Abstract
Life-history theory generally predicts that there should be no selection for longevity beyond the limit of reproductive capacity. However, the capacity to increase fitness may not end when individuals reach a state of functional sterility. Recent studies show that intergenerational transfers of resources from post-reproductive parents can increase the offspring’s fitness, and analytical theory shows that age-trajectories of transfers may shape the course of senescence in social organisms. In eusocial insects, female roles are partitioned so that one phenotype or “caste” reproduces while another is responsible for resource transfers: the reproductive “queens” are arrested in a continuous reproductive mode, while transfer-activities such as hygienic behaviors, guarding, foraging and further food processing (“nursing”) that increases the nutritional value of provisions are conducted by sterile “workers”. Worker honey bees normally perform these tasks in a sequence so that nursing inside the protected nest is conducted prior to more risky exterior hive activities such as guarding and foraging. However, foragers may revert to nurse-activity in response to demographic changes, and worker bees can also develop into a stress resistant survival form with a 10-fold increase in lifespan. This elastic division of parental functions is believed to increase colony fitness. Further, it generates a stage-dependent trajectory of senescence that is difficult to address with established theories of aging. In the following, we show how a recent theory that includes resource transfers can be used to elucidate patterns of senescence in eusocial, non-reproducing individuals like the honey bee worker.
Keywords: Intergenerational transfer, Physiological and chronological age, Eusocial insect
1. Introduction
Social demographers have often dealt with aging and fertility separately from parenting. Yet, a recent contribution to the evolutionary theories of aging that links age-specific patterns of intergenerational transfers to mortality (Lee, 2003), suggests a new approach in this line of research (Rogers, 2003). Lee (2003) focuses on parental investment and transfers of resources between individuals of different ages. The work joins an economic model of exchange between individuals with an evolutionary theory of aging, and has been referred to as the most comprehensive aging theory to date (Rogers, 2003). The formalism can generate strong testable predictions, but an empirical evaluation of the theory requires measurements of transfers that would ideally include numerous care behaviors (e.g., feeding, warming, fanning and guarding) (Lee, 2003). Thus, the divergence between Lee’s formalism and established theories that do not incorporate intergenerational resource transfers is challenging to assess.
Established evolutionary theories of aging, i.e., the mutation accumulation framework (Medawar, 1952) and the antagonistic pleiotropy (Williams, 1957) framework, including the disposable soma theory (reviewed by Kirkwood and Rose, 1991), seek to explain why mortality increases with age. The theories all predict that the principal determinant in the evolution of longevity is the level of extrinsic mortality. If this level is high, life expectancy in the wild is short, and there is little selection for high levels of somatic maintenance; if the level of extrinsic mortality is low, selection would postpone deleterious gene effects and direct greater investment into building and maintaining a durable soma (reviewed by Kirkwood and Austad, 2000). None of these theories incorporate resources transferred to offspring (Lee, 2003), and none of them were explicitly designed to address aging patterns in alloparental care givers, which may be of particular interest to aging research (Amdam and Omholt, 2002; Omholt and Amdam, 2004).
Eusocial organisms are characterized by the presence of a sterile worker caste that engages in alloparental care behaviors such as cleaning, nursing, guarding and foraging. Lee’s (2003) transfer theory predicts that social species with continuing care for offspring can show complex qualitative life-history patterns such as decline in juvenile mortality with age, and selection for reduced fertility as well as higher mortality. The formalism might not fully account for the evolution of sterile worker castes, but trajectories of senescence in a eusocial species may, nevertheless, serve as an important explanatory test-case for this theory of aging. The honey bee (Apis mellifera) is emerging as one key model for research on longevity in eusocial species (Omholt and Amdam, 2004; Rueppell et al., 2004). In the following, therefore, we use patterns of food transfers, senescence and frailty in the honey bee worker caste to exemplify how a theoretical framework that incorporates intergenerational transfers can be meaningfully applied to aging in a eusocial species.
