days and females an average of 35.7 days. These life expectancies are higher than those for Control A (full diet), which yielded life expectancies of 32.6 and 46.4 days for females and males, respectively, as well as for Control B (sugar-only), which yielded life expectancies of 32.5 and 31.0 for females and males, respectively. Thus both male and female life expectancies in the full diet (ad libitum) control cohorts are less than their respective life expectancies in nine of 12 of the treatment cohorts. Quadratic trends appear for analyses of life expectancies vs. expected lengths of sequences for both high-quality (full diet) food-days (p value for overall regression effect for quadratic fit, for females p = 0.2276 and for males p < 0.0001) and for low-quality (sugar-only) food-days (p value for overall regression effect for quadratic fit, for females p = 0.0014 and for males p < 0.0001).
Table 1.
Life expectancy (days) of female and male medflies subject to each of the 12 treatments resulting from the 3-by-4 combination1 of stochastic food availability (p) and food persistence (q); e0 = expectation of life at eclosion (days); SD = standard deviation and n = initial number in cohort2
|
q (persistence) |
||||||
|---|---|---|---|---|---|---|
| p (discovery) | 0.2 | 0.5 | 0.8 | Mean | ||
| Females | 0.05 | e0 | 31.7 | 32.0 | 36.0 | 33.2 |
| SD | 25.92 | 20.40 | 21.97 | |||
| n | 79 | 81 | 97 | |||
| 0.10 | e0 | 30.5 | 35.0 | 35.7 | 33.7 | |
| SD | 22.81 | 23.43 | 21.89 | |||
| n | 90 | 83 | 84 | |||
| 0.15 | e0 | 39.1 | 34.9 | 34.2 | 36.1 | |
| SD | 22.70 | 22.58 | 20.38 | |||
| n | 80 | 86 | 73 | |||
| 0.20 | e0 | 36.9 | 43.0 | 39.9 | 39.9 | |
| SD | 22.65 | 23.21 | 17.92 | |||
| n | 94 | 88 | 80 | |||
| Mean | e0 | 34.6 | 36.2 | 36.5 | 35.7 | |
| Males | 0.05 | e0 | 37.6 | 41.9 | 35.4 | 38.3 |
| SD | 19.69 | 23.94 | 24.83 | |||
| n | 95 | 80 | 75 | |||
| 0.10 | e0 | 47.3 | 50.7 | 50.0 | 49.3 | |
| SD | 24.39 | 28.80 | 27.17 | |||
| n | 89 | 88 | 83 | |||
| 0.15 | e0 | 47.9 | 48.6 | 51.8 | 49.4 | |
| SD | 25.62 | 27.21 | 29.57 | |||
| n | 93 | 78 | 98 | |||
| 0.20 | e0 | 51.8 | 55.7 | 48.0 | 51.8 | |
| SD | 28.78 | 25.82 | 24.60 | |||
| n | 71 | 81 | 82 | |||
| Mean | e0 | 46.2 | 49.2 | 46.3 | 47.2 | |
Life expectancy for Control A, ad libitum full diet (p = q = 1), was 32.6 (14.3) and 46.4 (26.8) days for females (n = 87) and males (n = 79), respectively, and for Control B, sugar-only diet (p = q = 0), was 32.5 (14.3) and 31.0 (15.0) days for females (n = 77) and males (n = 85), respectively.
Fitted model: lifetime = 29.5 + 62.6p2 + 14.8q(sex) + 46q2(sex).
The similarity in female life expectancies at age 0 (eclosion) in the Control A (ad libitum full; e0 = 32.6 days) and Control B (sugar-only; e0 = 32.5 days) cohorts differ from the finding in several previous medfly studies, including investigations on the effects of food pulses (Carey et al., 2002a) and on the effects of dietary switches from sugar-only to full diet (Carey et al., 1998). However, closer examination of the mortality data reveals that this inconsistency in life expectancy between the two control cohorts is evident only during the first 7–10 days and is the outcome of mortality differences at the youngest ages where, unlike earlier studies, mortality in the cohorts maintained on sugar-only diet exceeded mortality in cohorts maintained on a full diet. For all ages beyond 9 days the life expectancies in the Control B (sugar-only) cohort exceed those in the Control A (full diet) cohort. For example, at 10, 20 and 30 days, life expectancies in Control B exceed life expectancies of Cohort A by 2.1, 7.5 and 8.8 days, respectively. Thus the general findings in which the life expectancy of cohorts maintained on sugar-only diet exceeds the life expectancy of cohorts maintained on a full diet is consistent with the findings in all previous studies even though there are some discrepancies at the youngest ages.
Statistical analysis (see fitted model in Table 1 footnote 2) reveals that the probabilities of discovery (p) and of persistence(q) and of their squares (the quadratic terms p2 and q2 in the model) have statistically significant effects on lifetime within sex (p < 0.0001; r2 = 0.0803). In other words, the higher values of p and q have statistically significant positive effects on life expectancy but that life expectancy improvements level off at higher values of each parameter (i.e. indicated by the statistical significance of the quadratic terms in the model). These results underline the complexity of the medfly DR response. On the one hand, the longevity of restricted medfly cohorts is significantly greater than the longevity of the ad libitum (Control A) cohort, but on the other, the longevity of cohorts is negatively correlated with the degree of restriction, as indicated by the Markov parameters – medfly cohorts with greater access to food (higher values of p and q) experienced greater longevity. In short, the complexity of the longevity response of medflies to a range of stochastic dietary environments emphasizes the importance of gathering and interpreting individual-level data on reproduction relative to changes in individual lifespan.
Reproduction
The total fertility rates (TFR) for female medflies in each of the 12 treatment cohorts and the two control cohorts reveal substantial differences in egg laying rates across cohorts (Table 2). The highest and lowest lifetime egg laying rates occur in the two control cohorts, with Cohort A females (ad libitum full diet) producing an average of nearly 640 eggs/female and Control B females (sugar-only diet) producing an average of slightly fewer than 55 eggs/female. Egg laying levels are positively correlated with the predicted number of high-quality food-days for both the discovery (p) and persistence (q) parameters. However, increases in the discovery parameter have a much greater effect on egg production than do increases in the persistence parameter. For example, averaged across all values of p (discovery), the mean number of lifetime eggs increases from around 140/female at p = 0.05 to around 370/female for p = 0.20, a 2.6-fold increase. In contrast, the mean number of lifetime eggs increases from 208 eggs/female at q = 0.2 to 314 eggs/female at q = 0.8, a 1.5 fold increase in egg production across all values of q (persistence).
