Abstract
Objectives
To understand the potential role of P2X7 as biomarker of endometrial cancer, and the molecular mechanisms by which cancerous epithelial cells maintain low expression of P2X7.
Methods
Feasibility clinical experimental study. Normal (28), simple or complex hyperplasia (7), complex hyperplasia with atypia (6) and cancer endometrial discarded tissues (40) were obtained from a total of 81 women, ages 25–75. Endpoint for P2X7 protein was average pixel signal density of tissue immunoreactivity with anti-P2X7 antibody. Endpoint for P2X7 mRNA was one-step quantitative Real-Time PCR. Experiments in-vitro included normal (hEVEC) and cancerous cervical epithelial cells (HeLa) transfected with reporter plasmid containing luciferase-3′ untranslated region (3′UTR)-P2X7 cDNA, using as endpoint steady-state luciferase mRNA levels.
Results
Levels of P2X7 protein and mRNA were significantly lower in vivo, in tissues of complex hyperplasia with atypia or endometrial adenocarcinoma, than in tissues of normal endometrium, simple hyperplasia or complex hyperplasia tissues (sensitivity and specificity of 89–100%, p<0.0001–0.01). Steady-state levels of luciferase mRNA increased over a 6 h incubation period in hEVEC cells transfected with the 3′ UTR-P2X7-luciferase vector, but decreased in HeLa cells transfected with the reporter plasmid.
Conclusions
Tissue levels of P2X7 protein and mRNA can differentiate effectively and accurately between normal and benign hyperplastic endometrial tissues from pre-cancerous and cancer tissues. Cancerous epithelial cells degrade P2X7 mRNA by activation of instability domains located at the 3′UTR of the P2X7.
Keywords: Human, Endometrium, mRNA, Protein, Cytokeratin-18, E-Cadherin
Introduction
The receptor P2X7 is membrane-bound, ligand-operated channel [1]. In epithelia, the P2X7 receptor is expressed mainly by proliferating (germinative) epithelial cells [2,3], and its main function is induction of apoptosis [1]. ATP is the naturally occurring ligand [1], and it is present in the extracellular space at concentrations that suffice to activate the P2X7 receptor [4–6]. In epithelial cells ATP can activate also other types of purinergic receptors and cellular signaling [1] but ATP-induced apoptosis can be blocked with P2X7 antagonists [4,7]. These data led to a novel apoptosis model which predicts that the growth of epithelial cells in vivo is controlled constitutively by P2X7-mediated apoptosis of proliferating epithelial cells [2,4,7].
Recent studies showed that P2X7 expression is lesser in uterine epithelial cancer cells compared to normal cells, and that P2X7-mediated apoptosis is abrogated in the cancer cells [2,3]. Based on these findings we hypothesized that decreased expression of P2X7 and the abrogated P2X7-mediated apoptosis predispose proliferating uterine epithelial cells to the effects of carcinogens and may lead to the development of cancer.
Endometrial epithelial cancers contribute significantly to the morbidity and mortality of women. In the USA about 6% of all cancer deaths in women are caused by endometrial cancers [8], most of which are Type 1 endometrioid adenocarcinomas. Despite this relatively high prevalence, little is known about the cellular and molecular mechanisms that lead to the development of endometrial cancers in women. P2X7 expression in endometrial epithelial cancer cells is lesser than in normal endometrial cells [3], suggesting that P2X7 plays a role in endometrial cancer pathogenesis. The main objective of the study was to better understand the role of P2X7 in the cancer biology of endometrial adenocarcinoma, and to improve P2X7 protein and mRNA assays for potential use as biomarkers of endometrial cancer in women. The second objective was to better understand the mechanism that controls expression of the P2X7 in cancer epithelial cells.
Materials and methods
Tissues
Discarded human uterine tissues were obtained from women undergoing curettage or hysterectomy for indications unrelated to the present study. Tissues were obtained according to approved IRB protocols 12-03-50 and 03-90-300 from two sources: The Human Tissue Procurement Facility of University Hospital CASE Medical Center/the Comprehensive Cancer Center Tissue Procurement Core Facility (CTPC) at CASE (Case Western Reserve) University, Cleveland, Ohio; and from the Cooperative Human Tissue Network (CHTN) (National Cancer Institute) through the Human Tissue Resource Network (HTRN), Department of Pathology at the Ohio State University, Columbus, Ohio. Tissues were collected based on availability and were used for assay development and proof-of-concept experiments. The data presented in this paper are considered preliminary, and no formal Power Analysis was performed to determine the number of tissues to be included in testing any specific hypotheses. No attempt was made to categorize results by patients’ ethnic origin or race.
Tissues were obtained by one of three methods: a) Cross-sections of uterine segments were obtained from paraffin embedded blocks that were prepared to establish the patient’s diagnosis by the Departments of Pathology. Additional sections for the purpose of the present study were cut and slides were made by the Departments of Pathology according to standard procedures; b) discarded unprocessed tissue segments from hysterectomy specimens. Upon removal by surgery, the bulk of the uterine specimen was used to establish the patient histological diagnosis. Leftover discarded tissues were collected by the pathologist at the site, and tissues were either snap frozen in liquid N2 and delivered on dry ice to the lab and stored at −80 °C until assayed, or cut into pieces of <0.5 cm and immersed in a 5 ml volume of RNAlater (Qiagen Inc, Valencia, CA) at room temperature; c) in some cases the pathologist sectioning the uterus upon its removal used a glass slide to collect cells by touching the endometrial lining (Touch-Preps). The glass slide containing cells was immersed in cold methanol and delivered to the lab. Histological diagnoses of the tissues were made by the Departments of Pathology at University Hospital CASE Medical Center in Cleveland or at the Ohio State University.
Cell cultures
Primary cultures of human Ectocervical–Vaginal Epithelial Cells (hEVEC) were generated from discarded normal ectocervical–vaginal tissues. HeLa cells were obtained from the ATCC. Cultures of normal human keratinocytes were generated from discarded foreskins that were obtained following non-ritual circumcision of newborn males at the nursery of the Department of Obstetrics and Gynecology, University Hospital CASE Medical Center according to approved IRB protocol 08-06-28. Methods of cell cultures were described [9].
Protein methods
The methods of Western blots, immunostaining and densitometry were described [10,11]. P2X7 receptor polypeptides were visualized using rabbit polyclonal anti P2X7 receptor (Alomone Laboratories, Jerusalem, Israel) [11,12]. The anti cytokeratin-18 (CK-18) antibody was from Cell Signaling Technology (Danvers, MA). The anti-E-Cadherin antibody as well as the secondary antibodies were described [3,11,12].
The novel method for image analysis of immunofluorescence data is provided in the “Supplementary Material” section. In the paper data are presented in terms of P2X7/E-Cadherin or P2X7/CK-18 average pixel density.
