Introduction
Aging is a universal process in living organisms, yet its rate among organisms is highly varied. A few of the identified mechanisms of aging have proven to be general (or public) to a number of species, including humans. Therefore, the use of a diverse array of species in research on the biology of aging has been advocated repeatedly (1–3), and the strength of comparative analyses has recently become apparent (4,5). The classic model organisms yeast (Saccharomyces cerevisiae), nematodes (Caenorhabditis elegans), fruit flies (Drosophila melanogaster), and laboratory rodents have played the central role in research on aging and life span (6). These models have many advantages, such as knowledge about husbandry and basic biology, availability of diverse stocks, short life spans, and an array of genetic tools and technologies that have substantially improved our understanding of aging, ranging from the molecular to the individual level (4,6). However, despite the advantages of these models and their biological and phylogenetic diversity, they collectively lack certain important biological characteristics reflected in a broader array of organisms (1). One central life history characteristic that is missing in all of the classic model organisms is sociality, which we believe is crucial in the context of research on aging(7)
Here we argue that social insects, one of the few groups of organisms that can compete with the broad ecological and evolutionary success of the human species (8), will make important contributions to our overall understanding of aging in biological systems across all levels of biological organization. The experimental opportunities will be of particular value in deciphering the effects of social systems on aging in various organisms, including humans (7). To support our claims, we outline three key reasons that coincide with different research approaches in the study of aging and demography in social insects.
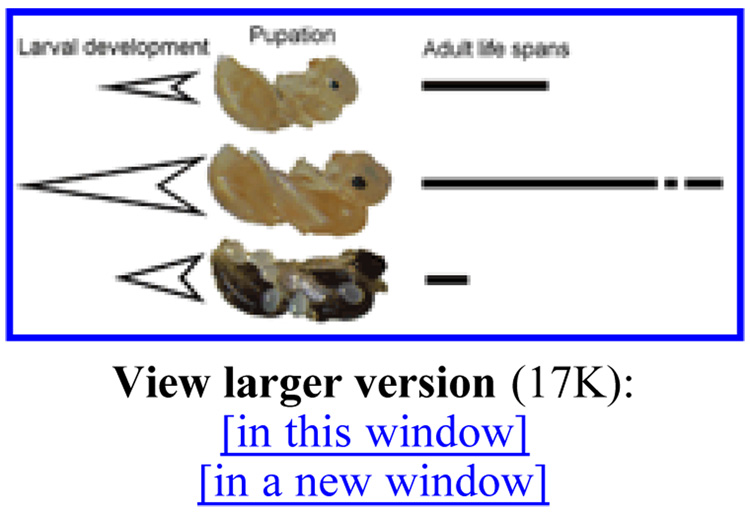
First, social evolution has shaped facultative gene expression programs capable of producing an array of discrete phenotypes from identical genotypes [for example, see (9,10)] (Fig. 1). These phenotypes differ profoundly in development, behavior, morphology, and longevity (11) and have mortality and life span characteristics that are the end results of interactions between inherent gene expression profiles and extrinsic mortality risks (12). Life spans may vary up to 100-fold between the different castes (13). In contrast to the mutant phenotype studies performed with classic model organisms, the genetic architecture of the differences in longevity observed in social insects has evolved naturally. In addition, the determination of causes of longevity differences among social castes should be more feasible and realistic than investigations of the proximate causation of longevity differences among species.
Fig. 1.
Derived from the same genomes, the pupae of ant workers (top), ant queens (middle), and males (bottom) have developed at different speeds, and they will give rise to adults that differ dramatically in life span. The width of the arrows represents larval size increase and their length represents the duration of larval development. The bars on the right represent adult life span, with the queen bar interrupted to indicate their much longer life spans.
As an example, Leptothorax rugatulus is shown. The pupae are 1 to 2 mm long. Eggs, laid by a queen, are attached to the male pupa.
Second, the evolution of a social system and kin structure has introduced new factors that have a direct bearing on aging and mortality, including cooperation and conflict [although the social life style promotes cooperation on a number of tasks, conflicts of interests exist over some aspects of colony life, such as reproduction, that are resolved over time through aggression or manipulation (14)], resource transfers [among colony members, resources are exchanged, for example in the form of nutrition or care (15)], and polyethism [behavioral specialization (16)]. Social evolution also adds a unique level in the hierarchical organization of biological systems: Colonies that are made up of semi-autonomous individuals. These colonies have been regarded as superorganisms (17–19) that offer a useful balance between the integrity of the whole system and experimental accessibility and manipulation of the particular units (20,21). The interpretation of individual rates of aging within the superorganism concept may provide unique tests of the various theories of aging (3,22).
Third, social insects provide unprecedented opportunities for probing mechanisms of aging over a range of social configurations. This is because the group as a whole displays a vast diversity in social organization, life histories, and life spans (16,23). Collectively, termites, ants, wasps, and bees range from facultative sociality (some members of a species are solitary, whereas others form social groups) to unicoloniality (the whole population acts as one cohesive social unit without discrimination among colonies) (24). Within each social setting, caste demography and polyphenism, mortality risks, resource flows, and life span differentials can vary dramatically (12, 13, 24). Specific life history phenomena with a bearing on aging that are exclusively found in social species include social parasitism (exploitation of a societal system or its resources by members of a different species to the detriment of the first, social species), facultative sterility of the majority of individuals, and extreme behavioral specialization.
We suggest that the above considerations, combined with the availability and ecological importance of social insects, put aging and biodemographic research within this group in a prime position to contribute to future scientific progress.