Table 2.
Total fertility rate (TFR) for female medflies subject to each of the 12 treatments resulting from the 3-by-4 combination1 of stochastic food availability (p) and persistence (q). SD = standard deviation; n = initial number of individuals in cohort. Values for controls are given in footnote1
|
q (persistence) |
|||||
|---|---|---|---|---|---|
| p (discovery) | 0.2 | 0.5 | 0.8 | Mean | |
| 0.05 | TFR | 106.3 | 123.2 | 198.7 | 142.7 |
| SD | 99.97 | 133.40 | 201.84 | ||
| n | 75 | 77 | 93 | ||
| 0.10 | TFR | 189.4 | 199.1 | 274.5 | 221.0 |
| SD | 191.86 | 188.13 | 232.00 | ||
| n | 86 | 78 | 81 | ||
| 0.15 | TFR | 242.4 | 289.0 | 336.0 | 289.1 |
| SD | 184.48 | 248.56 | 301.37 | ||
| n | 71 | 82 | 70 | ||
| 0.20 | TFR | 295.2 | 371.7 | 447.1 | 371.3 |
| SD | 256.68 | 267.71 | 305.62 | ||
| n | 89 | 84 | 78 | ||
| Mean | TFR | 208.3 | 245.8 | 314.1 | 256.1 |
Total fertility rates (TFR) for Control A, ad libitum full diet (p = q = 1) and for Control B, sugar-only diet (p = q = 0), were 638.7 (SD = 384.30; n = 84) and 54.4 (SD = 50.77; n = 75), respectively.
Fitted model: total number of eggs = 57 + 1768p − 1187p2.
Statistical analysis (see fitted model in Table 2, footnote 2) reveals that the probability of discovery, p, and its square, p2, have a statistically significant effect on total number of eggs (p < 0.0001; r2 = 0.2558) but that the probability of persistence (q-parameter) does not have an effect on lifetime egg production. Thus like the fitted statistical model for life expectancy with respect to the discovery parameter, p, the model of reproduction reveals that p has statistically significant positive effects on reproduction but that lifetime reproduction in medflies levels off at higher values of each parameter (i.e. as indicated by the statistical significance of the quadratic terms in the model). Because access to high-quality food (full diet) is associated with increasing values of p, the analysis shows that the lifetime egg production of flies is positively correlated with the frequency of access to full diet but with a non-linear relationship. Quadratic trends appear for the expected lengths of sequences of both low-quality (sugar-only) and high-quality (full diet) food-days (p value for quadratic fits p < 0.0001 for both). This general relationship of increasing egg production with increasing food availability is similar to the findings from studies on the relationship of the timing and frequency of food pulses on lifetime egg production in the medfly (Carey et al., 2002a) – egg production was greater in cohorts with more frequent access to a full diet.
The egg laying patterns for all of the individual female medflies and their respective survival for the 12 treatment cohorts are shown in the event history chart given in Fig. 1. Several aspects of these graphs merit comment. First, the characteristics of the stochastic patterns of the availability of full diet are reflected in the extent of intermittent egg laying in several of the cohorts. Consider, for example, the differences in egg laying patterns in the cohorts shown in Fig. 1(c,j) where the p* values for both of these cohorts was p* = 0.20 (the proportion of a fly’s lifetime for which it had access to high-quality food) but the patterns of availability of high-quality food differed. Flies in the cohort that generated the reproductive and survival patterns shown in Fig. 1(j) had more frequent access to new ‘patches’ of high-quality food than did the cohort of flies that generated the data visualized in Fig. 1(c). However, the persistence of these food patches was less in the former than in the latter cohort. These differences in food availability are manifested as differences in: (1) frequency of egg-laying – the females laid eggs on an average of 28% of the days in the Fig. 1(c) cohort whereas females laid eggs on an average of 48% of the days in the Fig. 1(j) cohort; and (2) intensity of egg laying – an average of 199 and 295 eggs/female in the Fig. 1(c) and Fig. 1(j) cohorts, respectively. In other words, increased access to new patches of high-quality food (high p values) is more important in determining the frequency and intensity of egg laying than is the degree of persistence of the high-quality food patch (high q values).
Fig. 1.
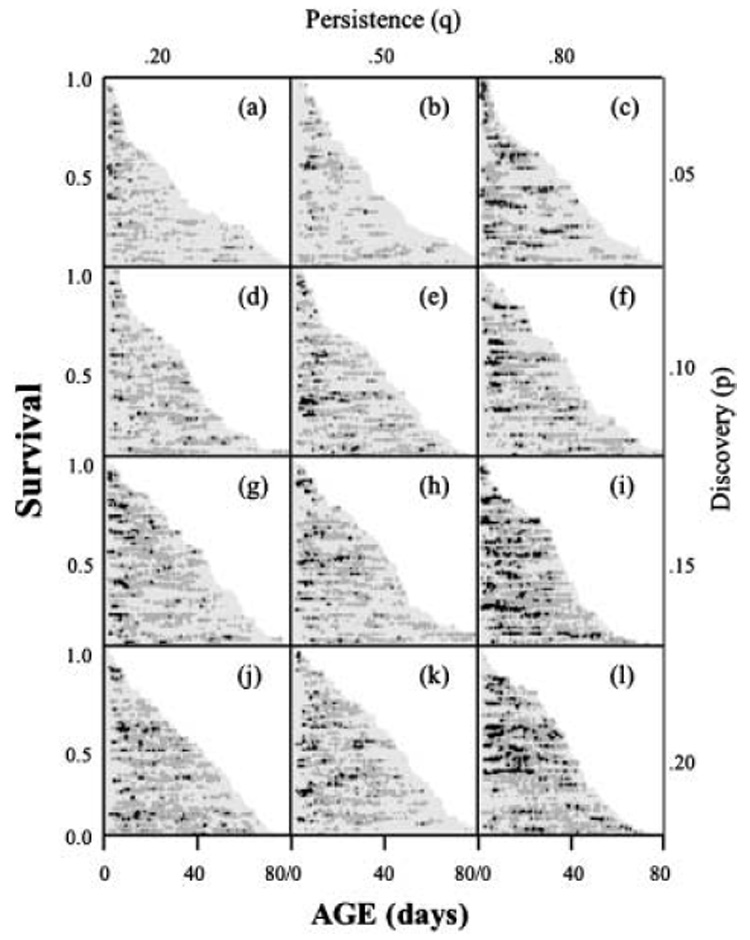
Event-history diagrams (Carey et al., 1998) visualizing the demographic response of individual medflies subjected to one of 12 different stochastic dietary treatment regimes (see text). Each horizontal line denotes a female ‘life line’, the length of which is proportional to her lifespan. The age-specific egg laying intensity corresponds to the shading: light gray = zero eggs/day; medium gray = 1–30 eggs/day; black = > 30 eggs/day.