RNA methods
The method for isolation of total RNA from frozen tissues was described [3]. The method of total RNA isolation using the RNAlater technique is provided in the “Supplementary Material” section. The quality of all RNA samples was assessed for concentration and purity by means of optical density measurement, and further monitored by 1.2% denaturing agarose gel electrophoresis and RT-PCR by using the transcript of CK-18 as template.
The methods of P2X7 and CK-18 RT-PCR, as well as two-step and one-step quantitative Real-time RT-PCR (qPCR) were previously described [1–3], and are provided in detail in the “Supplementary Material” section. Relative gene expression levels were calculated using the Comparative Threshold Cycle (Ct) method of Relative Quantitation (RQ). Verification of reverse transcription was done as described [3].
The method for preparation of Reference Standards for P2X7 and CK-18 RNA is provided in the “Supplementary Material” section. Curves of [Ct values] vs. [Copy-Number] were generated for the standards, and the copy numbers in the endometrial samples of the P2X7 and CK-18 mRNA was determined from the standards curves.
3′UTR-P2X7 steady-state assays
The methods for generation of control reporter plasmids (luciferase) or test reporter plasmids (P2X73′UTR-luciferase), transfections into hEVEC or HeLa cells, and determinations of luciferase mRNA levels by qPCR are provided in the “Supplementary Material” section. Luciferase mRNA steady-state levels in cells transfected with the 3′UTR-P2X7-luciferase vector were normalized to 0 hr results in cells transfected with the control vector.
Chemicals and supplies
All chemicals and supplies, unless specified otherwise, were obtained from Sigma Chemical (St. Louis, MO).
Data analysis
Data were analyzed using GraphPad Instat, GraphPad Software Inc., San-Diego, CA. Significance of differences between groups was estimated by two-tail t-test or by one way ANOVA with Tukey–Kramer Multiple Comparisons post test analysis. Composite data were tabulated in Contingency Tables and significance of differences between groups was estimated by Fisher’s exact test.
Results
Tissue samples
Endometrial tissues were obtained from a total of 81 women, ages 25–75, and were grouped as follows: 28 histologically normal samples, 7 simple or complex hyperplasia, 6 complex hyperplasia with atypia, and 40 cancer samples (39 Type-1 endometrioid adenocarcinomas and one carcinosarcoma). Assays utilized specimens that were either retrieved from different patients, or specimens that were retrieved from the same uterus, from the diseased site and from an adjacent histologically normal site. Some specimens were used for more than one assay.
P2X7 protein assays
Western blots
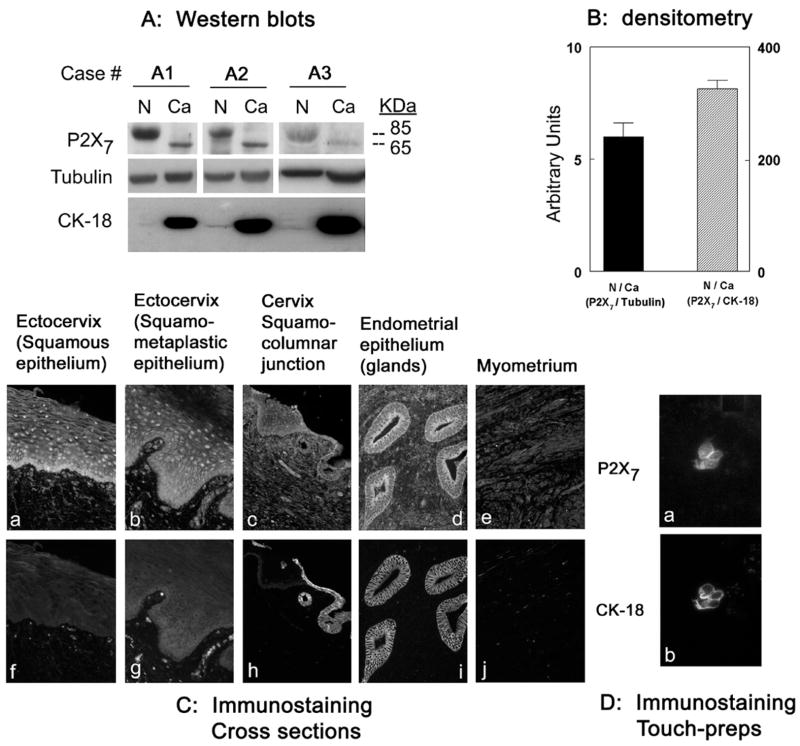
A recent study, showing lesser expression of the P2X7 in cancer tissues than in normal uterine epithelial tissues [3], normalized P2X7 protein data by tubulin. Since P2X7 is expressed mainly in epithelial cells while tubulin is expressed by all types of uterine cells including stromal cells, the previous data may have underestimated the expression of P2X7 protein. In this paper P2X7 data were normalized to cytokeratin 18 (CK-18), which is a specific cytokeratin of columnar epithelia [12]. In broken cells preparations, the ratio of P2X7/tubulin densitometry was 6 fold higher in normal than in cancer tissues, while the ratio of P2X7/CK-18 densitometry was 360 fold higher in normal than in cancer tissues (Figs. 1A and B). The likely explanation is that tissues obtained from segments of uteri containing endometrial adenocarcinomas were composed predominantly of CK-18-rich epithelial cells. In contrast, tissues obtained from normal uterine segments contained relatively few epithelial cells, and the abundance of non-epithelial stromal cells masked the contributions of P2X7 by the epithelial component of the tissues.
Fig. 1.
P2X7 protein assays. (A) Tissue samples were obtained from normal (N) and cancer (Ca) sections of uteri of three women (A1–A3) diagnosed with endometrial adenocarcinoma. Tissues were homogenized, and lysates fractionated by gel electrophoresis were immunoblotted with anti P2X7 antibody. Membranes were reprobed with anti tubulin antibody and anti CK-18 antibody. (B) Densitometry of the data in A the 85 kDa P2X7 bands were normalized to the tubulin bands (left bar) or to the CK-18 bands (right bar), and ratios were expressed in terms of relative N/Ca densities. (C) Uterine cross-sections co-immunostained with anti P2X7 and CK-18 antibodies. (D) Endometrial touch-preps co-immunostained with anti P2X7 and CK-18 antibodies. In C and D the magnification was ×20.
