Abstract
OBJECTIVES
To determine the extent to which preoperative performance on tests of executive function and memory was associated with delirium after coronary artery bypass graft (CABG) surgery.
DESIGN
Prospective observational cohort study.
SETTING
Two academic medical centers and one Department of Veterans Affairs medical center in Massachusetts.
PARTICIPANTS
Eighty subjects without preoperative delirium undergoing CABG or CABG-valve surgery completed baseline neuropsychological assessments with validated measures of memory and executive function.
MEASUREMENTS
Beginning on postoperative Day 2, a battery to diagnose delirium was administered daily. Confirmatory factor analysis (CFA) was used to define two cognitive domain composites (memory and executive function). The loading pattern of neuropsychological measures onto the latent cognitive domains was determined a priori. Poisson regression was used to model the association between neuropsychological performance and cognitive domain composite scores and risk of postoperative delirium. The association was expressed as the difference between impaired (0.5 standard deviations (SDs) below mean) and nonimpaired (0.5 SDs above mean) performers.
RESULTS
Forty subjects (50%) developed delirium. Measures of memory function were not significantly related to delirium. Of the executive function measures, verbal fluency, category fluency, Hopkins Verbal Learning Test learning, and backward recounting of days and months were significantly related to delirium. Preoperative mental status was a strong predictor of postoperative delirium. After controlling for age, sex, education, medical comorbidity, mental status, and the other cognitive domain, CFA cognitive domain composites suggest that risk for delirium is specific for executive functioning impairment (relative risk (RR) = 2.77, 95% confidence interval (CI) = 1.12–6.87) but not for memory impairment (RR = 0.49, 95% CI = 0.19–1.25).
CONCLUSION
Worse preoperative performance in executive function was independently associated with greater risk of developing delirium after CABG.
Keywords: aged, delirium, CABG surgery, executive function, cognitive impairment, factor analysis
Delirium is a common complication of coronary artery bypass graft (CABG) surgery, occurring in 32% to 73% of patients.1 Postoperative delirium is associated with greater mortality,2 morbidity,2,3 cost,4 and loss of independence in community-dwelling adults.3 Recognizing a patient’s preoperative risk for delirium is crucial to detect delirium proactively and limit its severity or prevent it altogether.5
Dementia has been identified as a risk factor for delirium in medical and surgical patients,6 but dementia is a complex disorder consisting of deficits in multiple cognitive domains, with considerable individual variability in how these domains are affected. Although memory deficits are the cornerstone of this diagnosis, impairments in executive function have recently been shown to be important predictors of functional decline,7 as well as predictors of the risk for dementia itself.8 Executive function is the term given to a spectrum of cognitive operations such as planning, working memory, impulse control, inhibition, and set shifting that are necessary for initiation, planning, and control of behavior9 and appear to be critically dependent on the frontal cortex and its connections to other cerebral and subcortical areas.10
Executive functions seem to be important in the level of functional impairment in dementia and may also be impaired in patients who do not meet the formal criteria for this diagnosis. For example, executive function may be impaired in patients with evidence of microvascular disease11 and even in patients who have risk for cerebrovascular disease.11,12 This is particularly important, because candidates for CABG are often subject to these very risk factors.
In this study, standard measures of memory and executive functions were administered preoperatively to a sample of patients undergoing CABG surgery who were monitored postoperatively for delirium. The purpose of this analysis was to determine whether preoperative performance on neuropsychological measures of memory and executive function is a risk factor for incident delirium in CABG patients. The investigators hypothesized that poor preoperative performance on measures of executive function would predict which subjects developed delirium.
METHODS
Participants
This prospective, observational cohort included subjects aged 60 and older who were undergoing CABG surgery or combined CABG-valve replacement surgery at two academic medical centers and one Department of Veterans Affairs medical center in Massachusetts. Institutional review boards at the three medical centers approved the study. One hundred eighty-two subjects were approached; of these, 97 provided informed consent, 83 refused to participate, and two were unable to provide informed consent. Two subjects were excluded from participation because of preoperative delirium, and two did not undergo an eligible surgery. Thirteen subjects were excluded because of inability to complete more than one baseline neuropsychological measure. Therefore, 80 subjects were included in the analysis. Excluded subjects developed postoperative delirium at a similar rate to included subjects.