Second, survival rates for the 12 cohorts shown in Fig. 1 increase in panels ordered from top-to-bottom (i.e. with increasing values of the discovery parameter, p) and, to a lesser extent, in panels ordered from left-to-right (i.e. with increasing values of the persistence parameter, q). Inasmuch as the average number of food-days also increases in the cohorts ordered in these two ways (top-to-bottom; left-to-right), the direction of longevity changes for medfly females relative to the extent of DR is opposite to the direction of longevity changes reported in most DR studies. This finding that the details of DR patterns and protocols have a profound impact on the sign and magnitude of change in life expectancy again underlines the complex relationship between DR and longevity; no simple relationship exists between the deleterious effects of reproduction on survival that occur when dietary conditions are favorable to reproduction, and the beneficial effects of survival itself.
Third, whenever the p* values of two cohorts are similar, reproduction is always greater in the cohort with the higher value of the discovery parameter, p. This supports the concept that medflies require frequent access to high-quality food to maximize their fertility rather than less frequent access to high-quality food patches but higher persistence of these patches once discovered. This stronger reproductive response to ephemeral food patches than to persistent food patches sheds light on the evolutionary ecology of food foraging in this species, which appears better adapted to thrive in environments in which food availability is frequent but ephemeral. In other words, different evolutionary pressures based on the various ways in which high-quality food is in short supply will select for between-sex as well as for between-species differences in their abilities to withstand food stress (Hoyenga & Hoyenga, 1981).
Discussion
The results of this study support the two hypotheses, that: (1) longevity is extended in individuals subjected to a stochastic DR feeding scheme; and (2) the food patch ‘discovery’ parameter, p, has a greater effect on longevity response than the ‘persistence’ parameter, q. The most important relationships that emerged from the current study in which individual medflies were subject to a Markov-chain feeding scheme (stochastic DR) include the following. First, medfly females reproduce even when extant sources of protein for egg production are exceedingly scarce, as indicated by both the present and previous (Carey et al., 1998, 2002a,b) findings. Individuals that do not attempt to reproduce under conditions of resource limitations may be selected against in nature for the simple reason that the duration of famine-like conditions may be greater than the extended lifespans of individuals whose life expectancy is increased in response to food restriction. Animals evolve an adaptive strategy in which they balance two factors – the time and energy already invested in the manufacture of eggs (off-spring) not yet laid and the risk of either starving or becoming so weak that they increase their own risk of dying (Bronson & Marsteller, 1985; Bronson, 1985). Thus the strategy of medfly females is to optimize the use of a scarce resource (protein) for maximizing reproduction subject to nutritional constraints (Tatar & Carey, 1995). Inasmuch as the concept of resource (dietary) scarcity is exceedingly complex and involves quality, quantity and availability, the life history response is necessarily complex – a unit of reproduction is not simply traded off for a unit of survival (Bell, 1984; Reznick, 1985; Bell & Koufopanou, 1986; Begon et al., 1996).
Second, medfly longevity was not extended under all stochastic DR conditions. One hypothesis for the absence of a universal response to all levels and types of food restriction is based on the complex relationship between reproduction and both DR and mortality. For example, mortality in both medflies (Carey et al., 1998) and Drosophila (Mair et al., 2003) changes abruptly when they are switched between ad libitum and restricted diets. Consequently, it is difficult to predict the net effect on longevity of multiple switches between the costly reproductive mode and the less costly waiting mode (Carey et al., 1998). That is, the net effects of some patterns will be to increase longevity relative to ad libitum controls whereas the net effects of other dietary protocols in which individuals are switched between reproductive and waiting modes will decrease longevity. This is consistent with the concept that the DR response probably evolved to adjust to short- rather than long-term periods of unpredictability (Shanley & Kirkwood, 2000).
Third, because of basic differences in the physiology, behavior and reproductive characteristics of males and females, the life history response of individuals to DR was sex-specific. These differences were evident in the current study, in which the patterns of the longevity response of males to the different stochastic treatments differed from that of females. In particular, male mortality increased sharply when they were subject to multiple switches between high- and low-quality diets during their lifetimes. In contrast, female mortality was largely unaffected by the multiple switches in diet. This is similar to differences reported in food-restricted mice (Hamilton & Bronson, 1985), in which females suspended all reproduction but males developed testes of normal size and were capable of mating successfully, even though stunted. In short, the evolutionary forces that have shaped the breeding success of males (including longevity) are probably fundamentally different from those acting on females.
Fourth, both reproduction and life expectancy in both sexes of the medfly were significantly higher in cohorts with high discovery parameters than with high persistence parameters but where the lifetime food-days were identical. This suggests that the physiological and reproductive systems of medflies are better adapted to ephemeral than to persistent environments, ceteris paribus. This is consistent with the so-call ‘r-selected’ species, which are adapted for rapid population growth in temporary environments. Thus the fitness of medflies is greater in environments in which they have frequent access to short-term food patches rather than infrequent access to more persistent food resources. This finding gives an important perspective because it provides depth and context for DR research inasmuch as it links the ecology of food foraging to the physiology of food restriction (Andrewartha & Birch, 1974; White, 1978; Hoyenga & Hoyenga, 1981; McNab, 2002). The findings also provide important context and perspective for developing theory and models concerned with the dynamics of populations in variable environments (Tuljapurkar, 1989; Tuljapurkar & Orzack, 1980).