Immunostaining assays
In uterine tissues the P2X7 protein is expressed predominantly in epithelial cells (Figs. 1Ca–d). To determine the potential role of CK-18 as background endometrial marker, uterine tissues were co-immunostained with anti P2X7 and CK-18 antibodies. In uterine cross-sections, P2X7 immunoreactivity was found predominantly in the epithelial cells of the ectocervix (squamous [Fig. 1Ca] and squamo-metaplastic [Fig. 1Cb] epithelia), the squamo-columnar junction (Fig. 1Cc), the endocervix (Fig. 1Cc) and the endometrium (Fig. 1Cd), and was absent in myometrial cells (Fig. 1Ce). CK-18 immunoreactivity was found only in the monolayered endocervical (Fig. 1Ch) and endometrial epithelia (Fig. 1Ci), and was absent in the squamous or squamo-metaplastic epithelia (Figs. 1Cf, g) or in myometrial cells (Fig. 1Cj). P2X7 and CK-18 co-staining was also found in endometrial surface touch-preps (Fig. 1Da, b). Co-localization of P2X7 staining with the CK18 (Figs. 2Aa, b) was similar to the co-localization of P2X7 with the general epithelial marker E-Cadherin (Figs. 2Ae, f). Collectively, these data indicate that CK-18 can be used to localize P2X7 staining in endometrial epithelial cells.
Fig. 2.
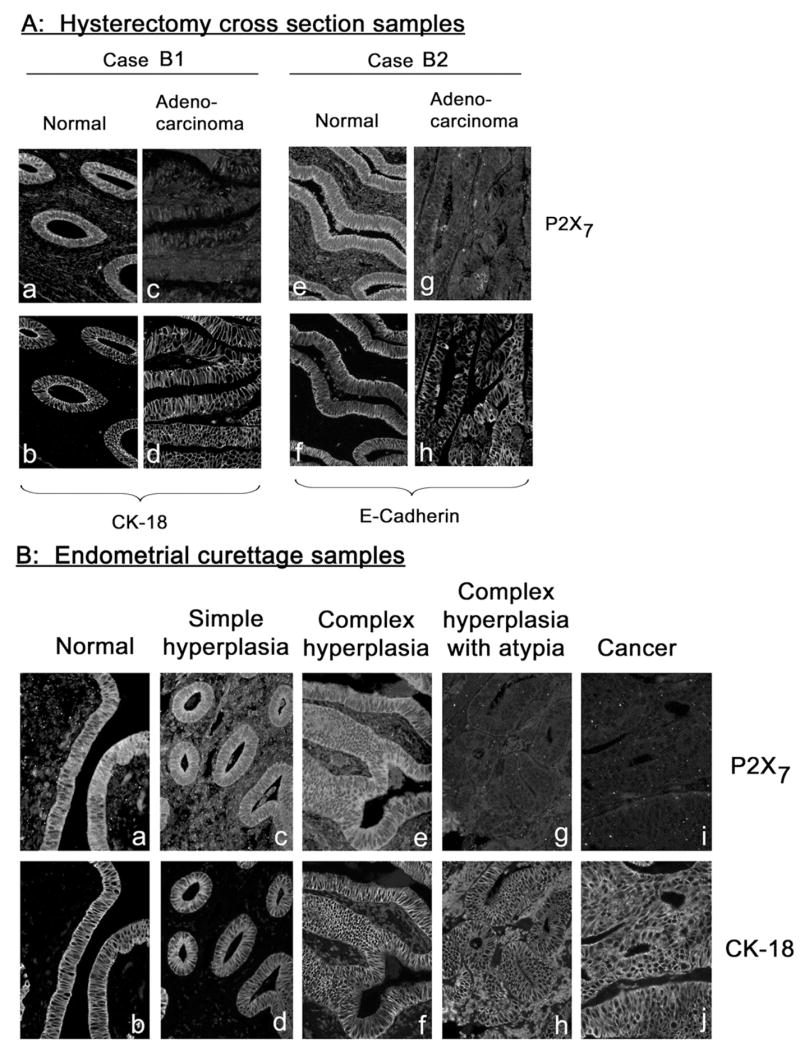
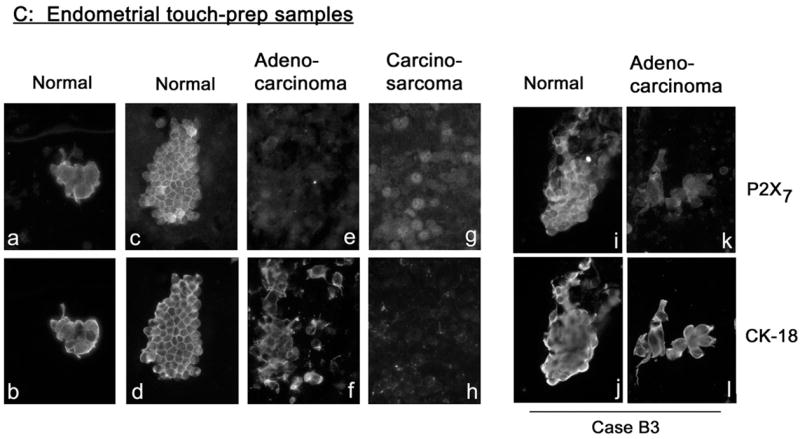
Co-immunostaining of endometrial tissues with anti P2X7 and CK-18 or anti P2X7 and E-Cadherin antibodies. (A) Cross-sections of paired normal and cancer regions from hysterectomy samples of two women (Cases B1 and B2) diagnosed with endometrial adenocarcinoma. (B) Cross-sections of endometrial tissues obtained by endometrial curettage and diagnosed as indicated. (C) Endometrial samples obtained by the touch-prep technique. Samples were obtained by touching exposed endometrial surface with a glass slide; cells were fixed on the slide and co-immunostained with anti P2X7 and CK-18 antibodies. Shown are clumps or sheets of endometrial epithelial normal and adenocarcinoma cells, and of carcino-sarcoma cells. Samples i–l were obtained from the same woman (Case B3). Magnification was ×20. Image analyses are summarized in Fig. 3.
P2X7 immunostaining in normal, pre-cancerous and cancer endometrial tissues
Irrespective of the method used to collect the endometrial tissues, namely by hysterectomy (Fig. 2A), endometrial curettage (Fig. 2B), or by the touch-prep technique (Fig. 2C), P2X7 immunoreactivity was found predominantly in normal endometrial epithelial cells (Figs. 2Aa, Ae, Ba, Ca, Cc, Ci) but was diminished or absent in cancer endometrial cells (Figs. 2Ac, Ag, Bi, Ce, Ck). Interestingly, P2X7 immunoreactivity was found in epithelial tissues of simple hyperplasia (Fig. 2Bc) and complex hyperplasia (Fig. 2Be), but not in tissues of complex hyperplasia with atypia (Fig. 2Bg). Endometrial carcinosarcoma cells showed negligible P2X7 immunoreactivity (Fig. 2Cg), but in contrast to adenocarcinoma they lacked CK-18 immunoreactivity (Fig. 2Ch).