2. Biological background
The honey bee has a long and rich history as an experimental organism. The activities within a bee colony are easy to quantify, and patterns of nursing and food exchange have been studied thoroughly (e.g., Riessberger and Crailsheim, 1997; Crailsheim et al., 1999). Honey bees have three types of colony members: queens, males (drones) and workers. A colony normally consists of 1 egg-laying queen, a few hundred drones and 10,000–30,000 workers during the favorable season. Queens and drones have limited behavioral repertoires, and engage exclusively in tasks related to reproduction. They are cared for by the workers, which are functionally sterile females that take care of the queen’s offspring by performing all remaining parental functions, i.e., foraging, food processing, feeding, warming, fanning and guarding. The workers generally specialize in different tasks sequentially. Activities inside the nest are therefore performed by young individuals (3–45 days old), and more risky exterior hive tasks like guarding and foraging are carried out by relatively older workers (18–65 days old) (Free, 1965). However, workers may also develop into a stress resistant survival form, the “diutinus” stage (Omholt and Amdam, 2004), that survives the unfavorable season. Diutinus workers will engage in nursing and foraging activities the next year, when most of them may be as old as 280 days (reviewed by Omholt and Amdam, 2004). The colony reproduces as a unit through colony fission (production of swarms during the favorable season), and by making drones that mate with virgin queens from other colonies (see Winston, 1987 for a review on honey bee biology).
3. Patterns of age- and stage-dependent food transfers
Bees engage in trophallaxis, which is the transfer of food by mouth from one individual to another. Trophallactic interactions are non-random, and they depend on factors such as sex and age of the receiver and donor, food availability and quality, time of day, weather and season (Riessberger and Crailsheim, 1997; Crailsheim, 1998; Crailsheim et al., 1999). Two main commodities are transferred by trophallaxis: (i) floral nectar, which provides the bees with carbohydrates. Nectar is passed on from returning foragers to bees that consume it, pass it on to other bees or store it as honey in the hive. (ii) Proteinacious royal jelly, which is a nutritious secretion produced by nurse bees. Jelly is the most important source of easy digestible protein for honey bees (Crailsheim, 1990). It is used as provisions for developing larvae and for feeding every adult bee in the colony (Crailsheim, 1992).
All bees are able to consume and digest nectar or honey that is passed to them or stored in the hive, although the individuals that provide this resource, as well as pollen from the field, are older workers on average. The brain-structure of foragers is unique (Fahrbach et al., 1995), and foragers eat less pollen (Jaycox et al., 1974) and store less nutrients in their blood and tissues compared to other bees (Engels and Fahrenhorst, 1974). Individuals that produce jelly are also physiologically specialized, and they are the only colony members that are able to digest pollen well (pollen is the sole source of amino acids for the production of jelly): workers can become nurse bees as 3–5-day-olds when the level of digestive endopeptidases in their guts is high enough to efficiently process ingested pollen (Crailsheim, 1988). However, bees that receive few or no transfers of jelly during the first 3 days of adult life are unable to become nurses (Naiem et al., 1999). Such workers appear to bypass the nurse-stage altogether and start foraging precociously. Bees normally lose the ability to produce jelly when they shift from nursing to foraging, and the foragers’ endopeptidase activity is strongly reduced (Crailsheim, 1988).
The above dynamics generate a temporal transfer pattern (Fig. 1) in which larvae and young adults are exclusive receivers of nectar and proteinacious jelly. Five- to 12-day-old nurse bees receive jelly more often than they donate it, whereas the opposite is true after day 16 (Crailsheim, 1998); this is because nurse bees preferentially donate jelly to any worker that is younger than themselves. Finally, workers end up as exclusive receivers of jelly and exclusive providers of nectar and pollen as they transform into foragers for the colony.
Fig. 1.
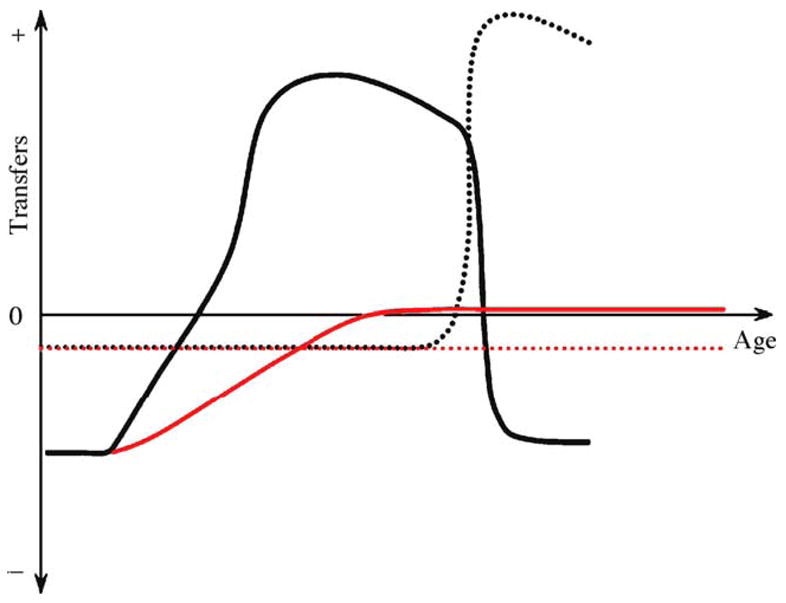
Temporal transfer dynamics in honey bee workers. The black full line is the net transfer of proteinacious jelly by age, and the black dotted line is the net transfer of foraged foods by age. These patterns are typical during the favorable season. The red full line is the net transfer of jelly by a bee that develops into a diutinus worker at the onset of an unfavorable period. The diutinus workers also consume stored honey; thus, the red dotted line depicts this negative net transfer of foraged foods. As conditions improve, the diutinus workers transform into nurse bees and foragers. At this time, they develop the temporal transfer dynamics shown by the black lines.