The current research is the fifth medfly study that has involved some form of DR, most of which elicited different longevity responses. These studies and the actuarial results include: (1) intermittently denying individuals access to diet, which reduced the life expectancy of medflies, because mortality increased when flies were starved even for 1 day (Carey et al., 1999); (2) reducing the quality of diet available to individuals at young ages, which extended the life expectancy of female medflies in all cohorts due to the absence of protein in their (sugar-only) diet, causing flies either to arrest or greatly to reduce their egg production (Carey et al., 1998); (3) reducing the total amount of diet available to individuals, which lowered life expectancy in all cohorts with the extent of reduction being conditional on the degree of food restriction (Carey et al., 2002b); (4) systematically switching between periods of either high- or low-quality food, which extended the life expectancy of medfly cohorts under some treatments but reduced it under other treatments depending upon the particular sequence of the two types of food-days (Carey et al., 2002a); and (5) subjecting medflies to a Markov chain feeding scheme (current study on stochastic DR), which manifested a complex longevity response whereby the life expectancy of most (but not all) cohorts subjected to stochastic DR increased relative to ad libitum control cohorts. The collective results of these five medfly studies reveal that the longevity response of medflies to food restriction is highly sensitive to the particular method of restriction. This, in turn, suggests that the widespread claim that longevity extension is a universal response to DR (Bertarand et al., 1999; Masoro, 2001, 2003) may need to be re-visited – the sign and magnitude of longevity extension in other species may also be highly dependent upon the specific details of the DR method. This is emphasized by recent research on D. melanogaster showing that, contrary to earlier findings on the medfly, mortality rates in cohorts maintained on a protein-restricted (i.e. sugar-only) diet are higher than in cohorts maintained on ad libitum (full) diets (Good & Tatar, 2001).
Restriction studies that have not elicited a longevity extension response (David et al., 1971; LeBourg & Medioni, 1991; LeBourg & Minois, 1996) have often been ignored as has reference to negative aspects of DR. Although animals that are food-restricted in the wild may be physiologically adapted to live longer in the absence of natural enemies (predators; parasites; disease), the realities of life in the wild are harsh and risky and thus in most situations run counter to environments favoring extended longevity. Animals that are food-restricted or have minimal fat reserves are not only at greater risk of being killed by natural enemies because they are weak, but they are also unlikely to reproduce. For example, only medfly males with adequate nutritional reserves may join mating leks because of the prodigious energetic investment required (wing buzzing; pheromone emission) and exposure to predators that home in on male aggressions that lekking males commit to (Yuval et al., 1998). Furthermore, the copulation frequency of protein-deprived males that do gain access to females is substantially lower than for males that are maintained on an ad libitum protein-rich diet (Blay & Yuval, 1997). Although food-restricted fruit flies in the wild may, at least in theory, have the potential to live longer, this increase in survival will have no evolutionary advantage if females are egg limited (Papaj et al., 1989; Rosenheim, 1996; Papaj, 2000) as an outcome of their inability to find a necessary source of protein for egg production (Wheeler, 1996). For mammals subjected to stochastic environments, the reproductive investment of gestating or lactating animals will be lost if they are suddenly deprived of high-quality sources of protein (Begon et al., 1996). Indeed, the average life expectancy of most small mammals living in the temperate zone is measured in weeks, or a few months at best (Bronson, 1985), which means that small mammals must reproduce whenever there is any possibility of successfully meeting their lactational costs and must push hard against their energetic constraints. In short, reproductive opportunism in medflies as reported here as well as in a previous study (Carey et al., 2002b) is a good strategy for animals living in an unpredictable environment, despite the increased risk of early death due to reproductive costs and survival uncertainties.
Conclusions
The longevity extension response of animals to DR is viewed as one of the universal canons of gerontology because the published results of a large number of DR experiments appeared to be so consistent and because the arguments on why organisms evolved this capability were believed by most gerontologists to be so compelling; by decreasing the rate of aging through unfavorable periods, individuals could increase the likelihood that they will be alive to reproduce if/when conditions improve in the future (Holliday, 1989). However, the results presented in this paper on stochastic DR along with findings from earlier medfly DR studies reveal that the details of the DR experimental protocol determines the actuarial outcome (i.e. sign and magnitude of the longevity response relative to ad libitum), and that understanding how and to what extent reproduction is affected by the manipulation is critical for interpretation of results.
The connection between various sources of energy available to insects is complex and poorly understood (Warburg & Yuval, 1996) and the medfly system is no exception. Proteins and lipids may metaphorically represent an energetic trust fund (Yuval et al., 1994) that medflies need for reproduction, whereas carbohydrates are comparable to a readily accessible cash account on which medflies can survive but not reproduce. In general, how dietary components are allocated is clearly more complicated than straightforward energetic–growth–maintenance budgets and involves interactive effects at multiple levels (Raubenheimer & Simpson, 2004). The broad implication of this and previous research on medfly DR is that the evolutionary premise for (Holliday, 1989) and the physiological response to (Masoro, 1988; Weindruch & Walford, 1988) DR may need to be re-visited because experimental results reveal that the particulars of the DR response cannot be understood without a deeper understanding of how organisms adjust their reproductive efforts to suboptimal and unpredictable nutrition environments in the wild.
Experimental procedures
Medfly natural history and ecology
Although the medfly originated in tropical west Africa, it is now found in virtually all regions of Africa, in Mediterranean regions of Europe where they can overwinter as either larvae (Papadopoulos et al., 1996) or adults (Mavrikakis et al., 2000) as well as in the Middle East and South and Central America (Bateman, 1972). Medfly adults emerge from pupae after a 2–3-week developmental period and immediately begin foraging for food and searching for mates (Hagen et al., 1981). Unlike Drosophila females, which lay their eggs on open, rotting fruit, medfly females seek out intact hosts in which to drill a hole and lay their eggs. Because egg laying can occur over an adult female’s lifetime and newly emerged adults have few larval reserves from which to manufacture eggs, females must search for food throughout their entire adult life (Drew et al., 1983; Vargas et al., 1983; Hendrichs & Hendrichs, 1990; Hendrichs et al., 1993), including carbohydrates for energy (e.g. nectar, aphid honeydew) and protein for maintenance and reproduction (e.g. bacteria, pollen, mold/yeast). The life history traits of the medfly are consistent with classical r-selected organisms (Begon et al., 1996) that are capable of explosive population growth, including wide host range (250 hosts), rapid developmental time (3 weeks), short period of sexual maturation (7–10 days) and high reproductive rate (> 1000 eggs/female).