To better understand the degree of which P2X7 immunoassays can differentiate normal and cancer endometrial tissues, P2X7 and CK-18 immunoreactivities in captured images were quantified and the ratio of P2X7/CK-18 signals was determined. In a set of 5 normal/adenocarcinoma paired specimens, P2X7/CK-18 signals were lower in cancer tissues than in the corresponding normal tissues (Fig. 3Aa, Table 1). Pooled results of 22 normal and 8 adenocarcinoma tissues showed average P2X7/CK-18 pixel densities of 2.08 vs. 1.09 (p<0.01, two-tail t-test) (Fig. 3Ab, Table 1). Image analyses of 8 specimens of simple or complex hyperplasia and of 6 specimens of complex hyperplasia with atypia showed average P2X7/CK-18 pixel densities of 1.82 vs. 1.02, respectively (p =0.03, two-tail t-test) (Fig. 3Ab, Table 1). The average P2X7/CK-18 pixel density of samples composed of complex hyperplasia with atypia was significantly smaller than that of normal samples and was not different from that of adenocarcinoma samples (Fig. 3Ab, Table 1).
Fig. 3.
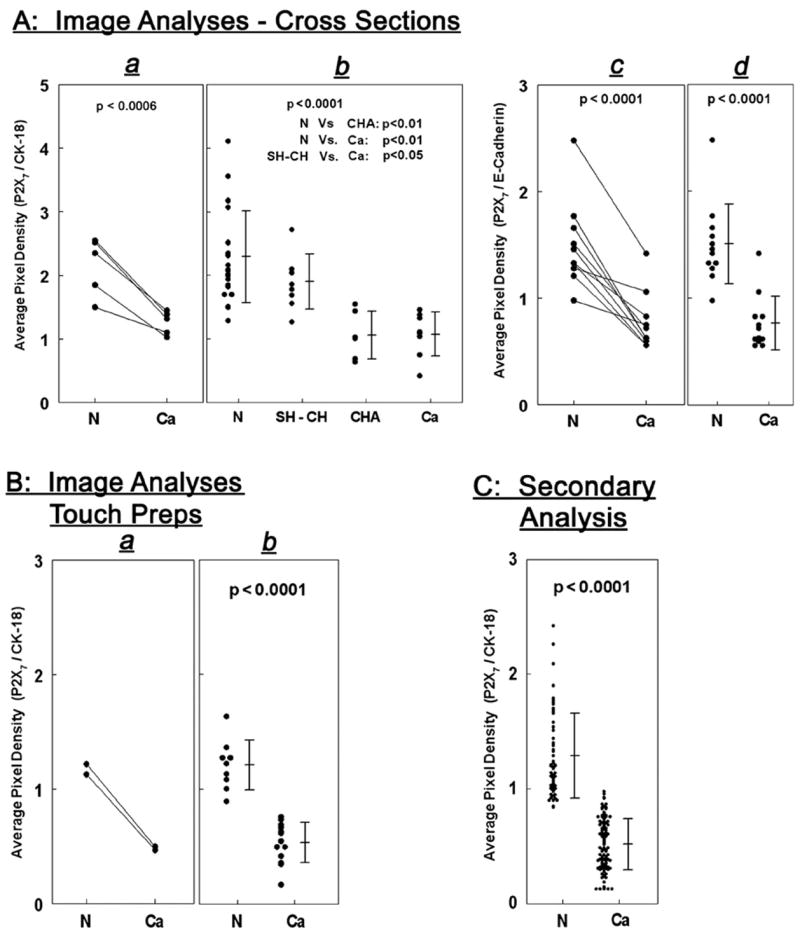
(A) Image analyses of tissue cross-sections co-immunostained with P2X7 and CK-18 antibodies (a, b) or with P2X7 and E-Cadherin antibodies (c, d). In a and c each line represents paired normal (N) and cancer (Ca, adenocarcinoma) samples obtained from the same uterus (n=5 for P2X7/CK-18 [a] and n =10 for P2X7/E-Cadherin [c]). N and Ca levels were compared by two-tail paired t-test. b, Pooled results of tissue cross-sections composed of 22 normal (N) specimens; 8 specimens of simple or complex hyperplasia (SH–CH); 6 specimens of complex hyperplasia with atypia (CHA); and 8 cancer (Ca, adenocarcinoma) tissues. Results were analyzed by one way ANOVAwith post test analysis using Tukey–Kramer Multiple Comparisons. d, Pooled results of 12 normal (N) and 12 cancer (Ca, adenocarcinoma) tissue cross-sections, analyzed by two-tail t-test. (B) Image analyses of touch-prep samples co-immunostained with the P2X7 and CK-18 antibodies. In a each line represents paired samples, normal (N) and cancer (Ca, adenocarcinoma), obtained from the same uterus (n=2). b shows pooled results of 9 normal (N) and 13 cancer (Ca, adenocarcinoma) samples, and data were analyzed by the two-tail t-test. (C) Secondary analysis of results pooled from 59 normal and 106 adenocarcinoma data points. Data were analyzed by the two-tail t-test.
Table 1.
| Endpoint | Data | Cut-off (N/Ca) | Sensitivity (%) | Specificity (%) | Significance |
|---|---|---|---|---|---|
| P2X7 protein | Fig. 3Ab a | 1.46 | 93 | 93 | p<0.0001 |
| Fig. 3Ad | 1.10 | 92 | 92 | p=0.0001 | |
| Fig. 3Bb | 0.80 | 100 | 100 | p<0.0001 | |
| Fig. 3C | 0.90 | 93 | 95 | p<0.0001 | |
| P2X7 mRNA | Fig. 5D | 45 | 89 | 90 | p=0.001 |
Accuracy of P2X7 protein and mRNA levels in differentiating normal (N) and cancer (Ca, adenocarcinoma) endometrial tissues. Data of Figs. 3A–C and 5D were categorized in 2×2 Contingency Tables by comparing Normal and Cancer histology data versus experimental Normal (data>cut-off) and Cancer (data<cut-off) results. Cut-off points for the experimental Normal and Cancer values were determined from Figs. 3 and 5. Significance of differences between groups and levels of sensitivity and specificity were estimated by Fisher’s exact test.
Simple Hyperplasia (SH) and Complex Hyperplasia (CH) data were included among the Normal data. Complex Hyperplasia with Atypia (CHA) data were included with the Cancer data (Fig. 3Ab).
Experiments also compared the value of CK-18 to that of E-Cadherin as background markers for the P2X7 assays. In a set of 10 paired normal/adenocarcinoma specimens, P2X7/E-Cadherin staining was lesser in the cancer than in the corresponding normal tissues (Fig. 3Ac, Table 1). Pooled results of 12 normal and 12 adenocarcinoma tissues showed average P2X7/E-Cadherin pixel densities of 1.44 vs. 0.68, respectively (p<0.0001, two-tail t-test) (Fig. 3Ad, Table 1). These data indicate that CK-18 and E-Cadherin performed equally well as epithelium P2X7 background markers.