Measures
Before surgery, subjects were administered a 45-minute neuropsychological battery consisting of five tests of executive function and one test of memory. The battery was chosen in accordance with the Statement of Consensus on Assessment of Neurobehavioral Outcomes after Cardiac Surgery.13 Based on conventions in the literature10 and the consensus of three neuropsychologists (LJG, WPM, CBB), the instruments were assigned to the memory or executive function domain, a priori. Some neuropsychological instruments were recognized to draw upon more than one cognitive domain and were assigned to the dominant domain.
Measures assigned to the executive function domain included the Trail Making Test Part B (TMT-B),14 digit span backwards,15 days of the week and months of the year backwards, semantic fluency, and phonemic fluency. The TMT-B is a test of executive function that involves shifting attentional abilities.14 Digit span of the Wechsler Adult Intelligence Scale, Revised15 and days of the week and months of the year backwards are tests of working memory requiring participants to sustain attention and manipulate information.10 Fluency tasks are measures that assess language and knowledge storage patterns by requiring the subject to generate words spontaneously in a category (semantic)16 or beginning with a specific letter (phonemic).16
The Hopkins Verbal Learning Test (HVLT), a 12-item verbal learning, retention, and recall measure,17 was administered. HVLT subscales (number of items identified in the initial three learning trials (learning score), number of spontaneously recalled items divided by the number of items learned after the third trial (retention percentage), and percentage of items correctly identified from a list (recognition discrimination)) were calculated in accordance with the manual. HVLT retention percentage and HVLT recognition discrimination are measures of short-term memory. HVLT learning score is primarily a measure of working memory with some organization, strategy and short-term memory contribution. Working memory, which is associated with frontal lobe function, is considered an executive function.10 Thus, HVLT learning score was classified as an executive function.
Delirium Assessment
A brief delirium assessment (< 15 minutes) was performed preoperatively and daily during the postoperative period, beginning on Day 2. Subjects were not assessed on postoperative Days 0 or 1 because of the intensive medical care required after the CABG procedure. Delirium was assessed using the diagnostic algorithm of the Confusion Assessment Method (CAM).18 Before completing the CAM, a standardized mental status interview was conducted that included the Mini-Mental State Examination (MMSE, a screening assessment of mental status),19 digit span (a test of working memory and attention that asks patients to repeat a series of random digits forward and backward), the Delirium Symptom Interview (an interview for eliciting eight key symptoms of delirium),20 and the Memorial Delirium Assessment Scale (a severity scale for delirium).21 This combined assessment for delirium has been shown to be highly reliable (κ= 0.95)22 when administered by trained, non-clinician interviewers. In the event of a postoperatively intubated subject, delirium was assessed using the CAM– intensive care unit, which is validated for use in intubated patients.23
Covariates
Demographic covariates such as age, sex, and educational level were collected from the patient before surgery. Information on medical comorbidity was collected from the medical record, and the Charlson Comorbidity Index was used to calculate the medical comorbidity.24 The type of surgery was abstracted from the surgical report.
Confirmatory Factor Analysis
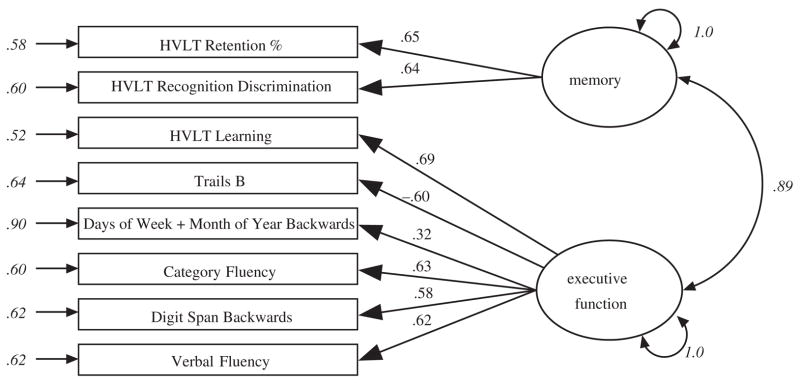
Based on an a priori assignment of neuropsychological tests to distinct cognitive domains (memory and executive function), a confirmatory factor analysis (CFA) model with two latent cognitive domains was created (Figure 1). To place the neuropsychological test performance scores on the same metric, scores were transformed using the Blom transformation.25 The latent cognitive domains were allowed to correlate freely, and their variances were freely estimated. The inclusion of a mixture of binary and continuous performance measures and missing data was accommodated by using Mplus software (Muthén & Muthén, Inc., Los Angeles, CA) and the maximum likelihood estimator. Parameters were estimated using Monte Carlo numerical integration. As such, conventional estimates of model fit reported in CFA are not available. Factor scores were saved from these models, which were rescaled to a mean of 0.5 unit variance and used as predictors of postoperative delirium, as were the individual performance indicators.