Nurse bees, but not foragers, consume more pollen during days of bad weather (Riessberger and Crailsheim, 1997). These resources are not transferred, but appear to be stored in the nurse bees’ bodies as storage protein (Amdam and Omholt, 2002). This typically occurs at the end of the favorable season, and an increase in an individual’s storage protein content is linked to the transition to the long-lived diutinus stage (Omholt and Amdam, 2004). Diutinus workers take care of the queen and thermoregulate the colony at 28–35 °C during periods of drought, rain and snow. As soon as conditions improve, they use their protein reserves to nurse new cohorts of larvae (see also Fig. 1). The colony therefore rapidly grows to the point where colony fission, and thus reproduction, is possible (reviewed by Omholt and Amdam, 2004).
4. Patterns of stage-dependent senescence and physiological frailty
Aging in bees may be described as “polyphenic”, which signifies a genome’s ability to produce two or more alternative phenotypes in response to environmental stimuli (West-Eberhard, 2003). This is because the honey bee genome can generate four female phenotypes characterized by separate aging patterns; the queen caste that senesces at negligible rates is one (Finch, 1990), while the other three are the workers’ nurse-, forager-and diutinus-stage (Omholt and Amdam, 2004) (see also Fig. 2).
Fig. 2.
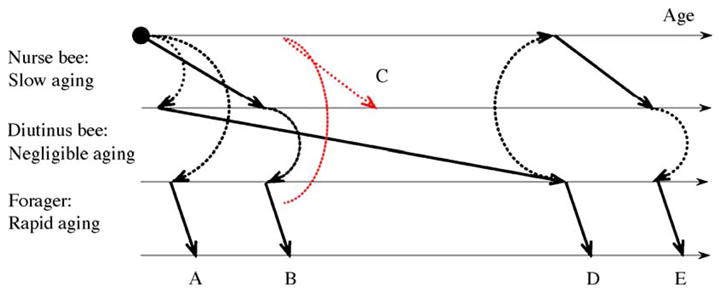
Plastic senescence in honey bee workers. A worker can switch between three phenotypes with distinct aging rates. Inter-individual variation in the timing of these shifts generates aging patterns where the physiological and chronological age of the bees are largely decoupled. (A) A worker that receives little or no transfers of jelly during the first days of adult life will bypass the nurse-stage and become a precocious forager. (B) During the favorable season, a worker will normally engage in nurse-activities before it initiates foraging. (C) Foragers in colonies that experience a dramatic loss of young nurse bees can revert to nursing. Diutinus workers initiate foraging (D) and nurse-activities (E) after the unfavorable season.
As outlined above, nurse bees live within the protected nest and experience low extrinsic mortality risk. Nurses have an upper lifespan of 75–135 days if they are prevented from shifting to foraging activities (Haydak, 1963), and they are characterized by moderate blood levels of the putative storage protein vitellogenin (~30 μg/μl; see subsequent sections for more information about this protein) (Amdam et al., 2004b). Nurse bees also have intermediate numbers of functional immune cells in their blood (4500–8000 μl−1) (Amdam et al., 2004b), and senesce as 30–75-day-olds when their ability to provide for the brood declines (Haydak, 1963; Eischen et al., 1984). Recent research shows that nurse bees are less susceptible to the oxidative-damage agent paraquat than newly emerged bees (i.e., bees that are less than 24 h old) (Seehuus et al., submitted for publication). Oxidative-stress resistance is an important biomarker of physiological frailty, and the results of Seehuus et al. therefore suggest that a bee’s frailty decreases during the first days of adult life.
Workers that perform risky foraging activities appear to senesce rapidly (Tofilski, 2000; Omholt and Amdam, 2004; Amdam et al., 2004b). Their performance as collectors of nectar and pollen may go down after as little as 5 days (Tofilski, 2000). Foragers do not have detectable levels of vitellogenin, and may therefore be deprived of storage protein for self-maintenance (Amdam and Omholt, 2002; Amdam et al., 2003). These bees also have few or no functional immune cells (0–1000 μl−1) (Rutz et al., 1976; Amdam et al., 2004b). The foragers’ frailty to paraquat is considerable, and not significantly different from the strong susceptibility of newly emerged bees (Seehuus et al., submitted for publication). Overall, most foragers survive only 7–14 days (Neukirch, 1982; Visscher and Dukas, 1997).