Operational framework
Although protocols for DR are outlined elsewhere (Yu et al., 1985; Weindruch & Walford, 1988; Masoro, 1995; Bertarand et al., 1999), we generalize the concept by considering DR in the broader context of food environments. We classify restriction environments according to food quantity defined as the relative or absolute amount of food available to an individual, food quality defined as dietary composition relative to a predetermined idealized standard, and temporal aspects of food availability defined according to whether an individual’s daily access to food is either constant, deterministically variable or stochastically variable.
The feeding scheme that was designed for this study follows a Markov chain stochastic process (Chiang, 1980; Markov, 1906), in which the conditional probability of the future status given the past history depends only upon the immediate past and not upon the remote past. Here the ad libitum food discovery probability, p, denotes the probability of an individual switching to a high-quality diet from a low-quality diet, which is also called the transition probability; and the persistence probability, q, denotes the probability of staying in a high-quality diet. The limiting probability (asymptotic probability) for an individual to be fed a high-quality diet on any given day, which is independent of the time and denoted as p*, is given by:
| (1) |
The Markov chain feeding scheme is designed to reflect feeding patterns in the wild that are assumed to be governed by random discovery of high-quality food sources, the persistence of which is also governed by a random process. For example, consider the value of p* = 0.200 for the following two cases: Case I – p = q = 0.2; Case II – p = 0.05, q = 0.80. Thus the average fly would have access to high-quality food an average of 20% of its lifetime for both Case I and Case II. However, the pattern of high-quality food availability would be substantially different in each case, the former consisting of many ephemeral food patches and the latter consisting of fewer but more persistent patches.
The expected lengths of high-quality food patches follow geometric distributions with switching probability 1 − q, so the expected length of a high-quality food patch is 1/(1 − q). Similarly, the expected lengths of low-quality (sugar-only) food patches also follows geometric distributions with switching probability p, so the expectation of length of low-quality food patches is 1/p. Thus the expected lengths of high-quality food patches available to the cohorts subject to the persistence parameters q = 0.2, 0.5 and 0.8 are 1.25, 2.0 and 5.0 days, respectively, and to the discovery parameters p = 0.05, 0.10, 0.15 and 0.20 are 20, 10, 6.7 and 5.0 days, respectively.
Experimental details
The study design of this stochastic experiment involved the use of two controls in which fly cohorts were maintained on either a full diet (Control A) or a sugar-only diet (Control B), and 12 treatments based on a design of four p values (0.05, 0.10, 0.15, 0.20) combined with three q values (0.20, 0.50, 0.80). A schematic that visualizes the density and patterns of food-days for each of the 12 treatments is given in Fig. 2 with corresponding values of p* computed from Eq. (1) (see above for details).
Fig. 2.
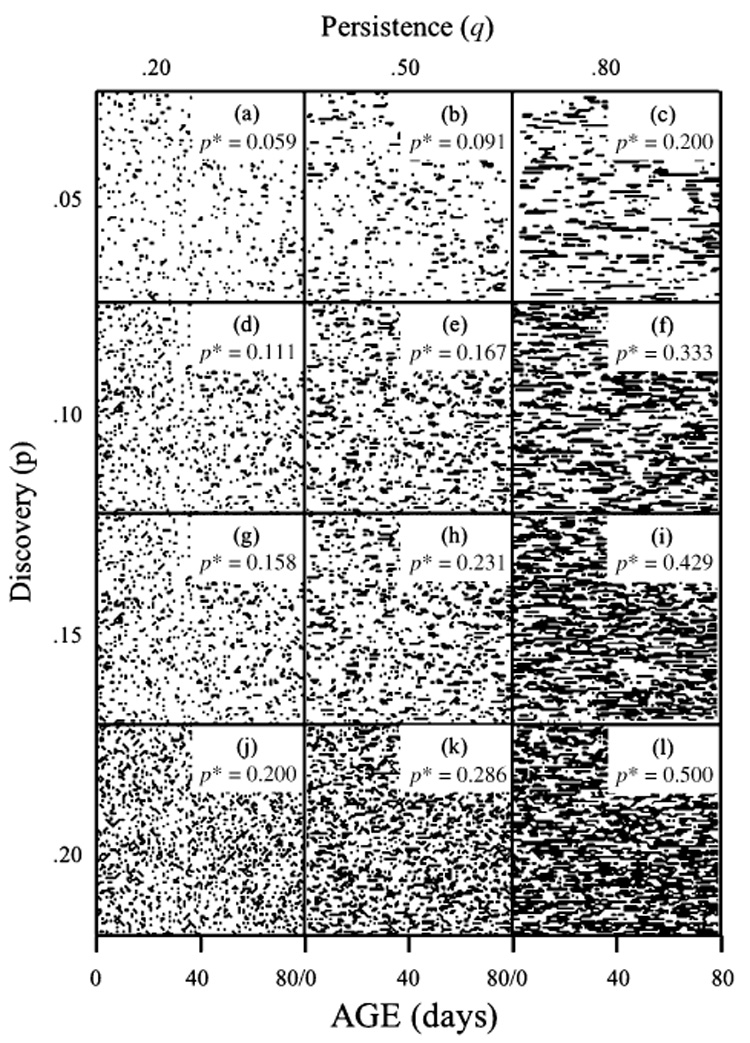
Schematic graphs showing the stochastic food availability patterns in the 12 treatment cohorts of the medfly DR study. Each panel corresponds to the food availability patterns generated for 100 individuals represented by imaginary horizontal ‘lines’ moving left to right from day 0 through day 80. The density and pattern of black ticks (or series of ticks) represent the food-days that were generated with simulations using one of four values of the discovery parameter, p, and one of three values of the corresponding persistence parameter, q. For example, the upper-left panel (p = 0.05, q = 0.20) shows a sparse pattern of food availability with most patches available to individuals only one day at a time (i.e. non-persistent). In contrast, the lower-right panel (p = 0.20, q = 0.80) shows a more dense pattern of food availability with most patches available to individuals over a series of days (i.e. persistent). The insets containing p* in each panel give the predicted number of high-quality food-days (asymptotic probability) for each feeding regime calculated using Eq. (1). For example, p* = 0.200 indicates that individuals in a cohort received high-quality food an average of 20% of the time.