Figure 1.
Path diagram and standardized parameter estimates for confirmatory factor analysis model. This path diagram summarizes the confirmatory factor analysis model and presents the estimated standardized coefficients (standardized with respect to the mean and variance of the observed and latent variables). Parameters shown in italics reflect estimated parameters; all others were fixed to unit weight in unstandardized solution (parameters not shown were fixed to zero). HVLT = Hopkins Verbal Learning Test.
Modeling Risk of Postoperative Delirium
Poisson regression was used to derive risk estimates for postoperative delirium in the multivariable analysis. Continuous neuropsychological test scores (and domain composites) were reversed (with the exception of TMT-B time) so that higher values implied more-impaired cognitive functioning and standardized to a mean of 0.5 and a standard deviation (SD) of 1.0. Given the Poisson regression model and data transformation, the exponentiated regression coefficients express the relative risk (RR) of postoperative delirium between persons scoring 0.5 SDs below the mean to those 0.5 SDs above the mean. The multivariate analyses adjust for age, sex, education, and medical comorbidity. Independent CFA composite analyses examined the relationship between the two cognitive domain composites (executive function and memory) after adjustment for the above demographic variables and for the other composite (i.e., the effect for memory performance is adjusted for executive function performance). Independent composite analyses are presented with and without adjustment for baseline mental status (MMSE score).
RESULTS
Descriptive statistics for those who developed delirium and those who did not are provided in Table 1. Overall, subjects were mostly male (78%), older (mean age ± SD 74.9 ± 6.2), and well educated (47% > 12 years). Forty subjects (50%) developed delirium after CABG surgery. Subjects who developed delirium after CABG surgery were more likely to be female and have high comorbidity (Table 1).
Table 1.
Characteristics of Those Who Developed Delirium and Those Who Did Not
| Characteristic | Did Not Develop Delirium (n = 40) | Developed Delirium (n = 40) | F or χ2, P-value |
|---|---|---|---|
| Age, mean ± standard deviation | 73.5 ± 5.9 | 75.7 ± 6.3 | F = 2.6, .11 |
| Male, % | 87.5 | 67.5 | χ2 = 4.6, .03 |
| Education, % | |||
| <High school | 15.0 | 15.0 | |
| High school | 37.5 | 37.5 | |
| >High school | 47.5 | 47.5 | χ2 = 0, 1.00 |
| High comorbidity (Charlson Comorbidity Index ≥3), % | 20.0 | 52.5 | χ2 = 9.1, .002 |
| Valve surgery, % | 12.5 | 15.0 | χ2 = 0.1, .74 |
Note: Test of equivalent means (for continuous characteristics) or distribution (for discrete characteristics) across groups denned by incident delirium assessed with one-way analysis of variance and F test or chi-square (χ2) test, respectively.
Table 2 describes the association between neuropsychological test performance and risk of postoperative delirium. The effects are expressed as differences in the risk of developing delirium between those who were poor performers (0.5 SDs below the mean) and those who were high performers (0.5 SDs above the mean). The unadjusted risk of delirium is higher in subjects with poor performance on measures of working memory, fluency, naming, and learning. After adjustment for age, sex, education, and high medical comorbidity, subjects with poor performance on measures of working memory, fluency, naming, and memory were at higher risk of developing delirium. Poor performance on the MMSE was related to delirium risk before and after adjustment.
Table 2.