Intraspecific Variability of Longevity
In the social Hymenoptera, which include bees, ants, and wasps, males are typically short-lived as compared to the female queens and workers (1). The male drones arise parthenogenetically from unfertilized eggs; thus, both sexes contain the same genetic material, although males are haploid and females diploid (25). In the other major group of social insects, the termites (Isoptera), sex appears to be determined by sex chromosomes akin to the XY system in humans (26). The termites are a separate case from Hymenoptera because they are hemi-metabolous (developing gradually without metamorphosis during a pupal stage) insects living in colonies that contain a complex mixture of males and females at various developmental stages. At the individual level, termites demonstrate great physiological and morphological flexibility. They may thus be of substantial value in aging research (27), for example, through studies on age progression in juvenile stages that have been arrested at various stages of development. However, this group is challenging experimentally; thus we focus our discussion on the social Hymenoptera.
In the social Hymenoptera, females can develop into different castes, a process that is generally triggered by epigenetic factors. In most wasps, female castes directly reflect different physiological states that result from differences in nutrition. In bees and many ants, castes are produced by discrete developmental cascades triggered by epigenetic factors, such as food. Thus, most of the intraspecific variability is generated by environmental factors that result in differential (developmental) programs characterized by different gene expression profiles (9, 10). This is somewhat analogous to the differentiation of cell types in multicellular organisms, with workers constituting the soma and reproductively active individuals representing the germ line of the superorganism (22). The replicating tissue (queens) may live 100 times longer than the units of the soma (workers). This is an astonishing example of how the same nuclear genome can be programmed during development to yield very short-lived or very long-lived adults that represent the extremes in the spectrum from rapid to negligible senescence (1).
The variation in longevity among the different worker castes is as intriguing as the differences linked to reproductive division of labor. In honeybees, mortality rates change dramatically with the transition from nursing to foraging tasks (28). The temporary worker castes (nurses and foragers) are morphologically similar, and their rate of senescence changes in response to switches in behavior and physiology (1, 29). Workers performing in-hive duties may live more than 130 days if they remain continuously exposed to larvae in a colony where the emergence of new workers is prevented (30). Similarly, bees that emerge in late summer and autumn (so-called winter bees) show little foraging activity and may live for 6 to 8 months (31). In comparison, workers in the spring and summer age rapidly after their transition from hive bees to foragers and die within 1 to 2 weeks (29).
This dramatic change in longevity potential is presumably driven by switching genetic circuitry that results in specific physiological changes. When nurses switch to foraging, their cellular defense machinery is down-regulated by a dramatic reduction in the number of functioning haemocytes (immunocytes, cells of the immune system) (32). At the same time, the yolk precursor vitellogenin, which constitutes a substantial proportion of the storage protein fraction in the nurse bees, is down-regulated to undetectable levels (33). The exact effects of these changes on aging have yet to be determined. However, we believe that this concerted drop in somatic maintenance provides an entry point for research on regulatory mechanisms that underlie differential rates of aging in behavioral as well as morphological castes.
Another level at which caste differences must be addressed is the cellular level. Most cells in insects are postmitotic and undergo mechanical senescence (34). Thus, the extremely long-lived, sexually reproductive females (23) should have efficient DNA repair and molecular protection mechanisms. Alternatively, cells may be replaced more frequently than previously thought. In fact, even in the shorter-lived worker bees, mitosis does occur in the intestinal crypts (35) and possibly in the central nervous system (36, 37). Strong differences in the proliferation capacity of the various cell types are to be expected among the castes and sexes of social Hymenoptera if their longevity is due to cell replacement.
With respect to cellular senescence, it is also noteworthy that queens mate only at the beginning of their relatively long lives and store sperm in a specialized spermatheca to inseminate their eggs as they are laid throughout their existence. Thus, the sperm cells survive their originators (males die after copulation) without refrigeration for years, a feat that human biotechnology has yet to achieve (38, 39). Although the preservation mechanism is not yet understood, it is clear that, apart from intrinsic properties of the sperm cells, the controlled environment of the spermatheca serves a critical role, because isolated sperm suffer a dramatic reduction in survivability (39, 40). This form of environmental facilitation of longevity is paralleled at the superorganism level by the social facilitation of queen longevity, which will be discussed in the next section.
Regardless of the level of biological organization, two important distinctions have to be kept in mind when working in the framework of comparative analysis: (i) What factors are correlative and which are causal for longevity differences? (ii) Among the causal factors, which are responsible for initial (and overall) differentiation between groups [determination of group membership; for example, the primary sex determination signal, the csd gene in honeybees (25)], and which specifically generate different patterns of life span? Castes differ in numerous aspects of their physiology and behavior that may not be directly related to their longevity; thus, we expect that many genes are differentially expressed that have no direct connection to aging rate or life span.
Widely applicable genetic and genomic approaches that have recently become available are of crucial importance for investigating the molecular basis of the different rates of aging in social insects. Their combination will facilitate comprehensive insight into the molecular mechanisms that underlie the huge, yet naturally evolved, life span differences among social insect castes. First and foremost, the honeybee genome sequence (41), particularly after full assembly and annotation, will provide crucial background information for gene identification and will enable all subsequent analyses. Complete sequence information will help identify candidate genes and genetic markers (microsatellites, single-nucleotide polymorphisms, etc.) and allow the construction of honeybee microarrays to compare large-scale gene expression patterns across sexes, castes, and ages.
Although the latter will be helpful in the identification of numerous differentially expressed genes at various life stages of long-lived and short-lived individuals, it will be difficult to pinpoint the specific genes responsible for different aging rates. It is important to note that the time window selected for investigation is critical, because the expression of genes that lead to permanent morphological or physiological change may be restricted in both time and physiological space (22). Functional analyses with small inhibitory RNA molecules (25) or the transfer of genes (and thus genetic effects) to laboratory strains of Drosophila will be essential in establishing genetic causation. Finally, specific expression studies of candidate genes may also be a fruitful avenue to understanding the caste differences in longevity, which must ultimately derive from differential gene expression, but this approach will not lead to the discovery of new mechanisms of the evolution of extreme longevity.