In two sets of paired normal/adenocarcinoma touch-prep specimens the P2X7/CK-18 staining was lower in the cancer cells than in the corresponding normal cells (Fig. 3Ba, Table 1). Pooled results of touch-prep specimens obtained from 22 uteri, 9 normal and 13 adenocarcinoma cases showed average P2X7/CK-18 pixel densities of 1.22 vs. 0.55, respectively (p<0.0001, two-tail t-test) (Fig. 3Bb, Table 1).
Fig. 3C shows secondary analysis of a total of 36 specimens obtained from the 22 uteri samples (13 from the normal cases and 23 from the cancer cases) and a total of 165 microscopic fields images (59 of normal cases and 106 of cancer cases). Combined data analysis showed average P2X7/CK-18 pixel density of 1.17 for normal cases versus 0.49 for cancer cases (Fig. 3C, p <0.0001, two-tail t-test; Table 1).
P2X7 RNA assays
To better understand the potential value of P2X7 mRNA in differentiating normal and cancer endometrial cells, tissue levels of P2X7 mRNA were normalized either to GAPDH or to CK-18 mRNA levels. Fig. 4A shows that levels of P2X7/GAPDH mRNA in normal endometrial tissues were 20 fold higher than in cancer tissues. In contrast, in the same specimens, P2X7/CK-18 mRNA levels in the normal endometrial tissues were on the average 270 fold higher than in the cancer tissues (Fig. 4A). The likely explanation, as discussed above, is that tissues obtained from segments of uteri containing endometrial adenocarcinomas were composed predominantly of epithelial cells while tissues obtained from normal uterine segments contained relatively few epithelial cells and abundance of non-epithelial stromal cells.
Fig. 4.
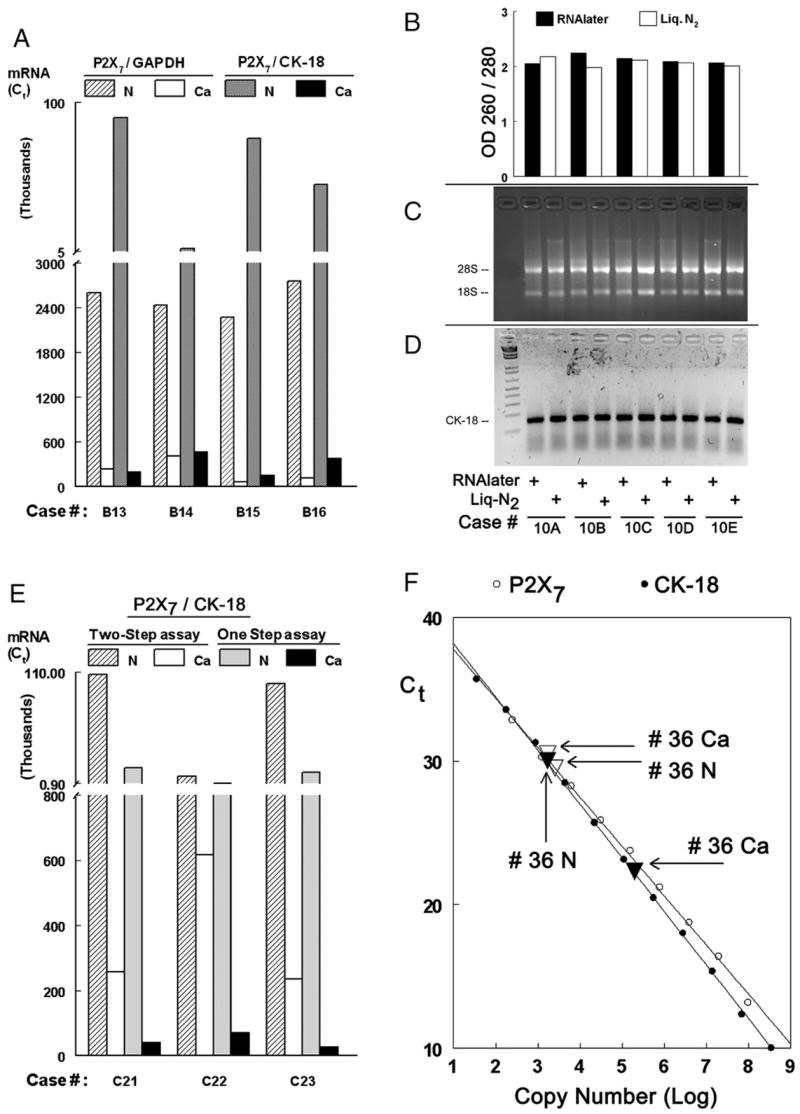
P2X7 mRNA assays. (A) Normal (N) and Cancer (Ca, adenocarcinomas) paired tissue samples obtained from 4 uteri were assayed by two-step qPCR for either P2X7/GAPDH or P2X7/CK-18 mRNA levels. (B–D) Normal endometrial tissues obtained from 4 uteri were divided into two equal parts: one part was preserved by freezing in liquid N2/−80 °C; the second part was immersed in RNAlater at room temperature. The condition of the extracted RNA was determined by OD 260/280 (B); by fractionation of RNA in 2% denaturing agarose gel electrophoresis and determinations of the 28S/18S ratio (C); and by reverse transcription capacity of the extracted RNA as template for CK-18 synthesis (D). (E) P2X7/CK-18 mRNA levels were determined in normal (N) and Cancer (Ca, adenocarcinomas) paired tissue samples obtained from 3 uteri, using either the two-step qPCR or one-step qPCR methods. (F) qPCR using reference standards. Solutions containing P2X7 (○) and CK-18 (●) plasmids at the indicated amounts (expressed as log copy number) were assayed by One-step qPCR. The curves of [Ct] vs. [Copy Number] for both the P2X7 and the CK-18 could be fit into straight lines (r2>0.999, p<0.0001 in both cases). Arrows show P2X7 and CK-18 mRNA qPCR results of normal (N △) and cancer (Ca, adenocarcinoma ▲) samples from the same uterus (# 36) that were assayed in parallel.
Optimization of endometrial P2X7 mRNA assays
Two steps were employed to simplify the P2X7 mRNA assays. First, in order to avoid the use of freezing, tissues were preserved at room temperature in RNAlater (Qiagen). Tissues obtained from four uteri (normal endometrium) were divided upon removal into two equal parts: one part was snap frozen in liquid N2 and maintained at −80 °C until assayed; the other part was immersed in RNAlater and maintained in that solution at room temperature until assayed. The results of the experiment were as follows: A) the RNA yield in both methods was similar (not shown); B) The quality of extracted RNA by both methods was similar, as is evident by preserved RNA integrity, and the capacity of the extracted RNA to serve as template for CK-18 synthesis by RT-PCR (Figs. 4B–D); C) Tissues could be maintained in RNAlater at room temperature for up to 7 days prior to assays (not shown).