The diutinus workers, as nurse bees, remain highly protected inside the colony. They are characterized by very high levels of the vitellogenin protein (~100 μg/μl), and high numbers of functional immune cells (8000–10,000 μl−1) (Fluri et al., 1982; Amdam et al., 2004b). Diutinus workers as old as 200 days are resistant to paraquat (S. Seehuus, K. Norberg, U. Gimsa, T. Krekling, G.V. Amdam, unpublished data), and their rate of senescence appears to be negligible (reviewed by Omholt and Amdam, 2004).
5. Implications for established theories of aging
The stage-dependent profiles of senescence and physiological frailty outlined above translate into a plastic aging pattern as workers shift between behavioral roles (Fig. 2). Workers usually perform tasks associated with rapid senescence (foraging) after stages of slow (nursing) or negligible senescence (diutinus function), but foragers are able to revert to nursing activities if a colony’s nurse bees are removed (Huang and Robinson, 1996) (see also Fig. 2C). Moreover, workers of the same age-cohort do not shift from one behavioral stage to another at the same time, and similarly aged bees can develop into both short-lived foragers and long-lived diutinus workers (Huang and Robinson, 1995). This demonstrates that senescence is not a simple function of chronological age in honey bees.
Evolution of stage-dependent aging in non-reproducing individuals is not trivial to explain (Omholt and Amdam, 2004). Specifically, the mutation accumulation and antagonistic pleiotropy theories were designed to account for patterns of senescence in a situation where replicating genotypes are under strong negative selection if they have too many life-shortening- or too many longevity-promoting alleles relative to the level of extrinsic mortality. Aging in functionally sterile eusocial individuals that show temporal division of parental functions is, therefore, beyond the immediate explanatory scope of these frameworks (Amdam and Omholt, 2002; Omholt and Amdam, 2004). The fact that nurse bees and diutinus workers, which are exposed to comparable levels of extrinsic mortality risk, are characterized by different rates of senescence is in accordance with this conclusion.
The disposable soma theory focuses on optimal allocation of resources between reproduction and somatic maintenance, a mechanism that can plausibly generate antagonistic pleiotropy (Rogers, 2003). By exploiting the regulatory dimension of the disposable soma framework, and by describing honey bee workers as somatic cells in a super-organism (i.e., the social insect society; see Oster and Wilson (1978) for further information on this concept), Amdam and Omholt (2002) were able to address aspects of honey bee worker senescence. However, the use of explanatory modules derived from theory on rates of senescence in cell lines is an analogy in the context of organismal aging within a eusocial group. The authors’ rational was also largely based on patterns of resource transfer within the super-organism (also analysed by Oster and Wilson, 1978), which in effect are transfers between individual bees of different stages (reviewed by Omholt and Amdam, 2004). The disposable soma theory may, thus, be less useful as an explanatory framework for honey bee aging than a theory that addresses inter-individual transfers directly.
6. Is aging theory that includes intergenerational transfers valid for eusocial species?
Lee (2003) states that the age-specific selection pressure on mortality depends on a weighted average of remaining fertility and remaining intergenerational transfers to be made to others. The formalism shows that only transfers shape senescence in social species with continuing care for offspring (under the likely assumption of optimal-quantity tradeoff for offspring). This implies that remaining fertility can be tentatively disregarded as explanatory factor for patterns of senescence in social species, and we suggest that Lee’s result also holds for eusocial organisms. Specifically, only transfers shape senescence in social species that are characterized by reproductive division of labor and group level fitness.
Still, Lee’s concept of intergenerational transfers cannot be directly reassigned to eusocial life histories. This is because the term ‘offspring’, and thus ‘generation’, is used differently. Specifically, a factual honey bee offspring at the population level is a colony rather than a bee, although fitness (successful fission plus drone production) is achieved through the parental roles of single individuals. The colony level approach to honey bee reproduction (e.g., Oster and Wilson, 1978) is in most cases both meaningful and correct. We propose, however, that it is less valid as a theoretical basis for aging. This is partly because a concept of “colony- or group-level aging” is an abstraction, and also redundant when the social dimension can be incorporated in a theory of organismal aging. Further, the fact that reproductive efforts by queens and parental investments by workers result in colony level reproduction does not change the fact that individuals give birth and provide care for juveniles. Finally, the concept of intergenerational transfers cannot be assigned to the colony level, unless when used as a metaphor for interactions that occur between individual bees. The honey bee colony is therefore better described as a unit of overlapping generations of individuals, which is an established characteristic of a eusocial group. We think this opens for a consistent use of Lee’s framework.