Social Dimensions of Aging
Average life expectancy clearly differs between castes and sexes, but more fundamental are the distinct roles of the individuals in societal organization that ultimately determine not only the rate and pattern of their own mortality, but general colony demographics. In social Hymenoptera, males serve an exclusively reproductive function, which occurs when males leave their colonies (during "mating flights"). While in the colony, males represent a sink for communal resources. In many ant species, mating flights occur only once, and males are not readmitted into their colonies, leading to their rapid death. In contrast, males of some bee and wasp species fly out repeatedly until they die because of mating or other causes. Little is known about the social regulation of male life history, except for factors that influence their rearing in the context of sex ratio theory (42) and their elimination at the end of the reproductive season (a phenomenon that could be paraphrased "social apoptosis").
Queens of social insects are also raised in the colony as part of the reproductive effort and generally leave the colony only to mate. The mortality rate during mating and subsequent colony founding is orders of magnitude higher than during all other stages of the queen's life (43). Once queens are established in a colony, they are shielded from many external mortality hazards and produce new colony members: sexuals (queens and males for reproduction) and workers. Queens of social insects are morphologically specialized for reproduction. Compared to females of nonsocial Hymenoptera, their enlarged abdomen contains oversized ovaries, and they reach egg-laying rates of up to 3000 eggs per day in the honeybee (44) and 50 million per year in the African army ant Dorylus (45). At the same time, these queens may live between 2 and more than 20 years (11, 16, 23), which is over 100 times longer than the life spans of their sons and sterile daughters (the workers).
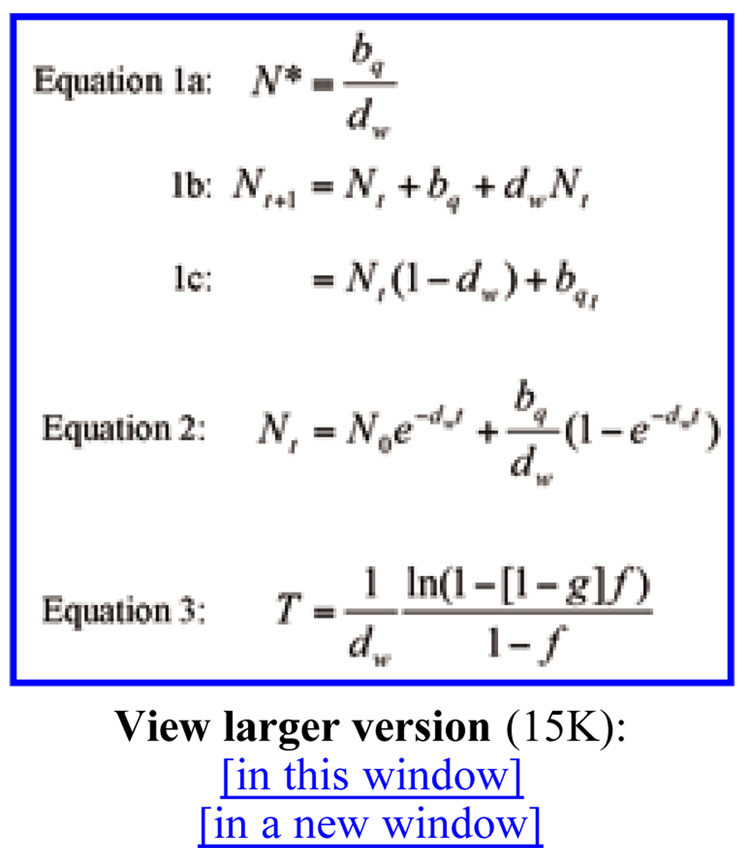
This high reproductive output is necessary to allow for replacement of dying workers and for colony growth. The colony (superorganism) grows and reproduces according to birth and death rates at both lower (workers and queens) and higher (colony) levels of individualism (46). The relation of individual- and colony-level demography was developed by Carey (46) and Tuljapurkar et al. (47), who noted that eusocial insect colonies constitute a special kind of population in that virtually all births are directly attributable to a single individual (the queen) whereas virtually all deaths are attributable to the group (workers). Thus, unlike populations in which each female has the potential to reproduce, the contribution toward growth through births owing to the queen is offset by the sum of death in the population of workers. This relation is given as shown in Eq. 1 (Fig. 2), where N*,bq, and dw denote the saturation (maximum) population of workers, the daily egg production rate of the queen, and the per capita death rate of workers, respectively. Two implications emerge: (i) An upper limit for colony size must exist, owing to demographic constraints; and (ii) once N* is reached for a colony, the only way for growth to continue is by the addition of more reproductives either to stay within the colony or to swarm (to create a new colony).
Fig. 2.
The following equations describe the hierarchical population model of social insects (see text). For Eq. 1, N*, bq, and dw denote the saturation (maximum) population of workers, the daily egg production rate of the queen, and the per capita death rate of workers, respectively. For Eqs. 1a, 1b, and 2, bq, and dw are as above, t denotes time, and N0 is the initial colony size. In Eq. 3, Ns is the swarming size (the number of nonreproductives at which the colony will issue a swarm); f is the swarming ratio, which is the number of workers that leave with the swarm (Ns), relative to the maximum number possible (N*); and the swarming fraction is denoted (1 - g), which is the proportion that remains with the original colony N0, relative to the number in the colony at swarming Ns. The generation time T for an established colony may be defined as the time interval in which the numbers increase from the initial N0 to the swarming size Ns. For the basic model, T is determined by setting t = infinity and Nt = Ns in Eq. 2 to get Eq. 3.
The basic model to describe the within-colony dynamics is given by Eqs. 1a and 1b (Fig. 2), which results in the time course of the population being given by Eq. 2 (Fig. 2), where t denotes time and N0is the initial colony size. Note that Eq. 2 is different from the discrete version of the Malthusian equation (which describes unlimited population growth), because the birth rate is independent of population number. Thus, population regulation is intrinsic to a colony with one reproductive (or any fixed number). Note also that as t becomes large, the colony size climbs toward the saturation number [Eq. 1 in Fig. 2]. Thus, N* is rederived and is equal to the earlier derivation.