As with all previous medfly research published by Carey and co-workers (e.g. Carey, 2003), the current study was conducted at the Moscamed medfly mass rearing facility near Tapachula, Mexico – a facility constructed in 1979 as a joint enterprise funded by the US Department of Agriculture and the Mexico Ministry of Agriculture to rear large numbers (i.e. 500 million to 1 billion per week) of medfly adults for sterilization and subsequent release as a tactical component of a program to prevent the spread of the pest further into Mexico. The rearing technology involves collecting several liters (i.e. tens of millions) of eggs from reproducing adults each day that are used to ‘seed’ diet-filled trays held in cafeteria-style racks. The mature larvae are separated from the rearing medium after 1 week and distributed in special holding trays for pupation. A predetermined number of these pupae are removed from the production facility a few days before eclosion (around 2 weeks) for use in the medfly aging studies. General details have been described concerning all aspects of medfly mass rearing at this facility (Vargas, 1989) and the specific details on the use of the medfly model system for aging research (Carey & Liedo, 1999; Carey, 1999).
A 3-µL droplet of food and a 6-µL droplet of water were supplied to the flies on glass slides using separate Ependorf® needles. The slides of the previous day were removed, placed on a metallic grid and washed. Newly emerged (virgin) individual flies were housed in 4 × 4 × 10-cm plexiglass cages, each of which was part of a 24-unit cage. Distribution of treatments was made in a randomized block design, using different color codes to identify both the treatment and the food slides. Females and males were placed in alternate cages to eliminate the possibility of eggs from two females overlapping on the egg collection surface. Females laid eggs through organdy mesh fastened to the front of the cage and were counted daily using an electronic image analysis system that automatically records the number of medfly eggs that fall in a 4.5 × 4.5-cm2 field. Methods used for analysis of the demographic data are outlined in Carey (1999). A miscommunication between technicians and the principal investigator that was not discovered until after the study was finished resulted in the use of unequal numbers of individuals across some treatments (n = 71–98 flies per treatment).
Statistical methods
We use both parametric and non-parametric approaches for data analysis. Linear regression as a parametric model is used to characterize linear relationships between response and predictors. Assume are the data and there exists a linear relationship between X and Y, such that Y = Xβ + ε, where ε is an error term. The estimate of β can be obtained by least squares. We assume independent, Gaussian and homoscedastic errors for inference.
Define the local linear scatterplot smoother as the minimizer (minimizing with respect to (b0, b1,…, bd)), where Kd is a d-dimensional kernel function and h is the bandwidth. Then Data-adaptive methods for bandwidth choice are available, such as one-leave-out cross-validation. The smoothing methods have been described in detail in Chiou et al. (2003). Note that we investigate the significance of non-parametric relationships by fitting parametric regression functions with similar shape, notably quadratic regressions.
Acknowledgments
We thank A. Oropeza, R. Bustamante, E. de la Cruz, S. Rodriguez, R. Rincón, A. Villela and G. Rodas for technical assistance and the Moscamed program in Mexico for allowing us use of their facilities in Metapa. This research was supported by grants from the National Institute on Aging (P01-AG022500-01; P01-AG08761-10).
References
- Andrewartha HG, Birch LC. The Distribution and Abundance of Animals. Chicago: University of Chicago Press; 1974. [Google Scholar]
- Austad SN. Life extension by diet restriction in the bowl and doily spider. Frontinella pyramitela. Exp. Gerontol. 1989;24:83–92. doi: 10.1016/0531-5565(89)90037-5. [DOI] [PubMed] [Google Scholar]
- Austad SN, Kristan DM. Are mice calorically restricted in nature? Aging Cell. 2003;2:201–207. doi: 10.1046/j.1474-9728.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- Bateman MA. The ecology of fruit flies. Annu. Rev. Entomol. 1972;17:493–518. [Google Scholar]
- Begon M, Harper JL, Townsend CR. Ecology: Individuals, Populations and Communities. 3rd edn. Oxford: Blackwell Science Ltd.; 1996. [Google Scholar]
- Bell G. Evolutionary and nonevolutionary theories of senescence. Am. Nat. 1984;124:600–603. [Google Scholar]
- Bell G, Koufopanou V. The cost of reproduction. In: Dawkins R, Ridley M, editors. Oxford Surveys in Evolutionary Biology. Oxford: Oxford University Press; 1986. pp. 83–131. [Google Scholar]
- Bertarand HA, Herlihy JT, Ikeno Y, Yu BP. Dietary restriction. In: Yu BP, editor. Methods in Aging Research. Boca Raton: CRC Press; 1999. pp. 271–300. [Google Scholar]
- Blay S, Yuval B. Nutritional correlates of reproductive success of male Mediterranean fruit flies (Diptera: Tephritidae) Anim. Behav. 1997;54:59–66. doi: 10.1006/anbe.1996.0445. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Mammalian reproduction: an ecological perspective. Biol. Reprod. 1985;32:1–26. doi: 10.1095/biolreprod32.1.1. [DOI] [PubMed] [Google Scholar]
- Bronson FH, Marsteller FA. Effect of short-term food deprivation on reproduction in female mice. Biol. Reprod. 1985;33:660–667. doi: 10.1095/biolreprod33.3.660. [DOI] [PubMed] [Google Scholar]