7. Can transfer theory explain stage-dependent aging?
In the following, we do not engage in an analytical deduction of the force of selection on mortality in the honey bee. Rather, we discuss whether transfer theory can elucidate the patterns of senescence outlined in the previous sections. We assume that the key component that has been driving selection on mortality in honey bee workers at an age a is the remaining lifetime transfers to be made to others after age a (Lee, 2003). Selection will in this case favor the survival of individuals that will make larger investments in others in the future; or equivalently, individuals that embody a larger investment of resources than others are under positive selection for survival (Lee, 2003).
Much of the nutrients in honey bee larvae and pupae are directly recyclable (i.e., through cannibalism of developing brood (Woyke, 1977; Webster et al., 1987)), whereas the newly emerged bee embodies a notable, non-consumable investment. However, 0–5-day-old bees are exclusive receivers of nutritional resources, and considerable amounts of proteinacious jelly have to be invested in them before they can become nurse bees themselves. During the favorable season, the net investment, or remaining lifetime transfers to be made to others, will normally increase until day 12, and then decline after day 16, as the bee becomes a net donor of jelly to younger bees (Fig. 3). When the worker initiates foraging after a period as nurse bee, she becomes one of the colony’s exclusive providers of nectar and pollen. The bee experiences a considerable extrinsic mortality risk as a forager. This implies that the remaining lifetime transfers to be made by any single forager are relatively few. The dynamics change during the unfavorable season, however: the diutinus workers develop as the colony’s production of larvae declines, and they are therefore not required to care for large numbers of younger bees until the following year. Their remaining lifetime transfers to be made to others therefore increase after emergence, and then plateaus out at constant high level throughout the unfavorable period (Fig. 3).
Fig. 3.
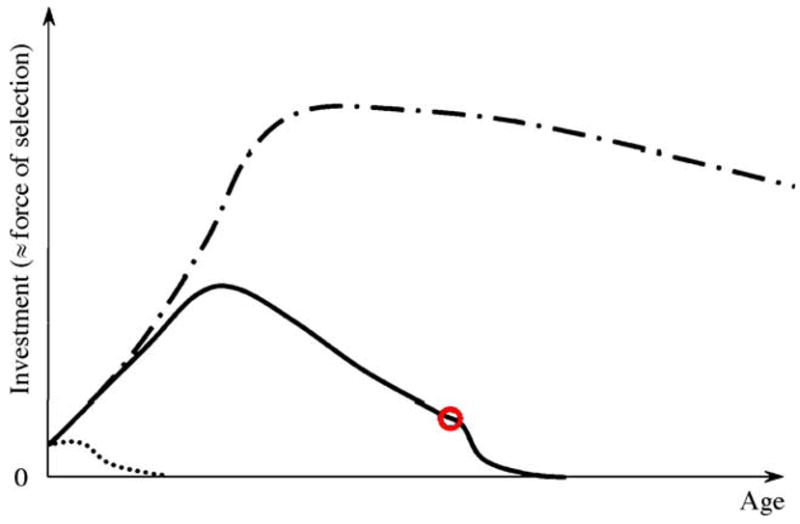
Representation of the investment embodied in honey bee workers. Patterns during the favorable and unfavorable season are depicted by full and dash-dotted lines, respectively. The red circle identifies the relative time of onset of foraging. The dotted line exemplifies the investment in a bee that receives little or no proteinacious jelly after emergence. The dynamics match the relative vitellogenin concentrations of the bees accurately (e.g., Engels and Fahrenhorst, 1974; Fluri et al., 1982; Amdam et al., 2004a).
Accordingly, selection for survival is weaker in the juvenile bee and the forager compared to the age-interval when bees are functional nurses (Fig. 3). Further, the force of selection on the nurse-stage declines with time as the probability of being a nurse instead of a forager at an age a decreases, and less lifetime resource transfers remain. It is also intuitive that the survival of individuals that delay resource transfers until after the unfavorable season is strongly selected for. A stage-dependent pattern of slow aging during the nurse bee stage, rapid aging in foragers and negligible senescence throughout the diutinus stage would therefore emerge if only transfers shape senescence in honey bee workers. Lee’s approach also predicts that newly emerged bees and foragers are frailer than nurse bees, which appears to be the case (Seehuus et al., submitted for publication).