This basic model is extended to incorporate colony-level dynamics by considering the following concepts: the swarming size Ns, given by the number of nonreproductives at which the colony will issue a swarm; the swarming ratio f, which is the number of workers that leave with the swarm, Ns, relative to the maximum number possible, N*; and the swarming fraction, denoted (1 - g), which is the proportion that remain with the original colony N0, relative to the number in the colony at swarming, Ns. Thus, generation time T for an established colony may be defined as the time interval T in which the numbers increase from the initial N0 to the swarming size Ns. For the basic model, T is determined by setting t=infinity and Nt = Ns in Eq. 2 to get Eq. 3 (Fig. 2).
The notable feature of Eq. 3 is that it relates the demographic parameters that describe events within colonies to the generation time that controls the rate of creation of new colonies. This is the crux of what is referred to as a hierarchical demographic relationship. This equation describes the quantitative tradeoffs between swarming size, offspring size, birth rate, and death rate in determining generation time. Note that insects with a high death rate will produce offspring colonies at a faster rate than will insects with a low death rate. Note also that a colony can reproduce very frequently by swarming at low swarm numbers and by putting most of the colony's individuals in the offspring colony.
The importance for research on aging of this hierarchical population model of social insects is that it provides important insights into how individual-level birth and death rates change colony-level dynamics. One of the most counterintuitive results of this model is that, when the egg production rate of an individual queen is fixed, lower rates of aging in workers will decrease rather than increase the growth rate of the colony because the threshold for swarming (fission) is increased. Because the hierarchical population model is a multilevel extension of the basic Lotka model, it possesses many of the same properties as other stable population models, as well as some unique ones. That is, social insect populations structured by individual age within a colony as well as by age of colony that are subject to a fixed regime of worker survival rates, queen reproduction, swarming thresholds, and colony survival rates will eventually attain a stable individual age-by-colony distribution, a stable distribution of colonies by age, and a constant rate of colony increase. The resulting population will consist of a fixed overall age distribution of individual workers within a fixed distribution of differently aged colonies.
We believe that this demographic model has several implications for research on aging. First, queens and workers in colonies are the insect analogs of mitotic and postmitotic cells in culture. If the senescence phase (phase III) is characterized as the cessation of cell growth in fibroblast cell cultures used to study replicative senescence, then at least part of the reduction in cell culture growth is likely due not to the disappearance of dividing cells but to the dilution of the culture with postmitotic cells (48). The ratio of reproductives (mitotic cells; queens) to nonreproductives (postmitotic cells; workers) decreases as the size of the population (culture; colony) increases, so that the number of births (new cells; eggs) equals the number of deaths, and, thus, the growth rate is zero. Second, the social insect model has the potential to shed important light on the relation among aging at several levels, including the aging of components (cells; workers), the germ line (queen; gonads), and of the whole (individual; colony). For example, the social insect biodemographic model revealed that the death rate of worker bees is a primary life history trait of individuals that determines colony growth rate. This is because the lower the death rate of workers, the greater the fraction of new births that will contribute to growth rate and not simply to replacement. The same relation between death rate and aging at lower levels of biological organization for individuals might apply.
In contrast to their nonsocial relatives, social insect queens have overcome the negative association of reproduction and life span [known as the longevity cost of reproduction (1)]. In some cases, assuming a reproductive role even enhances longevity (49). However, the longevity of the reproductively active animals is facilitated through the transfer of some reproductive costs to nonreproductive colony members: The workers assist the queen in multiple ways, an overall process that is probably best described as the "social facilitation of longevity." Queens of some species are constantly attended by a retinue of workers for protection and hygiene (16, 50) (Fig. 3). They live in a climate-controlled environment erected mostly by the workers (20, 51). Moreover, queens are fed preprocessed high-quality food in the form of either nutritional worker eggs or proteinaceous secretions produced by adult workers or worker-destined larvae. In honeybee workers, these secretions are partly derived from the yolk protein vitellogenin (52), which is produced in large amounts by nurse bees (53). This non-oogenic use of vitellogenin for provisioning of the queen clearly shows that the regulatory machinery for oocyte maturation has been exploited by natural selection to enable sterile workers to enhance the reproductive output of the colony (52). Through the collection of nectar and pollen and the conversion of these resources to jelly used to feed the queen and her brood, honeybee workers take on a major part of the overall reproductive cost, and this might ultimately contribute to their relatively short life span. It is currently unclear whether social insect queens senesce. Most queens are killed by their own workers when they show signs of fertility decline, the most common cause of which seems to be the depletion of stored sperm (54).
Fig. 3.
Within the hive, the honeybee queen is always attended by a number of workers who feed and clean her, adding further protection from environmental hazards.
The fact that sterile workers live much shorter lives than reproductive queens cannot be explained by individual life history theory (55). However, it is satisfactorily explained when one regards the workers as disposable soma in the colony superorganism (18, 20). In order to minimize colony resource loss, the physiology of the superorganism should allow tight regulation of the investment of resources in the maintenance of individual somatic units (for example, by resource transfers), according to the extrinsic mortality risk of the units. Because foraging is risky (56), colony investments in foragers should be restricted, further reducing the life span of these individuals. This seems to be the case in ants (12). The temporal nature of the division of labor and the seasonal colony life cycle in temperate honeybees lead to the prediction of a facultative worker age-determination mechanism (22) in the framework of the disposable soma theory. Because of its role in somatic maintenance, the vitellogenin titer of honeybee workers may play a central regulatory role in the generation of such a flexible life span-determination machinery (22). The link between vitellogenin levels and longevity in honeybees also presents an interesting comparison to the role that serum lipoproteins play in human aging (57).