Second, experiments utilized one-step qPCR for the P2X7/CK-18 mRNA assay. This technique involves fewer technical steps compared with the traditional two-step qPCR. The results in Fig. 4E show that tissue P2X7 and CK-18 mRNA levels determined by the one-step method were comparable with those determined by the two-step method, and that the P2X7/CK-18 mRNA ratios in all three cases were similar. Thus, respectively, for cases C21, C22, and C23, P2X7/CK-18 mRNA levels using the two-step qPCR method were 415, 14, and 420; and using the one-step qPCR method those levels were 391, 13, and 410 (Fig. 4E).
To further optimize the P2X7 RNA assays, Reference Standards were developed for qPCR P2X7 and CK-18 mRNA assays in order to enable comparison of results among different experiments. To this aim, qPCR assays included constructs containing known amounts of P2X7 and CK-18 cDNAs. Fig. 4F shows a linear relationship for the known standard controls between cycle number (Ct) and the amount (copy number) of the P2X7 cDNA (Fig. 4F, empty circles), and between Ct and the copy number of the CK-18 cDNA (Fig. 4F, filled circles). Fig. 4F also shows that levels of P2X7 and CK-18 mRNA of normal (N) and adenocarcinoma samples (Ca) from the same uterus (# 36) which were run in parallel were located at the middle of the linear portion of the curves. Using those curves as reference, the P2X7/CK-18 mRNA ratio of copy numbers of sample # 36 as determined from the measured Ct values were 150.3 (Normal) and 0.9 (Cancer) (Fig. 4F).
The validity of this method was tested further using 5 additional paired sets of normal–adenocarcinoma specimens (Fig. 5, total of 6 normal–adenocarcinoma paired specimens [including # 36]). The results show the same trends in all 6 sets of tissues: CK-18 mRNA copy numbers in the normal samples were lower than in the cancer samples while P2X7 mRNA copy numbers in the normal samples were higher than in the cancer samples (Figs. 5A and B). Thus the P2X7/CK-18 mRNA ratio levels were 3 to 170 fold higher in normal than in cancer tissues (Fig. 5C). Pooled results of tissues obtained from 19 uteri, 9 normal and 10 cancers (adenocarcinomas) showed average P2X7/CK-18 copy numbers of 122 vs. 4, respectively (p=0.002, two-tail t-test) (Fig. 5D, Table 1).
Fig. 5.
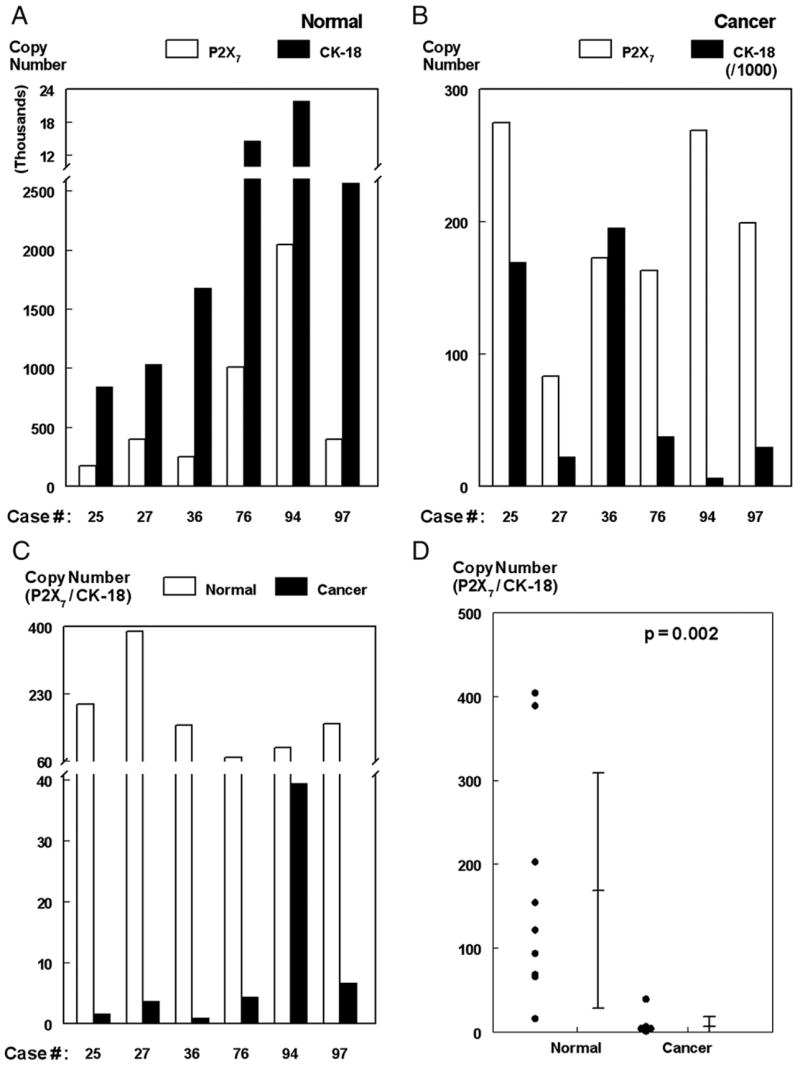
P2X7 mRNA assays. Normal (A) and cancer (adenocarcinomas, B) paired tissue samples were obtained from 6 uteri and assayed for P2X7/CK-18 mRNA levels by the one-step qPCR as in D. Tissues P2X7 and CK-18 mRNA levels were determined from the P2X7 and CK-18 [Ct] vs. [Copy Number] reference standards curves. (C) P2X7/CK-18 mRNA ratios for each of the 6 tissues. (D) Pooled results of 9 normal and 10 adenocarcinoma tissues (analyzed by two-tail t-test).
Enhanced degradation of 3′UTR-P2X7 in cancer epithelial cells
The findings of lower P2X7 protein and P2X7 mRNA levels in cancer cells than in normal epithelial cells suggest pre-translational control of P2X7 in cancer cells. Based on recent results [2] we tested the hypothesis that regions at the 3′UTR of P2X7 confer instability to the transcript.
Experiments were conducted using hEVEC (normal) and HeLa (cancer) uterine cervical epithelial cells. The choice of cells was based on the fact that they represent well characterized models of human uterine P2X7-expressing epithelial cells [2,3,9–11].
For experiments, a cDNA corresponding to the 3′UTR region of the human P2X7 gene was ligated upstream of SV40 promoter/luciferase reporter vector, and the construct cDNA was transfected into hEVEC or HeLa cells. Control experiments included cells transfected with the luciferase reporter vector only.
In hEVEC or HeLa cells transfected with the control luciferase reporter vector, steady-state levels of luciferase mRNA did not change significantly over a 6 h incubation period (Fig. 6A). In hEVEC cells transfected with the 3′UTR-P2X7-luciferase vector, steady-state levels of luciferase mRNA also did not change significantly over a 6 h incubation period (Fig. 6B). In contrast, in HeLa cells transfected with the 3′UTR-P2X7-luciferase vector, steady-state levels of the luciferase mRNA decreased significantly (Fig. 6B).