8. Concluding remarks
We have argued that the theory of aging put forward by Lee (2003) is in accordance with the well-built link between temporal parental functions and aging in the honey bee worker caste. Like the more established theoretical models of aging, the formalism was not specifically built to account for patterns of senescence in eusocial species. The outline presented here, thus, suggests that the explanatory scope of Lee’s idea is particularly plastic and can be fruitfully applied over a wide range of levels of social organization. Therefore an interesting parallel study-case may be colonial organisms such as corals. The branching coral Acropora palmata, for example, exhibits rapid growth at the edges of colonies and a higher level of frailty and mortality in basal regions: a pattern that nicely corresponds to distal translocation of nutrients and calcium (Meesters and Bak, 1995).
Yet, the hypothesis that stage-dependent senescence in worker bees is the outcome of selection on transfers does not provide useful information about the evolutionary trajectory of aging in queens and drones (see Keller and Genoud (1997) for more information on the longevity of social insect queens). Moreover, the idea does not present a mechanistic basis for honey bee senescence. Current knowledge on the proximate regulation of honey bee aging, on the other hand, may provide information on how a causal link between transfers and longevity can come about in mechanistic terms. This is because the bees’ transfers of proteinacious jelly are copied by changes in the hemolymph vitellogenin level: the vitellogenin titer increases during the period when workers are net receivers of jelly (from emergence until 10–12 days of age), and declines in older nurse bees that are net donors (Amdam and Omholt, 2002). The pattern appears to emerge because vitellogenin itself is used for jell-production (Amdam et al., 2003). The fact that diutinus workers do not nurse brood has likewise been used to explain why vitellogenin accumulates in this caste (reviewed by Amdam and Omholt, 2002). The production of vitellogenin is finally switched off when the bees initiate foraging (Pinto et al., 2000). It has been suggested that vitellogenin promotes survival in honey bees through a storage protein function (Engels et al., 1990; Amdam and Omholt, 2002), and a positive effect on the bees’ immune system (Amdam et al., 2004b) and level of stress resistance (Amdam and Omholt, 2002; Seehuus et al., submitted for publication). This mechanistic perspective is further compatible with the patterns of longevity in queens and drones: the long-lived queens (lifespan 1–5 years) have very high vitellogenin levels throughout life, including the unfavorable seasons when they do not lay eggs (Engels and Fahrenhorst, 1974). The drones, on the other hand, normally survive for less than 3 weeks and synthesize vitellogenin at basal levels only up until days 7–10 of adult life (Trenczek et al., 1989).
The link between the vitellogenin level and the lifespan of honey bees can be elucidated more explicitly by the finding that vitellogenin gene expression has an inhibitory effect on the juvenile hormone titer of worker bees (Guidugli et al., submitted for publication). Juvenile hormone, which rapidly increases in drones and is found only at basal level in queens, is a systemic hormone that causes accelerated aging in the fruit fly Drosophila melanogaster (reviewed by Tatar and Yin, 2001). In the worker caste, the juvenile hormone level increases rapidly in association with the cessation of vitellogenin production at onset of foraging (Pinto et al., 2000). This may imply that the vitellogenin concentration of a bee communicates the level of investment embodied in her; or equivalently, if the bees’ trajectories of transfers and mortality are joined at the physiological level through the functions of vitellogenin, the protein may exemplify an evolutionary end point connection between investments, transfers and senescence. Yet, future research on social invertebrates, including ants that represent another major eusocial model for aging and longevity (Keller and Genoud, 1997; Chapuisat and Keller, 2002), is needed to establish whether resource transfers have been of importance for the evolution of senescence in social insects.
Acknowledgments
Founding was provided by the Norwegian Research Council, project no. 157851/432 to G.V.A. and NIA PO1 AG 22500 to R.E.P.