Performance on Neuropsychological Testing and Risk for Delirium
| RR of Delirium* (95% Confidence Interval)
|
||
|---|---|---|
| Measure | Raw | Adjusted† |
| Memory | ||
| HVLT retention % | 1.15 (0.95–1.40) | 1.08 (0.89–1.31) |
| HVLT recognition discrimination | 1.17 (0.97–1.41) | 1.14 (0.93–1.41) |
| Executive function | ||
| Verbal fluency | 1.37 (1.09–1.72)‡ | 1.56 (1.18–2.06)‡ |
| Digit Span backwards score | 1.33 (1.00–1.77) | 1.32 (0.98–1.78) |
| Categorical fluency | 1.48 (1.19–1.84)‡ | 1.42 (1.12–1.81)‡ |
| HVLT learning score | 1.33 (1.10–1.59)‡ | 1.25 (1.01–1.55)‡ |
| Backwards days and months | 1.83 (1.25–2.67)‡ | 1.63 (1.07–2.49)‡ |
| Trails B time | 1.11 (0.86–1.43) | 1.05 (0.79–1.38) |
| Mental status | ||
| Mini-Mental State Examination score | 1.33(1.15–1.52)‡ | 1.25 (1.05–1.49)‡ |
| CFA composites | ||
| Memory | 1.29(1.01–1.64)‡ | 1.51 (1.08–2.12)‡ |
| Executive function | 1.38(1.09–1.75)‡ | 1.93 (1.26–2.96)‡ |
| Independent CFA composites§ | ||
| Without mental status adjustment | ||
| Memory | 0.45 (0.14–1.43) | |
| Executive function | 5.02 (1.28–19.68)‡ | |
| With mental status adjustment | ||
| Memory | 0.49 (0.19–1.25) | |
| Executive function | 2.77 (1.12–6.87)‡ | |
Relative risk (RR) estimates derived from exponentiated regression coefficients from Poisson regression model. Performance variables were rescaled to describe the difference in risk between persons with impaired performance (0.5 standard deviations (SDs) below the mean) and those with better performing (0.5 SDs above the mean). An exception was the backwards days and months, which was 0 if incorrect and 1 if correct.
Adjusted for age, sex, education (<high school, high school, > high school) and high comorbidity (Charlson Comorbidity Index ≥3).
P<.05.
Independent confirmatory factor analysis (CFA) composites adjusted for age, sex, education, high comorbidity, and the other composite. The composites are presented with and without adjustment for mental status.
HVLT = Hopkins Verbal Learning Test.
A CFA model (Figure 1) was constructed with the neuropsychological performance indicators loading onto two latent cognitive domains: memory and executive function. The unadjusted analysis of the cognitive domain composites for memory function and executive function revealed that poor memory performance (RR = 1.29, 95% CI = 1.01–1.64) and poor executive function (RR = 1.38, 95% CI = 1.09–1.75) were related to postoperative delirium risk. After adjusting for sociodemographic and health factors and the other composite, the results indicate that risk for delirium is specific for executive function impairment (RR = 5.02, 95% CI = 1.28–19.68) but not memory impairment (RR = 0.45, 95% CI = 0.14–1.43). When the effect of baseline mental status was included in the analysis, poor performance on executive function was independently associated with a significantly greater risk of delirium (RR = 2.77, 95% CI = 1.12–6.87), and poor memory performance was not associated with delirium risk (RR = 0.49, 95% CI = 0.19–1.25).
DISCUSSION
This study of older patients undergoing CABG surgery analyzed preoperative memory and executive function performance on neuropsychological measures as risk factors for delirium after CABG. A CFA model was created to develop memory and executive function composites, and the association between these composites and development of postoperative delirium was validated. After adjustment for age, sex, education, comorbidity, baseline cognition, and memory, poor performance on measures of executive function was independently associated with greater risk of developing delirium. The effect of memory on delirium was significant until executive function was adjusted for, suggesting that executive function may mediate the effect of memory on delirium. Adjustment for baseline mental status did not change the mediating effect of executive function on delirium.
Previous studies of delirium have determined that dementia is a risk factor for delirium.6 Criteria for dementia require impairment in memory and another cognitive impairment,26 but those with memory impairment or other cognitive impairments have not been studied with respect to delirium. Although established dementia is a risk factor for delirium, the current data suggest that impairments in preoperative performance on measures of executive function may also be predictive.
The association between impairment in executive function and delirium may have a pathophysiological basis. Atherosclerosis is known to impair cognitive abilities, particularly in measures of frontal lobe function.11 In addition, risk factors for atherosclerosis are known to affect performance on measures of executive function adversely.11,12 In the CABG population, atherosclerosis has been associated with delirium.27 Delirium and atherosclerosis share common risk factors such as older age,2 male sex,28 hypertension,29 and peripheral vascular disease.2 On the basis of these reports and these findings, it may be that the underlying atherosclerosis pathology that affects the frontal lobes impairs preoperative performance of executive functions. Preoperative executive function impairment predisposes patients to postoperative difficulty processing reorienting stimuli, which may present as deficits in attention or altered thought patterns that are required for the diagnosis of delirium.