The social context of life span can be dissected in the social insects by an array of available methods. Their colonies provide countless opportunities for manipulating social organization [also called sociotomy (58) or pseudomutation of the superorganism (59)] and studying the effects of the changes on individual mortality rates. The ratio of age classes (for example, of brood to nurses) can be changed, stored resources controlled, and reproduction manipulated. Various colony age structures can be created with far-reaching consequences (for example, in honeybees, uniform worker age results in precocial foragers and overaged nurses) (60). In addition, group size can be manipulated (61); social signals altered (such as brood pheromone) (62); the influence of nature and nurture dissociated by cross-fostering (63); and the genetic composition of colonies controlled by artificial insemination, at least in some species (44). Finally, the social context can be exploited to manipulate nutritional status, work load, social connectedness, mechanical wear, and activity patterns at the individual level to study the influences of these parameters on mortality rates.
The Variety of Lifestyles
Natural selection has resulted in tremendous variability in social organization and life history within and among social Hymenoptera species, allowing the use of a wide variety of comparative approaches to gain insight into the evolution of aging (13,23,64). It would be especially valuable to investigate the relation between degree of sociality and the differences in life span between workers and queens. Morphological and reproductive caste divergence and colony size are variable parameters that need to be studied. Another important aspect is the number of reproducing queens in a given colony. Particularly in ants and wasps, colonies with several or many queens are found (polygyny) instead of single-queen colonies, exemplified by the honeybee. In ants, the societal parameter "queen number" correlates negatively with queen longevity among species (23). However, carefully designed studies within species will be necessary to disentangle the cause and effect of this correlation. The presence of several queens per colony indicates an important change in reproductive tactic. In most polygynous systems, workers become part of the future reproductive investment, because new queens return to their natal colony, either to serve as additional egg-layers or to bud off with a part of the workers to form a new colony. In both cases, workers increase the fitness of the new queens directly (65). Thus, in contrast to queen longevity, workers longevity is expected to show a positive correlation with queen number. Such findings reveal that reproductive tactics create interesting predictions at the individual level, and they can also be related to mortality at the colony level (65,66).
Social dominance hierarchies are a consequence of reproductive competition within multiqueen societies (there is sometimes also competition among workers) (14). These hierarchies, initially established by physical aggression, are maintained over extended time periods (up to a year). The effect on individual longevity of individual social rank or within-group intensity of social competition can thus be addressed. As in vertebrates, social rank seems to be correlated with hormone titers in social insects (67) and tied to reproductive output (14), but these correlations can be overcome experimentally (68). It is therefore possible to decouple social rank and reproduction and study their effects on aging separately. Good candidates for these kinds of studies would be Ponerine ants (49), in which workers have retained, over evolutionary times, full reproductive capacity. After queen death, social dominance among workers determines who assumes the reproductive role in a given colony, and in some species queens do not exist at all.
Another special opportunity to study reproductive effects on longevity is provided by species in which some workers reproduce, such as the Cape honeybee (Apis mellifera capensis). Its reproductive workers have been compared to cancer cells on the basis of their proliferation propensity (69), and it would be interesting to know whether this analogy can be extended to their aging pattern as well. Likewise, in other races of honeybees and in many wasp and ant species, workers start to become reproductively active when kept without a queen (70), and it would be interesting to decipher the effect of reproduction on their aging pattern. The longevity consequences of facultative reproduction of workers have not yet been experimentally addressed. Honeybee workers from queenless colonies are believed to be long-lived (71). However, it is unclear whether this is due to low brood numbers or to their own reproductive activity.
The variety of lifestyles and ecological specialization has led to the evolution of physically distinct worker castes in many ant species (16). Different worker castes specialize in different tasks within the colony, but they also differ in body size, often allometrically (that is, a disproportionate growth of some body parts leads to changes in absolute size and in size relative to other body parts). Although one simple comparison between castes has already produced interesting results (12), the full potential of research on physical castes has not yet been explored. Allometric growth may prove to be particularly interesting, because the link between the relative size of different organ systems or relative tissue mass may be related to life span. On the other hand, morphologically homologous castes, such as seed millers and soldiers in the ant genus Pheidole (72), have very different tasks with distinct extrinsic mortality risks that should select for dissimilar aging patterns. Likewise, the repletes of honeypot ants, a caste that serves as living food receptacles (16), are expected to reach much older ages than other workers, purely due to their societal role, presumably without a genetic component. Numerous ant species exist with extreme intraspecific size variation (up to 500-fold) (16) that is often related to behavioral differentiation. Experimentally, it is possible to gain insight into the (separate) effects of behavioral and morphological parameters (for example, size) on aging within species, independent of factors that confound comparative analyses among different species.
Probably unique to humans and social insects is the phenomenon of slave making, which results in some interesting demographic predictions. Slave making is one of the many forms of social parasitism found in social insects (16). One species (of ants) raids other species' nests to steal some of the brood, which are subsequently raised and kept in the slave-maker colony to perform housekeeping tasks (16). The slave survival value for the slave-makers depends on the cost of acquisition (energy and time invested in raiding other colonies). Thus, we predict that the longevity of slaves in slave-maker colonies is dependent on the abundance of colonies that can be raided. The more colonies to raid that surround a slave-making colony, the more dispensable individual slaves become, and thus manipulation by the slave-makers should maximize the short-term utility rather than longevity of slaves. Because the slaves' fitness is zero, their life span characteristics have to be regarded as the outcome of an interaction between their nonenslaved phenotype and the slave-maker colony environment. If housekeeping tasks are costly, we expect that slave-makers who exist in colonies with many slaves will live longer than those with few slaves, especially in slave-making species whose workers cease most activities (except for slave raiding) after slaves are captured (73). Furthermore, socially parasitic queens, which enter alien colonies and exploit the existing work force for their own reproduction, produce fertile offspring much earlier than their nonparasitic counterparts and consequently should be much shorter-lived.