References
- Amdam GV, Hartfelder K, Norberg K, Hagen A, Omholt SW. Altered physiology in worker honey bees (Hymenoptera: Apidae) infested by the mite Varroa destructor (Acari: Varroidae): a factor in colony loss during over-wintering? J Econ Entomol. 2004a;97:741–747. doi: 10.1093/jee/97.3.741. [DOI] [PubMed] [Google Scholar]
- Amdam GV, Simões ZLP, Hagen A, Norberg K, Schroder K, Mikkelsen O, Kirkwood TBL, Omholt SW. Hormonal control of the yolk precursor vitellogenin regulates immune function and longevity in honeybees. Exp Gerontol. 2004b;39:767–773. doi: 10.1016/j.exger.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Amdam GV, Norberg K, Hagen A, Omholt SW. Social exploitation of vitellogenin. Proc Natl Acad Sci USA. 2003;100:1799–1802. doi: 10.1073/pnas.0333979100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Omholt SW. The regulatory anatomy of honeybee lifespan. J Theor Biol. 2002;216:209–228. doi: 10.1006/jtbi.2002.2545. [DOI] [PubMed] [Google Scholar]
- Chapuisat M, Keller L. Division of labour influences the rate of ageing in weaver ant workers. Proc R Soc Lond B. 2002;269:909–913. doi: 10.1098/rspb.2002.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crailsheim K. Transport of leucine in the alimentary canal of the honeybee (Apis mellifera L.) and its dependence on season. J Insect Physiol. 1988;34:1093–1100. [Google Scholar]
- Crailsheim K. Protein synthesis in the honeybee (Apis mellifera L.) and trophallactic distribution of jelly among imagos in laboratory experiments. Zool J Physiol. 1990;94:303–312. [Google Scholar]
- Crailsheim K. The flow of jelly within a honeybee colony. J Comp Physiol B. 1992;162:681–689. [Google Scholar]
- Crailsheim K. Trophallactic interactions in the adult honeybee (Apis mellifera L.) Apidologie. 1998;29:97–112. [Google Scholar]
- Crailsheim K, Riessberger U, Blaschon B, Nowogrodzki R, Hrassnigg N. Short-term of simulated bad weather conditions upon the behaviour of food-storer honeybees during day and night (Apis mellifera carnica Pollmann) Apidologie. 1999;30:299–310. [Google Scholar]
- Eischen FA, Rothenbuhler WC, Kulincevic JM. Some effects of nursing on nurse bees. J Apic Res. 1984;23:90–93. [Google Scholar]
- Engels W, Fahrenhorst H. Alters- und kastenspezifische Veränderungen der Haemolymph-Protein-Spektren bei Apis mellificia. Roux Arch. 1974;174:285–296. doi: 10.1007/BF00573233. [DOI] [PubMed] [Google Scholar]
- Engels W, Kaatz H, Zillikens A, Simões ZLP, Truve A, Braun RP, Dittrich F. Honey bee reproduction: vitellogenin and caste-specific regulation of fertility. In: Hoshi M, Yamashita O, editors. Advances in Invertebrate Reproduction. Vol. 5. Elsevier Science Publishers B.V; Amsterdam: 1990. pp. 495–502. [Google Scholar]
- Fahrbach SE, Giray T, Robinson GE. Volume changes in the mushroom bodies of adult honey bee queens. Neurobiol Learn Mem. 1995;63:181–191. doi: 10.1006/nlme.1995.1019. [DOI] [PubMed] [Google Scholar]
- Finch CE. Longevity, Senescence and the Genome. University of Chicago Press; Chicago: 1990. [Google Scholar]
- Fluri P, Lüscher M, Wille H, Gerig L. Changes in weight of the pharyngeal gland and haemolymph titres of juvenile hormone, protein and vitellogenin in worker honey bees. J Insect Physiol. 1982;28:61–68. [Google Scholar]
- Free JB. The allocation of duties among worker honeybees. Zool Soc Lond. 1965;14:39–59. [Google Scholar]
- Guidugli KR, Nascimento AM, Amdam GV, Barchuk AR, Angel R, Omholt SW, Simões ZLP, Hartfelder K. Vitellogenin regulates hormonal dynamics in the worker caste of a eusocial insect. FEBS Lett. doi: 10.1016/j.febslet.2005.07.085. submitted for publication. [DOI] [PubMed] [Google Scholar]
- Haydak MH. Age of nurse bees and brood rearing. Minnesota Agric Exp Sta, Sci J Ser. 1963;5122:101–103. [Google Scholar]
- Huang Z-Y, Robinson GE. Seasonal changes in juvenile hormone titers and rates of biosynthesis in honey bees. J Comp Physiol B. 1995;165:18–28. doi: 10.1007/BF00264682. [DOI] [PubMed] [Google Scholar]
- Huang Z-Y, Robinson GE. Regulation of honey bee division of labor by colony age demography. Behav Ecol Sociobiol. 1996;39:147–158. [Google Scholar]
- Jaycox ER, Skowronek W, Guynn G. Behavioral changes in worker honey bees (Apis mellifera) induced by injections of a juvenile hormone mimic. Ann Entomol Soc Am. 1974;67:529–534. [Google Scholar]