- Carey JR. Population study of mortality and longevity with Gompertzian analysis. In: Yu BP, editor. Methods in Aging Research. Boca Raton: CRC Press; 1999. pp. 3–24. [Google Scholar]
- Carey JR. Longevity. The Biology and Demography of Life Span. Princeton: Princeton University Press; 2003. [Google Scholar]
- Carey JR, Liedo P. Measuring mortality and reproduction in large cohorts of the Mediterranean fruit fly. In: Sternberg H, Timiras PS, editors. Studies of Aging. Berlin: Springer-Verlag; 1999. pp. 111–124. [Google Scholar]
- Carey JR, Liedo P, Harshman L, Liu X, Müller H-G, Partridge L, Wang J-L. Food pulses increase longevity and induce cyclical egg production in Mediterranean fruit flies. Funct. Ecol. 2002a;16:313–325. [Google Scholar]
- Carey JR, Liedo P, Harshman L, Müller H-G, Partridge L, Wang J-L, Zhang Y. Life history response of Mediterranean fruit flies to dietary restriction. Aging Cell. 2002b;1:140–148. doi: 10.1046/j.1474-9728.2002.00019.x. [DOI] [PubMed] [Google Scholar]
- Carey JR, Liedo P, Müller H-G, Wang J-L, Chiou J-M. Mortality oscillations induced by periodic starvation alter sex-mortality differentials in Mediterranean fruit flies. J. Gerontol.: Biol. Sci. 1999;54A:B424–B431. doi: 10.1093/gerona/54.10.b424. [DOI] [PubMed] [Google Scholar]
- Carey JR, Liedo P, Müller H-G, Wang J-L, Vaupel JW. Dual modes of aging in Mediterranean fruit fly females. Science. 1998;281:996–998. doi: 10.1126/science.281.5379.996. [DOI] [PubMed] [Google Scholar]
- Chapman T, Partridge L. Female fitness in Drosophila melanogaster: an interaction between the effect of nutrition and of encounter rate of males. Proc. R Soc. Lond.; Series B. 1996;263:755–759. doi: 10.1098/rspb.1996.0113. [DOI] [PubMed] [Google Scholar]
- Chiang CL. An Introduction to Stochastic Processes and Their Applications. Huntington, NY: Robert E. Krieger Publishing Co.; 1980. [Google Scholar]
- Chiou J-M, Müller H-G, Wang J-L, Carey JR. A functional multiplicative effects model for longitudinal data, with application to reproductive histories of female medflies. Stat. Sinica. 2003;13:1119–1133. [PMC free article] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Hafen E, Leevers SJ, Partridge L. Dietary restriction in long-lived dwarf flies. Science. 2002;296:319. doi: 10.1126/science.1069366. [DOI] [PubMed] [Google Scholar]
- Costa DP, Sinervo B. Field physiology: physiological insights from animals in nature. Annu. Rev. Physiol. 2004;66:209–238. doi: 10.1146/annurev.physiol.66.032102.114245. [DOI] [PubMed] [Google Scholar]
- David J, Van Herrewege J, Jouillet P. Quantitative under-feeding of Drosophila: effects on adult longevity and fecundity. Exp. Gerontol. 1971;6:249–257. doi: 10.1016/0531-5565(71)90037-4. [DOI] [PubMed] [Google Scholar]
- Drew RAI, Courtice AC, Teakle DS. Bacteria as a natural source of food for adult fruit flies (Diptera: Tephritidae) Oecologia. 1983;60:279–284. doi: 10.1007/BF00376839. [DOI] [PubMed] [Google Scholar]
- Good TP, Tatar M. Age-specific mortality and reproduction respond to adult dietary restriction in Drosophila melanogaster. J. Insect. Physiol. 2001;47:1467–1473. doi: 10.1016/s0022-1910(01)00138-x. [DOI] [PubMed] [Google Scholar]
- Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- Hagen KS, Allen WW, Tassan RL. Mediterranean fruit fly: the worst may be yet to come. Calif. Agric. 1981;36:5–7. [Google Scholar]
- Hamilton GD, Bronson FH. Food restriction and reproductive development in wild house mice. Biol. Reprod. 1985;32:773–778. doi: 10.1095/biolreprod32.4.773. [DOI] [PubMed] [Google Scholar]
- Hendrichs J, Hendrichs MA. Mediterranean fruit fly (Diptera: Tephritidae) in nature: location and diel pattern of feeding and other activities on fruiting and nonfruiting hosts and nonhosts. Annu. Entomol. Soc. Am. 1990;93:632–641. [Google Scholar]
- Hendrichs J, Lauzon CR, Cooley SS, Prokopy RJ. Contribution of natural food sources to adult longevity and fecundity of Rhagoletis pomonella (Diptera: Tephritidae) Annu. Entomol. Soc. Am. 1993;86:250–264. [Google Scholar]
- Holliday R. Food, reproduction and longevity: is the extended lifespan of calorie-restricted animals an evolutionary adaptation? Bioessays. 1989;10:125–127. doi: 10.1002/bies.950100408. [DOI] [PubMed] [Google Scholar]
- Hoyenga KB, Hoyenga KT. Gender and energy balance: sex differences in adaptations for feast and famine. Physiol. Behav. 1981;28:545–563. doi: 10.1016/0031-9384(82)90153-6. [DOI] [PubMed] [Google Scholar]
- Kenyon C. Environmental factors and gene activities that influence life span. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C. elegans II. Cold Spring Harbor: Cold Spring Harbor Press; 1997. pp. 791–813. [PubMed] [Google Scholar]
- Kirk KL. Dietary restriction and aging: comparative tests of evolutionary hypotheses. J. Gerontol.: Biol. Sci. 2001;56A:B123–B129. doi: 10.1093/gerona/56.3.b123. [DOI] [PubMed] [Google Scholar]
- LeBourg E, Medioni J. Food restriction and longevity in Drosophila melanogaster. Age Nutr. 1991;2:90–94. [Google Scholar]
- LeBourg E, Minois N. Failure to confirm increased longevity in Drosophila melanogaster submitted to a food restriction procedure. J. Gerontol. Biol. Sci. 1996;51A:B280–B283. doi: 10.1093/gerona/51a.4.b280. [DOI] [PubMed] [Google Scholar]
- Lin S-J, Kaeberlein M, Andalis AA, Sturtz LA, Defossez P-A, Culottas VC, Fink GR, Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- Mair W, Goymer P, Pletcher SC, Partridge L. Demography of dietary restriction and death in Drosophila. Science. 2003;301:1731–1733. doi: 10.1126/science.1086016. [DOI] [PubMed] [Google Scholar]
- Mair W, Goymer P, Pletcher SD, Partridge L. Response (to Rauser et al.) Science. 2004;303:1611–1612. [Google Scholar]