The lifestyle of social insects also makes them particularly suitable for biology of aging research in two further respects that are only indirectly related to their sociality. First, many social insects are suitable for demographic studies from a practical perspective because they can be kept under quasi-natural conditions, and these artificial observation nests allow for detailed monitoring of behavior and reliable censuses, as all social insects return regularly to their colony while alive. Second, the ecological success of some species that have become worldwide pests means that they live under very different ecological circumstances in which to study processes of local adaptation and differentiation. Life span, as a highly complex, integrative trait might be challenging, but it is certainly an interesting one to study in the hopes of understanding the evolution of life histories. Probably the best example of geographically varying life histories in social insects that lends itself to a comparative analysis are the different honeybee races (50). However, these studies are at a descriptive level, and the subsequent studies that will further our understanding of mechanisms of aging have not yet been conducted.
Conclusions
Social insects offer unique opportunities for experimental research on the biology of aging and biodemography that cannot be addressed in any of the classic model organisms. The recent development of broadly applicable genetic tools, genomic information, and general theories of aging at the molecular level provide many opportunities to investigate aging in social insects. Above we have outlined some approaches to such an endeavor. Although it is important to keep a clear view of the differences between insect and human social evolution, we are confident that the information gathered through studies of social insects will broaden our general understanding of the aging phenomenon and can be used to formulate more specific research hypotheses to help us understand aging in humans.
References
- 1.Finch CE. Longevity, Senescence, and the Genome. Chicago, IL: Univ. of Chicago Press; 1990. [Google Scholar]
- 2.Masoro EJ. In: Encyclopedia of Gerontology. Birren JE, editor. vol. 2. San Diego, CA: Academic Press; 1996. [Google Scholar]
- 3.Kirkwood TBL, Austad SN. Why do we age? Nature. 2000;408:233–238. doi: 10.1038/35041682. [CrossRef][Medline] [DOI] [PubMed] [Google Scholar]
- 4.Haussmann MF, Winkler DW, O'Reilly KM, Huntington CE, Nisbet IC, Vleck CM. Telomeres shorten more slowly in long-lived birds and mammals than in short-lived ones. Proc. R. Soc. London Ser. B. 2003;270:1387–1392. doi: 10.1098/rspb.2003.2385. [CrossRef][Medline] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am. J. Clin. Nutr. 78;2003:361–369. doi: 10.1093/ajcn/78.3.361. [Abstract/Free Full Text] [DOI] [PubMed] [Google Scholar]
- 6.Partridge L, Gems D. Mechanisms of aging: Private or public? Nat. Rev. Genet. 2002;3:165–175. doi: 10.1038/nrg753. [CrossRef][Medline] [DOI] [PubMed] [Google Scholar]
- 7.Carey JR, Judge DS. Life span extension in humans is self-reinforcing: a general theory of longevity. Pop. Dev. Rev. 2001;27:411–436. [CrossRef] [Google Scholar]
- 8.Wilson EO. Success and Dominance in Ecosystems: The Case of the Social Insects. Germany: Ecology Institute, Oldendorf/Luhe; 1990. [Google Scholar]
- 9.Evans JD, Wheeler DE. Differential gene expression between developing queens and workers in the honey bee, Apis mellifera. Proc. Natl. Acad. Sci. U.S.A. 1999;96:5575–5580. doi: 10.1073/pnas.96.10.5575. [Abstract/Free Full Text] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans JD, Wheeler DE. Gene expression and the evolution of insect polyphenisms. Bioessays. 2001;23:62–68. doi: 10.1002/1521-1878(200101)23:1<62::AID-BIES1008>3.0.CO;2-7. [CrossRef][Medline] [DOI] [PubMed] [Google Scholar]
- 11.Page RE, Jr, Peng CY-S. Aging and development in social insects with emphasis on the honey bee, Apis mellifera L. Exp. Gerontol. 2001;36:695–711. doi: 10.1016/s0531-5565(00)00236-9. [CrossRef][Medline] [DOI] [PubMed] [Google Scholar]
- 12.Chapuisat M, Keller L. Division of labour influences the rate of ageing in weaver ant workers. Proc. R. Soc. London Ser. B. 2002;269:909–913. doi: 10.1098/rspb.2002.1962. [CrossRef][Medline] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carey JR. Demographic mechanisms for the evolution of long life in social insects. Exp. Gerontol. 2001;36:713–722. doi: 10.1016/s0531-5565(00)00237-0. [CrossRef][Medline] [DOI] [PubMed] [Google Scholar]
- 14.Heinze J, Hoelldobler B, Peeters C. Conflict and cooperation in ant societies. Naturwissenschaften. 1994;81:489–497. [CrossRef] [Google Scholar]