- Keller L, Genoud M. Extraordinary lifespans in ants: a test of evolutionary theories of ageing. Nature. 1997;389:958–960. [Google Scholar]
- Kirkwood TBL, Austad SN. Why do we age? Nature. 2000;408:233–237. doi: 10.1038/35041682. [DOI] [PubMed] [Google Scholar]
- Kirkwood TBL, Rose MR. Evolution of senescence: late survival sacrificed for reproduction. Philos Trans R Soc Lond B. 1991;332:15–24. doi: 10.1098/rstb.1991.0028. [DOI] [PubMed] [Google Scholar]
- Lee RD. Rethinking the evolutionary theory of aging: transfers, not births, shape senescence in social species. Proc Natl Acad Sci USA. 2003;100:9637–9642. doi: 10.1073/pnas.1530303100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medawar PB. An Unsolved Problem of Biology. H.K. Lewis; London: 1952. [Google Scholar]
- Meesters EH, Bak RPM. Age-related deterioration of a physiological-function in the branching coral Acropora Palmata. Mar Ecol Prog Ser. 1995;121:203–209. [Google Scholar]
- Naiem E-S, Hrassnigg N, Crailsheim K. Nurse bees support the physiological development of young bees (Apis mellifera L.) J Comp Physiol B. 1999;169:271–279. [Google Scholar]
- Neukirch A. Dependence of the life span of the honeybee (Apis mellifera) upon flight performance and energy consumption. J Comp Physiol. 1982;146:35–40. [Google Scholar]
- Omholt SW, Amdam GV. Epigenic regulation of aging in honeybee workers. Sci Aging Knowl Environ. 2004;26:pe28. doi: 10.1126/sageke.2004.26.pe28. [DOI] [PubMed] [Google Scholar]
- Oster GF, Wilson EO. Caste and Ecology in the Social Insects. Princeton University Press; Princeton: 1978. [PubMed] [Google Scholar]
- Pinto LZ, Bitondi MMG, Simões ZLP. Inhibition of vitellogenin synthesis in Apis mellifera workers by a juvenile hormone analogue, pyriproxyfen. J Insect Physiol. 2000;46:153–160. doi: 10.1016/s0022-1910(99)00111-0. [DOI] [PubMed] [Google Scholar]
- Riessberger U, Crailsheim K. Short-term effect of different weather conditions upon the behaviour of forager and nurse honey bees (Apis mellifera carnica Pollmann) Apidologie. 1997;28:411–426. [Google Scholar]
- Rogers AR. Economics and the evolution of life histories. Proc Natl Acad Sci USA. 2003;100:9114–9115. doi: 10.1073/pnas.1733942100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueppell O, Amdam GV, Page RE, Carey JR. From genes to societies. Sci Aging Knowl Environ. 2004;5:pe5. doi: 10.1126/sageke.2004.5.pe5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutz W, Gerig L, Wille H, Lüscher M. The function of juvenile hormone in adult worker honeybees, Apis mellifera. J Insect Physiol. 1976;22:1485–1491. [Google Scholar]
- Seehuus SC, Norberg K, Gimsa U, Krekling T, Amdam GV. Reproductive protein protects sterile honey bee workers from oxidative stress. Proc Natl Acad Sci USA. doi: 10.1073/pnas.0502681103. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Yin CM. Slow aging during insect reproductive diapause: why butterflies, grasshoppers and flies are like worms. Exp Gerontol. 2001;36:723–738. doi: 10.1016/s0531-5565(00)00238-2. [DOI] [PubMed] [Google Scholar]
- Tofilski A. Senescence and learning in honeybee (Apis mellifera) workers. Acta Neurobiol Exp. 2000;60:35–39. doi: 10.55782/ane-2000-1323. [DOI] [PubMed] [Google Scholar]
- Trenczek T, Zillikens A, Engels W. Developmental patterns of vitellogenin haemolymph titre and rate of synthesis in adult drone honey bees (Apis mellifera) J Insect Physiol. 1989;35:475–481. [Google Scholar]
- Visscher PK, Dukas R. Survivorship of foraging honey bees. Insectes Soc. 1997;44:1–5. [Google Scholar]
- Webster TC, Peng Y-S, Duffey SS. Conservation of nutrients in larval tissue by cannibalizing honey bees. Physiol Entomol. 1987;12:225–231. [Google Scholar]
- West-Eberhard MJ. Developmental Plasticity and Evolution. Oxford University Press; New York: 2003. [Google Scholar]
- Williams GC. Pleiotropy, natural selection and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- Winston ML. The Biology of the Honey Bee. Harvard University Press; Cambridge: 1987. [Google Scholar]
- Woyke J. Cannibalism and brood-rearing efficiency in the honeybee. J Apic Res. 1977;16:84–94. [Google Scholar]