Fig. 6.
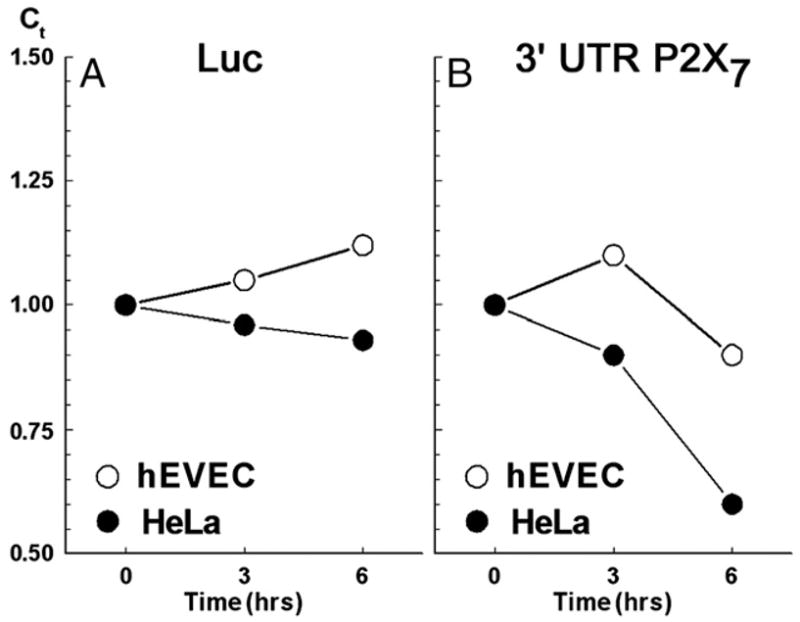
Steady-state levels (qPCR, means of two experiments) of luciferase mRNA in normal human ectocervical–vaginal epithelial cells (hEVEC, ○) and in cancer human cervical epithelial cells (HeLa, ●) transfected with either the luciferase reporter vector (Luc) or with the 3′UTR-P2X7-luciferase vector. The experiment is described in Materials and methods and Results.
Discussion
The two major findings of the study were that P2X7 expression is lesser in cancer and in pre-cancerous endometrial epithelial cells than in normal cells; and that overexpressed 3′ UTR of the P2X7 mRNA is degraded at a faster rate in cancer epithelial cells than in normal epithelial cells.
The P2X7 immunostaining assays effectively differentiated between normal tissues and benign hyperplasia from tissues of complex-hyperplasia with atypia or cancers. Recent reports suggested that complex-atypia cells are found in the background of overt cancers [13–15]. This possibility was ruled out in the present study as all cases diagnosed initially as complex hyperplasia with atypia were re-evaluated and the histology was confirmed. The present results show two-fold greater signal intensity in normal/benign hyperplasia tissues than in complex hyperplasia with atypia and cancer specimens, with sensitivity and specificity values of 92–100% (Table 1).
The P2X7 immunoassays yielded lower Normal to Cancer differentiation ratio than the Western blots. The possible explanation is that the method used for pixel quantification was constrained by the existing software used to identify regions of interest in the microscopic fields, and therefore overestimated the P2X7 signals in cancer cells. The method employed a-priori selection of CK-18 or E-Cadherin positive areas and determined the corresponding P2X7 total pixel density. The data showed that P2X7 immunoreactivity was nearly uniform across the surface of the cells, but the CK-18 or E-Cadherin immunoreactivities were limited to the plasma membrane and cytoplasm and were absent in regions corresponding to the nuclei. Since the nuclear/cytoplasm ratio is greater in cancer than in normal cells (e.g. Fig. 2A), and since the CK-18 or E-Cadherin signals included pixel values for dark areas (i.e. cell nuclei), the P2X7 determinations in cancer specimens may have been overestimated. This may have resulted in Normal/Cancer differentiation ratios that under-estimated the true differentiation power of the P2X7 method. Efforts are being made to improve P2X7 signal acquisition and data analysis.
The present study also describes an improved and a simplified P2X7 mRNA assay. The P2X7 mRNA results had similar accuracy to those of the P2X7 protein assay (Table 1), but the P2X7 mRNA assay is faster, about 6 h for completion, and it has the potential for automation.
One of our objectives was to better understand the mechanism that controls expression of the P2X7 in epithelial cancer cells. In uterine cells, P2X7 protein levels correlate with P2X7 mRNA levels (Ref. [2,3] and present data), suggesting regulation at the gene-RNA level. Human uterine epithelial cells express two splice variants of the P2X7: The 75 kDa-complex functional full-length P2X7, and a 44 kDa-complex truncated inactive P2X7-j [2]. Contrary to the present results showing reduced concentration of P2X7 mRNA in cancer cells, mRNA levels of the P2X7-j are similar in normal and cancer epithelial cells [2]. Since the P2X7 and P2X7-j mRNAs share the same promoter [2], it is unlikely that decreased transcription is the only mechanism that controls the low expression of the full-length P2X7 in cancer cells. An additional possible mechanism is that the P2X7 and P2X7-j mRNA have different 3′UTR, a region often important for maintenance of mRNA stability [16,17]. This hypothesis was tested by expressing in normal and cancer epithelial cells a SV40 promoter-luciferase reporter vector containing the human P2X7 3′UTR upstream of the promoter. The results showed greater degree of degradation of the P2X7 3′UTR in the cancer cells than in the normal cells, suggesting that regions at the 3′UTR of the P2X7 gene contain instability sites, and that the faster degradation of the P2X7 mRNA in cancer cells is the result of activation of instability domains located at the 3′UTR.
The present results could be important for our understanding of the role of P2X7 in the development of endometrial cancer. The background theorem is that defective apoptosis can lead to the development of cancer. The P2X7 mechanism regulates apoptosis of epithelial cells, and P2X7-mediated apoptosis critically depends on expression of the P2X7 receptor [4,7,10]. The observation that P2X7 expression is low not only in cancer endometrial cells but also in cells identified as complex hyperplasia with atypia resembles previous findings in cervical cancer/cervical dysplasia cells [2]. These data indicate decreased expression of P2X7 already in the pre-cancerous phenotypes, and therefore suggest that decreased expression of the P2X7 precedes cancer development in those tissues and may be mechanistically involved in the development of cancers.