- 15.Lee RD. Rethinking the evolutionary theory of aging: Transfers, not births, shape social species. Proc. Natl. Acad. Sci. U.S.A. 2003;100:9637–9642. doi: 10.1073/pnas.1530303100. [Abstract/Free Full Text] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoelldobler B, Wilson EO. The Ants. Cambridge, MA: Belknap Press of Harvard Univ. Press; 1990. [Google Scholar]
- 17.Wheeler WM. The ant-colony as an organism. J. Morphol. 1911;22:307–325. [Google Scholar]
- 18.Wilson DS, Sober E. Reviving the superorganism. J. Theor. Biol. 1989;136:337–356. doi: 10.1016/s0022-5193(89)80169-9. [Medline] [DOI] [PubMed] [Google Scholar]
- 19.Moritz RFA, Fuchs S. Organization of honey bee colonies: characteristics and consequences of a superorganism concept. Apidologie. 1998;29:7–21. [Google Scholar]
- 20.Oster GF, Wilson EO. Caste and Ecology in the Social Insects. Princeton, NJ: Princeton Univ. Press; 1978. [PubMed] [Google Scholar]
- 21.Fewell JH. Social insect networks. Science. 2003;301:1867–1870. doi: 10.1126/science.1088945. [Abstract/Free Full Text] [DOI] [PubMed] [Google Scholar]
- 22.Amdam GV, Omholt SW. The regulatory anatomy of honeybee lifespan. J. Theor. Biol. 2002;216:209–228. doi: 10.1006/jtbi.2002.2545. [CrossRef][Medline] [DOI] [PubMed] [Google Scholar]
- 23.Keller L. Queen lifespan and colony characteristics in ants and termites. Insectes Sociaux. 1998;45:235–246. [CrossRef] [Google Scholar]
- 24.Giraud T, Pedersen JS, Keller L. Evolution of supercolonies: the Argentine ants of southern Europe. Proc. Natl. Acad. Sci. U.S.A. 2002;99:6075–6079. doi: 10.1073/pnas.092694199. [Abstract/Free Full Text] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beye M, Hasselbach M, Fondrk MK, Page RE, Jr, Omholt SW. The gene csd is the primary signal for sexual development in the honeybee and encodes an SR-type protein. Cell. 2003;114:419–429. doi: 10.1016/s0092-8674(03)00606-8. [Medline] [DOI] [PubMed] [Google Scholar]
- 26.Roisin Y. Caste sex ratios, sex linkage, and reproductive strategies in termites. Insectes Sociaux. 2001;48:224–230. [Google Scholar]
- 27.Thorne BL, Breisch NL, Haverty MI. Longevity of kings and queens and first time of production of fertile progeny in dampwood termite (Isoptera; Termopsidae; Zootermopsis) colonies with different reproductive structures. J. Anim. Ecol. 2002;71:1030–1041. [CrossRef] [Google Scholar]
- 28.Sakagami SF, Fukuda H. Life tables for worker honeybees. Res. Pop. Ecol. 1968;10:127–139. [Google Scholar]
- 29.Neukirch A. Dependence of the life span of the Honeybee {Apis mellifera) upon flight performance and energy consumption. J. Comp. Physiol. 1982;146:35–40. [Google Scholar]
- 30.Haydak MH. Scientific Journal Series, Minnesota Agricultural Experimental Station No. 5122. St. Paul, MN: 1963. Age of Nurse Bees and Brood Rearing. [Google Scholar]
- 31.Maurizio A. The influence of pollen feeding and brood rearing on the length of life and physiological conditions of the honeybee. Bee World. 1950;31:9–12. [Google Scholar]
- 32.Rutz W, Luzio G, Wille H, Lüscher M. A bioassay for juvenile hormone (JH) effects of insect growth regulators (IGR) on adult worker honeybees. Bull. Soc. Entomol. Suisse. 1974;47:307–313. [Google Scholar]
- 33.Pinto LZ, Bitondi MMG, Simoes ZLP. Inhibition of vitellogenin synthesis in Apis mellifera workers by a juvenile hormone analogue, pyriproxyfen. J. Insect Physiol. 2000;46:153–160. doi: 10.1016/s0022-1910(99)00111-0. [CrossRef][Medline] [DOI] [PubMed] [Google Scholar]
- 34.Comfort A. The Biology of Senescence. ed. 3. London: Churchill Livingstone; 1979. [Google Scholar]
- 35.Snodgrass RE. Anatomy of the Honey Bee. Ithaca, NY: Cornell Univ. Press; 1956. [Google Scholar]
- 36.Haydak MH. Changes with age in the appearance of some internal organs of the honey bee. Bee World. 1957;38:197–203. [Google Scholar]
- 37.Technau GM. Fibre number in the mushroom bodies of adult Drosophila melanogaster depends on age, sex, and experience. J. Neurogenet. 1984;1:113–126. doi: 10.3109/01677068409107077. [Medline] [DOI] [PubMed] [Google Scholar]
- 38.Kaftanoglu O, Peng CY-S. Preservation of honey bee {Apis mellifera) spermatozoa in liquid nitrogen. J. Apicultural Res. 1984;23:157–163. [Google Scholar]
- 39.Harbo JR, Williams JL. Effects of above-freezing temperatures on temporary storage of honeybee spermatozoa. J. Apicultural Res. 1987;26:53–55. [Google Scholar]
- 40.Verma LR. Biology of honey bee {Apis mellifera) spermatozoa. Part 1, Effect of different diluents on motility and survival. Apidologie. 1978;9:167–174. [Google Scholar]
- 41. See http://www.hgsc.bcm.tmc.edu/press_releases/honeybee.html.