- Markov AA. Extension of the law of large numbers to dependent events (Russian) Bull. Soc. Phy. Math. Kazanstan. 1906;2:135–156. [Google Scholar]
- Masoro EJ. Mini review: food restriction in rodents: an evaluation of its role in the study of aging. J. Gerontol. 1988;43:B59–B64. doi: 10.1093/geronj/43.3.b59. [DOI] [PubMed] [Google Scholar]
- Masoro E. Design issues in dietary restriction. In: Hart RW, Neumann DA, Robertxon RT, editors. Dietary Restriction: Implications for the Design and Interpretation of Toxidity and Carninogenicity Studies. Washington, DC: ILSI Press; 1995. pp. 341–350. [Google Scholar]
- Masoro EJ. Caloric restriction’s effects on aging: opportunities for research on human implications. J. Gerontol. Biol. Med. Sci. 2001;00:56A. doi: 10.1093/gerona/56.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Subfield history: caloric restriction, slowing aging, and extending life. Sci. SAGE KE 2003 Re. 2003;2:1–7. doi: 10.1126/sageke.2003.8.re2. [DOI] [PubMed] [Google Scholar]
- Mavrikakis PG, Economopoulos AP, Carey JR. Continuous winter reproduction and growth of the Mediterranean fruit fly (Diptera: Tephritidae) in Heraklion, Crete, Southern Greece. Environ. Entomol. 2000;29:1180–1187. [Google Scholar]
- McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. J. Nutri. 1935;10:63–79. [PubMed] [Google Scholar]
- McNab BK. The Physiological Ecology of Vertebrates. Ithaca, NY: Comstock Publishing Associates; 2002. [Google Scholar]
- Papadopoulos NT, Carey JR, Katsoyannos BI, Kouloussis NA. Over-wintering of the Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae) in Northern Greece. Annu. Entomol. Soc. Am. 1996;89:526–534. [Google Scholar]
- Papaj DR. Ovarian dynamics and host use. Annu. Rev. Entomol. 2000;45:423–448. doi: 10.1146/annurev.ento.45.1.423. [DOI] [PubMed] [Google Scholar]
- Papaj DR, Roitberg BD, Opp SB. Serial effects of host infestation on egg allocation by the Mediterranean fruit fly: a rule of thumb and its functional significance. J. Anim. Ecol. 1989;58:955–970. [Google Scholar]
- Prokopy RJ, Roitberg BD. Foraging behaviour of true fruit flies: concepts of foraging can be used to determine how tephritids search for food, mates, and egg-laying sites and to help control these pests. Am. Sci. 1984;72:41–49. [Google Scholar]
- Raubenheimer D, Simpson SJ. Organismal stoichiometry: quantifying non-independence among food components. Ecology. 2004;85:1203–1216. [Google Scholar]
- Rauser WL, Mueller LD, Rose MR. Dietary restriction in Drosophila. Science. 2004;303:1610–1611. doi: 10.1126/science.303.5664.1610c. [DOI] [PubMed] [Google Scholar]
- Reznick D. Costs of reproduction: an evaluation of the empirical evidence. Oikos. 1985;44:257–267. [Google Scholar]
- Rosenheim JA. An evolutionary argument for egg limitation. Evolution. 1996;50:2089–2094. doi: 10.1111/j.1558-5646.1996.tb03595.x. [DOI] [PubMed] [Google Scholar]
- Shanley DP, Kirkwood TBL. Calorie restriction and aging: a life-history analysis. Evolution. 2000;54:740–750. doi: 10.1111/j.0014-3820.2000.tb00076.x. [DOI] [PubMed] [Google Scholar]
- Tatar M, Carey JR. Nutrition mediates reproductive trade-offs with age-specific mortality in the beetle, Calosobruchus maculatus. Ecology. 1995;76:2066–2073. [Google Scholar]
- Tu M-P, Tatar M. Juvenile diet restriction and the aging and reproduction of adult Drosophila melanogaster. Aging Cell. 2003;2:327–333. doi: 10.1046/j.1474-9728.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- Tuljapurkar S. An uncertain life: demography in random environments. Theor. Popul. Biol. 1989;35:227–294. doi: 10.1016/0040-5809(89)90001-4. [DOI] [PubMed] [Google Scholar]
- Tuljapurkar SD, Orzack SH. Population dynamics in variable environments I. long-run growth rates and extinction. Theor. Popul. Biol. 1980;18:314–342. [Google Scholar]
- Vargas R. Mass production of tephritid fruit flies. In: Robinson AS, Hooper G, editors. World Crop Pests. Fruit Flies: Their Biology, Natural Enemies and Control. Amsterdam: Elsevier; 1989. pp. 141–152. [Google Scholar]
- Vargas RI, Harris EJ, Nishida T. Distribution and seasonal occurrence of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) on the Island of Kauai in the Hawaiian Islands. Environ. Entomol. 1983;12:303–310. [Google Scholar]
- Warburg MS, Yuval B. Effects of diet and activity on lipid levels of adult Mediterranean fruit flies. Physiol. Entomol. 1996;21:151–158. [Google Scholar]
- Weindruch R, Walford RL. The Retardation of Aging and Disease by Dietary Restriction. Springfield: Charles C. Thomas; 1988. [Google Scholar]
- Wheeler D. The role of nourishment in oogenesis. Annu. Rev. Entomol. 1996;41:407–431. doi: 10.1146/annurev.en.41.010196.002203. [DOI] [PubMed] [Google Scholar]
- White TCR. Weather, food and plagues of locusts. Oecologia. 1976;22:119–134. doi: 10.1007/BF00344712. [DOI] [PubMed] [Google Scholar]
- White TCR. The importance of a relative shortage of food in animal ecology. Oecologia. 1978;33:71–86. doi: 10.1007/BF00376997. [DOI] [PubMed] [Google Scholar]
- Yu BP, Masoro EJ, McMahan CA. Nutritional influences on aging of Fischer 344 rats. I. Physical, metabolilc, and longevity characteristics. J. Gerontol. Biol. Sci. 1985;40:657–670. doi: 10.1093/geronj/40.6.657. [DOI] [PubMed] [Google Scholar]
- Yuval B, Holliday-Hanson M, Washingo RK. Energy budget of swarming male mosquitoes. Ecol. Entomol. 1994;19:74–78. [Google Scholar]
- Yuval B, Kaspi R, Shloush S, Warburg MS. Nutritional reserves regulate male participation in Mediterranean fruit fly leks. Ecol. Entomol. 1998;23:211–215. [Google Scholar]