This study has several advantages. The subjects were prospectively enrolled and did not have delirium at baseline. The neurocognitive battery was chosen in accordance with published concensus13 and has been used in other studies to detect cognitive deficits.30 Daily follow-up with a reliable delirium battery maximized capture of postoperative delirium.
There are also several limitations to this study. The population had symptomatic atherosclerosis, a known risk factor for executive function deficits. This may limit the generalizability of the findings to other medical and surgical populations. Delirium was not assessed for on postoperative Day 1, which would lead to a conservative estimate of the incidence of delirium in the population. Despite this, the incidence of delirium after CABG in this population is at the upper limit of published studies. Some subjects were unable to complete the full neuropsychological test battery because of illness, time commitments, and other wishes. Most of the subject recruitment was performed in the hospital, on the night before surgery; under these circumstances, compliance with the neuropsychological battery was gratifyingly high.
Within the battery, some neuropsychological measures assess multiple cognitive domains and therefore may not be specific for executive function or memory. The neuropsychological measures were assigned a priori to the predominant cognitive domain. The neuropsychological battery consisted of multiple measures of executive function, but the indicators of memory function both derive from the HVLT. Thus, the memory factor is underspecified. This analysis would benefit from an additional measure of visual memory function, which was not performed in the study. Finally, this study evaluated the independent effect of executive function and memory in terms of delirium risk; it was not designed to evaluate the relative contribution of executive function and memory on delirium risk.
This study has important clinical implications. Efforts to target populations for screening, intervention, or prevention of delirium should consider executive function impairment as a risk factor. The high incidence of delirium associated with CABG surgery may have some relationship to underlying microvascular disease of the brain that affects frontal function. Further studies in medical and surgical populations are needed to examine the role of mild executive function impairments in the development of delirium.
Acknowledgments
We are indebted to Christopher B. Brady, PhD, for his review of the assignment of cognitive domains.
Financial Disclosure: This research was funded by Older Americans Independence Center Grant 5 P60 AG08812-14 and K12 Mentored Clinical Scientist Award 5 K12 AG00294-18 from the National Institute on Aging.
Footnotes
Presented as an abstract at the 2005 American Geriatric Society Annual Conference.
Author Contributions: James L. Rudolph: study design, acquisition of subjects, data analysis, interpretation of data, preparation of manuscript. Richard N. Jones: study design, data analysis interpretation, preparation of manuscript. Laura J. Grande: study design, interpretation of results, preparation of manuscript. William P. Milberg: study design, interpretation of results, data analysis, preparation of manuscript. Emily G. King: study concept, acquisition of subjects. Lewis A. Lipsitx and Sue E. Levkoff: study concept, preparation of manuscript. Edward R. Marcantonio: study design and concept, data analysis, interpretation of data, preparation of manuscript.
Sponsor’s Role: The authors retained full independence of the data during this study.
References
- 1.Smith LW, Dimsdale JE. Postcardiotomy delirium: Conclusions after 25 years? Am J Psychiatry. 1989;146:452–58. doi: 10.1176/ajp.146.4.452. [DOI] [PubMed] [Google Scholar]
- 2.Marcantonio ER, Goldman L, Mangione CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994;271:134–139. [PubMed] [Google Scholar]
- 3.Marcantonio ER, Goldman L, Orav EJ, et al. The association of intraoperative factors with the development of postoperative delirium. Am J Med. 1998;105:380–384. doi: 10.1016/s0002-9343(98)00292-7. [DOI] [PubMed] [Google Scholar]
- 4.Franco K, Litaker D, Locala J, et al. The cost of delirium in the surgical patient. Psychosomatics. 2001;42:68–73. doi: 10.1176/appi.psy.42.1.68. [DOI] [PubMed] [Google Scholar]