The present data could also be developed in the future for screening of endometrial cancer in women at risk. Risk factors for endometrial cancer are relatively common and include conditions such as obesity, unfavorable glycemic index, diabetes, hypertension and unovulation [18–22]. Although only a small percentage of those women will develop endometrial cancer, the prevalence of risk factors and symptoms such as irregular uterine bleeding and the greater awareness for the risk factors have created a demand by women and caregivers for screening and early detection of endometrial cancer. At present there is no simple cost-effective method to screen for endometrial cancer and suspected cases are referred for endometrial biopsy or curettage [21,23]. The present data showed that regardless of the method used for collecting specimens, namely hysterectomy curettage or endometrial touch-preps, P2X7 immunoassays could differentiate normal/benign-hyperplastic cells from atypical-hyperplastic/cancer cells. These data indicate that the P2X7 assays can identify cancer cells, and suggest that the P2X7 assays could potentially be developed to screen for uterine endometrial precancerous conditions in women.
Supplementary Material
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ygyno.2007.03.032.
Acknowledgments
The study was supported in part by NIH grant AG15955 and by an unrestricted grant by CytoCore Inc. to GIG.
References
- 1.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–92. [PubMed] [Google Scholar]
- 2.Feng YH, Li X, Wang L, Zhou L, Gorodeski GI. A truncated P2X7 receptor variant (P2X7-j) endogenously expressed in cervical cancer cells antagonizes the full-length P2X7 receptor through hetero-oligomerization. J Biol Chem. 2006;281:17228–37. doi: 10.1074/jbc.M602999200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li X, Zhou L, Feng YH, Abdul-Karim F, Gorodeski GI. The P2X7 receptor: a novel biomarker of uterine epithelial cancers. Cancer Epidemiol, Biomarkers Prev. 2006;15:1–8. doi: 10.1158/1055-9965.EPI-06-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Q, Wang L, Feng YH, Li X, Zeng R, Gorodeski GI. P2X7-receptor mediated apoptosis of human cervical epithelial cells. Am J Physiol. 2004;287:C1349–58. doi: 10.1152/ajpcell.00256.2004. [DOI] [PubMed] [Google Scholar]
- 5.Grahames CBA, Michel AD, Chessell IP, Humphrey DPA. Pharmacological characterization of ATP- and LPS-induced IL-1β release in human monocytes. Br J Pharmacol. 1999;127:1915–21. doi: 10.1038/sj.bjp.0702732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loomis WH, Namiki S, Ostrom RS, Insel PA, Junger WG. Hypertonic stress increases T-cell Interleukin-2 expression through a mechanism that involves ATP release, P2 Receptor, and p38 MAPK activation. J Biol Chem. 2003;278:4590–6. doi: 10.1074/jbc.M207868200. [DOI] [PubMed] [Google Scholar]
- 7.Wang Q, Li X, Wang L, Feng YH, Zeng R, Gorodeski GI. Anti-apoptotic effects of estrogen in normal and in cancer human cervical epithelial cells. Endocrinology. 2004;145:5568–79. doi: 10.1210/en.2004-0807. [DOI] [PubMed] [Google Scholar]
- 8.American College of Obstetricians and Gynecologists. Practice bulletin, clinical management guidelines for obstetrician-gynecologists, number 65, August 2005: management of endometrial cancer. Obstet Gynecol. 2005;106:413–25. doi: 10.1097/00006250-200508000-00050. [DOI] [PubMed] [Google Scholar]
- 9.Gorodeski GI, Romero MF, Hopfer U, Rorke E, Utian WH, Eckert RL. Human uterine cervical epithelial cells grown on permeable support—a new model for the study of differentiation and transepithelial transport. Differentiation. 1994;56:107–18. doi: 10.1046/j.1432-0436.1994.56120107.x. [DOI] [PubMed] [Google Scholar]
- 10.Feng YH, Wang L, Wang Q, Li X, Zeng R, Gorodeski GI. ATP stimulates GRK-3-mediated phosphorylation and β-arrestin-2- and dynamin-dependent internalization of the P2X7-receptor. Am J Physiol. 2005;288:C1342–56. doi: 10.1152/ajpcell.00315.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Feng YH, Gorodeski GI. EGF facilitates epinephrine inhibition of P2X7-receptor mediated pore formation and apoptosis: a novel signaling network. Endocrinology. 2005;146:164–74. doi: 10.1210/en.2004-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 13.Soslow RA. Problems with the current diagnostic approach to complex atypical endometrial hyperplasia. Cancer. 2006;106:729–31. doi: 10.1002/cncr.21663. [DOI] [PubMed] [Google Scholar]
- 14.Trimble CL, Kauderer J, Zaino R, Silverberg S, Lim PC, Burke JJ, et al. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group Study. Cancer. 2006;106:812–9. doi: 10.1002/cncr.21650. [DOI] [PubMed] [Google Scholar]
- 15.Zaino RJ, Kauderer J, Trimble CL, Silverberg SG, Curtin JP, Lim PC, et al. Reproducibility of the diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer. 2006;106:804–11. doi: 10.1002/cncr.21649. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell P, Tollervey D. mRNA turnover. Curr Opin Biol. 2001;13:320–5. doi: 10.1016/s0955-0674(00)00214-3. [DOI] [PubMed] [Google Scholar]
- 17.Guhaniyogi J, Brewer G. Regulation of mRNA stability in mammalian cells. Gene. 2001;265:11–23. doi: 10.1016/s0378-1119(01)00350-x. [DOI] [PubMed] [Google Scholar]
- 18.Schmeler KM, Soliman PT, Sun CC, Slomovitz BM, Gershenson DM, Lu KH. Endometrial cancer in young, normal-weight women. Gynecol Oncol. 2005;99:388–92. doi: 10.1016/j.ygyno.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 19.Silvera SAN, Rohan TE, Jain M, Terry PD, Howe GR, Miller AB. Glycaemic index, glycaemic load and risk of endometrial cancer: a prospective cohort study. Public Health Nutr. 2005;8:912–9. doi: 10.1079/phn2005741. [DOI] [PubMed] [Google Scholar]
- 20.Pillay OC, Wong Te Fong LF, Crow JC, Benjamin E, Mould T, Atiomo W, et al. The association between polycystic ovaries and endometrial cancer. Hum Reprod. 2006;21:924–9. doi: 10.1093/humrep/dei420. [DOI] [PubMed] [Google Scholar]
- 21.Sonoda Y, Barakat RR. Screening and the prevention of gynecologic cancer: Endometrial cancer. Best Pract Res: Clin Obstet Gynaecol. 2006;20:363–77. doi: 10.1016/j.bpobgyn.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Weiss JM, Saltzman BS, Doherty JA, Voigt LF, Chen C, Beresford SAA, et al. Risk factors for the incidence of endometrial cancer according to the aggressiveness of disease. Am J Epidemiol. 2006;164:56–62. doi: 10.1093/aje/kwj152. [DOI] [PubMed] [Google Scholar]
- 23.Dijkhuizen FP, Mol BW, Brolmann HA, Heintz AP. The accuracy of endometrial sampling in the diagnosis of patients with endometrial carcinoma and hyperplasia: a meta-analysis. Cancer. 2000;89:1765–72. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ygyno.2007.03.032.