- 42.Hardy ICW. Sex ratios : concepts and research methods. Cambridge: Cambridge Univ. Press; 2002. [Google Scholar]
- 43.Whitcomb WH, Bhatkar A, Nickerson JC. Predators of Solenopsis invicta queens prior to successful colony establishment. Environ. Entomol. 1973;2:1101–1103. [Google Scholar]
- 44.Laidlaw HH, Jr, Page RE., Jr . Queen Rearing and Bee Breeding. Ceshire, CT: Wicwas Press; 1997. [Google Scholar]
- 45.Raignier A, van Boven J. Etude taxonomique, biologique et biometrique des Dorylus du sous-genre Anomma (Hymenoptera: Formicidae) Ann. Mus. Royal Congo Belge. 1955;2:1–359. [Google Scholar]
- 46.Carey JR., Jr . Applied Demography for Biologists. New York: Oxford Univ. Press; 1993. [Google Scholar]
- 47.Tuljapurkar S, Carey JR, Page RE. in preparation. [Google Scholar]
- 48.Bell E, Marek LF, Levinstone DS, Merrill C, Shur S, Young IT, Eden M. Loss of division potential in vitro: Aging or differentiation? Science. 1978;202:1158–1163. doi: 10.1126/science.725592. [DOI] [PubMed] [Google Scholar]
- 49.Hartmann A, Heinze J. Lay eggs, live longer: division of labor and life span in a clonal ant species. Evolution. 2003;57:2424–2429. doi: 10.1111/j.0014-3820.2003.tb00254.x. [Medline] [DOI] [PubMed] [Google Scholar]
- 50.Winston ML. The Biology of the Honey Bee. Cambridge, MA: Harvard Univ. Press; 1987. [Google Scholar]
- 51.Turner JS. The Extended Organism: The Physiology of Animal-Built Structures. Cambridge, MA: Harvard Univ. Press; 2000. [Google Scholar]
- 52.Amdam GV, Norberg K, Hagen A, Omholt SW. Social exploitation of vitellogenin. Proc. Natl. Acad. Sci. U.S.A. 2003;100:1799–1802. doi: 10.1073/pnas.0333979100. [Abstract/Free Full Text] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fluri P, Lüscher M, Wille H, Gerig L. Changes in weight of the pharyngeal gland and haemolymph titres of juvenile hormone and vitellogenin in worker honeybees. J. Insect Physiol. 1982;28:61–68. [CrossRef] [Google Scholar]
- 54.Butler CG. The process of queen supersedure in colonies of honeybees {Apis mellifera L.) Insectes Sociaux. 1957;4:211–223. [Google Scholar]
- 55.Roff DA. Life History Evolution. Sunderland, MA: Sinauer; 2002. [Google Scholar]
- 56.Visscher PK, Dukas R. Survivorship of foraging honeybees. Insectes Sociaux. 1997;44:1–5. [CrossRef] [Google Scholar]
- 57.Barzilai N, Atzmon G, Schechter C, Schaefer E-J, Cupples AL, Lipton R, Cheng S, Shuldiner AR. Unique lipoprotein phenotype and genotype associated with exceptional longevity. J. Am. Med. Assoc. 2003;290:2030–2040. doi: 10.1001/jama.290.15.2030. [Abstract/Free Full Text] [DOI] [PubMed] [Google Scholar]
- 58.Lenoir A. Feeding behavior in young societies of the ant Tapinoma eraticum L.: trophallaxis and polyethism. Insectes Sociaux. 1979;26:19–37. [Google Scholar]
- 59.Wilson EO. Caste and division of labor in leaf-cutter ants (Hymenoptera: Formicidae: Atta). I. The overall pattern. A. sexdens. Behav. Ecol. Sociobiol. 1980;7:143–156. [Google Scholar]
- 60.Robinson GE, Page RE, Jr, Strambi C, Strambi A. Hormonal and genetic control of behavioral integration in honey bee colonies. Science. 1989;246:109–111. doi: 10.1126/science.246.4926.109. [DOI] [PubMed] [Google Scholar]
- 61.Fewell JH, Ydenberg RC, Winston ML. Individual foraging effort as a function of colony population in the honey bee, Apis mellifera L. Anim. Behav. 1991;42:153–155. [Google Scholar]
- 62.Pankiw T, Page RE, Jr, Fondrk MK. Brood pheromone stimulates pollen foraging in honey bees {Apis mellifera) Behav. Ecol. Sociobiol. 1998;44:193–198. [CrossRef] [Google Scholar]
- 63.Pankiw T, Tarpy DR, Page RE., Jr Genotype and rearing environment affect honeybee perception and foraging behavior. Anim. Behav. 2002;64:663–672. [CrossRef] [Google Scholar]
- 64.Keller L, Genoud M. Extraordinary lifespan in ants: a test of evolutionary theories of ageing. Nature. 1997;389:958–960. [CrossRef] [Google Scholar]
- 65.Bourke AFG, Franks NR. Social Evolution in Ants. Princeton, NJ: Princeton Univ. Press; 1995. [Google Scholar]
- 66.Strassmann JE, Solis CR, Hughes CR, Goodnight KF, Queller DC. Colony life history and demography of a swarm-founding social wasp. Behav. Ecol. Sociobiol. 1997;40:71–77. [CrossRef] [Google Scholar]
- 67.Roeseler P-F, Roeseler I, Strambi A. Role of ovaries and ecdysteroids in dominance hierarchy establishment among foundresses of the primitively social wasp, Polistes gallicus. Behav. Ecol. Sociobiol. 1985;18:9–13. [Google Scholar]
- 68.Roeseler P-F, Roeseler I. Dominance of ovariectomized foundresses of the paper wasp, Polistes gallicus. Insectes Sociaux. 1989;36:219–234. [Google Scholar]
- 69.Oldroyd BP. The cape honeybee: an example of social cancer. Trends Ecol. Evol. 2002;17:249–251. [CrossRef] [Google Scholar]
- 70.Bourke AFG. Worker reproduction in the higher eusocial Hymenoptera. Q. Rev. Biol. 1988;63:291–311. [Google Scholar]
- 71.Harbo JR. The effect of population-size on worker survival and honey loss in broodless colonies of honey bees, Apis mellifera L. (Hymenoptera, Apidae) Environ. Entomol. 1983;12:1559–1563. [Google Scholar]
- 72.Wilson EO. The relation between caste ratios and division of labor in the ant genus Pheidole (Hymenoptera: Formicidae) Behav. Ecol. Sociobiol. 1984;16:89–98. [Google Scholar]
- 73.Wesson LG. Contributions to the natural history of Harpagoxenus americanus Emery (Hymenoptera: Formicidae) Trans. Am. Entomol. Soc. 1939;65:97–122. [Google Scholar]
- 74.We thank B. Hoelldobler for the clarification on ant male life history and various members of the NIH-funded Program Project "Biodemographic Determinants of Life Span" for discussion. Financial support came from NIH (grants P01 AG022500-01 and P01AG08761-10), the Center for the Economy of Demography and Aging, and the Norwegian Research Council (project no. 157851/432).Rueppell O, Amdam GV, Page RE, Carey JR. From Genes to Societies. Sci. Aging Knowl. Environ. 2004;2004(5):pe5. doi: 10.1126/sageke.2004.5.pe5.