- 5.Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: A randomized trial. J Am Geriatr Soc. 2001;49:516–522. doi: 10.1046/j.1532-5415.2001.49108.x. [DOI] [PubMed] [Google Scholar]
- 6.Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: A systematic review. J Am Geriatr Soc. 2002;50:1723–1732. doi: 10.1046/j.1532-5415.2002.50468.x. [DOI] [PubMed] [Google Scholar]
- 7.Royall DR, Palmer R, Chiodo LK, et al. Declining executive control in normal aging predicts change in functional status: The Freedom House Study. J Am Geriatr Soc. 2004;52:346–352. doi: 10.1111/j.1532-5415.2004.52104.x. [DOI] [PubMed] [Google Scholar]
- 8.Albert MS. Cognitive and neurobiologic markers of early Alzheimer disease. Proc Natl Acad Sci USA. 1996;93:13547–13551. doi: 10.1073/pnas.93.24.13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill EL. Executive dysfunction in autism. Trends Cogn Sci. 2004;8:26–32. doi: 10.1016/j.tics.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Stuss DT, Levine B. Adult clinical neuropsychology: Lessons from studies of the frontal lobes. Annu Rev Psychol. 2002;53:401–433. doi: 10.1146/annurev.psych.53.100901.135220. [DOI] [PubMed] [Google Scholar]
- 11.Kuo HK, Lipsitz LA. Cerebral white matter changes and geriatric syndromes: Is there a link? J Gerontol A Biol Sci Med Sci. 2004;59:818–826. doi: 10.1093/gerona/59.8.m818. [DOI] [PubMed] [Google Scholar]
- 12.Pugh KG, Kiely DK, Milberg WP, et al. Selective impairment of frontal-executive cognitive function in African Americans with cardiovascular risk factors. J Am Geriatr Soc. 2003;51:1439–1444. doi: 10.1046/j.1532-5415.2003.51463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murkin JM, Newman SP, Stump DA. Statement of consensus on assessment of neurobehavioral outcomes after cardiac surgery. Ann Thorac Surg. 1995;59:1289–1295. doi: 10.1016/0003-4975(95)00106-u. [DOI] [PubMed] [Google Scholar]
- 14.Trailmaking Tests A and B. Washington, DC: War Department Adjutant General’s Office; 1944. [Google Scholar]
- 15.Wechsler D. Wechsler Adult Intelligence Scale Revised Manual. New York: Psychological Corporation; 1989. [Google Scholar]
- 16.Benton AL, Hamsher K. Multilingual Aphasia Examination. Iowa City: University of Iowa; 1976. [Google Scholar]
- 17.Brandt J, Benedict RHB. Hopkins Verbal Learning Test Revised (HVLT) Lutz, FL: Psychological Assessment Resources, Inc.; 1991. [Google Scholar]
- 18.Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: The confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Albert MS, Levkoff SE, Reilly C, et al. The delirium symptom interview: An interview for the detection of delirium symptoms in hospitalized patients. J Geriatr Psychiatry Neurol. 1992;5:14–21. doi: 10.1177/002383099200500103. [DOI] [PubMed] [Google Scholar]
- 21.Breitbart W, Rosenfeld B, Roth A, et al. The Memorial Delirium Assessment Scale. J Pain Symptom Manage. 1997;13:128–137. doi: 10.1016/s0885-3924(96)00316-8. [DOI] [PubMed] [Google Scholar]
- 22.Simon SE, Bergmann MA, Marcantonio ER. Reliability of a comprehensive delirium assessment utilizing four instruments. Gerontologist. 2001;41 (Suppl):M365. [Google Scholar]
- 23.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Blom G. Statistical Estimates and Transformed Beta Variables. New York: John Wiley & Sons, Inc.; 1958. [Google Scholar]
- 26.Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 27.Rudolph JL, Babikian VL, Birjiniuk V, et al. Atherosclerosis is associated with delirium after coronary artery bypass graft surgery. J Am Geriatr Soc. 2005;53:462–466. doi: 10.1111/j.1532-5415.2005.53165.x. [DOI] [PubMed] [Google Scholar]
- 28.Elie M, Cole MG, Primeau FJ, et al. Delirium risk factors in elderly hospitalized patients. J Gen Intern Med. 1998;13:204–212. doi: 10.1046/j.1525-1497.1998.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubois MJ, Bergeron N, Dumont M, et al. Delirium in an intensive care unit: A study of risk factors. Intensive Care Med. 2001;27:1297–1304. doi: 10.1007/s001340101017. [DOI] [PubMed] [Google Scholar]
- 30.McKhann GM, Goldsborough MA, Borowicz LM, Jr, et al. Cognitive outcome after coronary artery bypass: A one-year prospective study. Ann Thorac Surg. 1997;63:510–515. doi: 10.1016/s0003-4975(96)01057-0. [DOI] [PubMed] [Google Scholar]