Abstract
This study examined whether endogenous extracellular adenosine acts to facilitate the adaptive response of the heart to chronic systolic overload. To examine whether endogenous extracellular adenosine can protect the heart against pressure overload induced heart failure, transverse aortic constriction (TAC) was performed on mice deficient in extracellular adenosine production as the result of genetic deletion of CD73. While there was no difference in left ventricular (LV) size or function between CD73 deficient mice (KO mice) and wild type (WT) mice under unstressed conditions, aortic constriction for 2 or 4 weeks induced significantly more myocardial hypertrophy, LV dilation and LV dysfunction in KO mice compared to WT mice. Thus, after 2 weeks of TAC, LV fractional shortening decreased to 27.4±2.5% and 21.9±1.7% in WT and KO mice respectively (p<0.05). Consistent with a role of adenosine in reducing tissue remodeling, KO mice displayed increased myocardial fibrosis and myocyte hypertrophy compared to WT mice. Furthermore, adenosine treatment reduced phenylephrine induced cardiac myocyte hypertrophy and collagen production in cultured neonatal rat cardiac myocytes and cardiac fibroblasts, respectively. Consistent with a role for adenosine in modulating cardiomyocyte hypertrophy, KO mice demonstrated increased activation of mTOR signaling, accompanied by higher expression of the hypertrophy marker atrial natriuretic peptide (ANP). Conversely, the adenosine analogue 2-chloro-adenosine significantly reduced cell size, mTOR/p70S6K activation, and ANP expression in cultured neonatal cardiomyocytes. These data demonstrate that CD73 helps to preserve cardiac function during chronic systolic overload by preventing maladaptive tissue remodeling.
Keywords: hypertrophy, heart failure, fibrosis, 5'-nucleotidase, adenosine
Introduction
Adenosine is a nucleoside that is released in response to stresses that increase ATP catabolism. In the heart, adenosine acts on multiple cell types through interaction with four different adenosine receptor subtypes1-3. In addition to its well known role in ischemic pre-conditioning3, 4, there is recent evidence that the adenosine analogue 2-chloroadenosine (CADO) or treatment that increases endogenous adenosine levels (e.g., inhibition of adenosine uptake with dipyridamole) can attenuate infarct-induced LV remodeling5. Adenosine may protect against heart failure induced by pressure overload, as administration of CADO attenuated hypertrophy, fibrosis and heart failure in mice exposed to transverse aortic constriction (TAC)6. Increasing interstitial adenosine levels by blocking adenosine uptake using dipyridamole was also reported to reduce hypertrophy in rats exposed to pressure overload.7 The anti-fibrotic effects of adenosine appear to involve activation of A2b receptors8, 9, while a role for cardiac adenosine A1 receptors has been suggested in the adenosine mediated reduction of cardiomyocyte hypertrophy6. Interestingly, endogenous adenosine levels rise during compensatory hypertrophy, but are diminished as hearts become decompensated 10, 11. It has thus been suggested that modulation of adenosine levels may be a target for treatment of ventricular dysfunction in failing hearts11. However, whether reduction of endogenous extracellular adenosine levels can contribute to the development of heart failure in the overloaded heart is not known.
The membrane-anchored cell surface enzyme CD73 catalyzes the conversion of extracellular AMP to adenosine, thereby increasing extracellular adenosine production 12. Darvish et. al. reported that CD73 activity accounts for approximately 46% of total adenosine production in rat heart homogenates 13, while other studies also demonstrated that CD73 mediated adenosine production is critical to ischemic pre-conditioning14,15, implying that CD73 contributes significantly to extracellular myocardial adenosine production under stress. The actual contribution of CD73 and the role of adenosine production in the development and progression of heart failure under chronic systolic overload are not known. Here we used CD73-KO mice to investigate the role of CD73 in protection against heart failure during chronic pressure overload. Our data demonstrate that CD73-KO exacerbated systolic overload induced ventricular hypertrophy, fibrosis and dysfunction. Furthermore, collagen production, cardiomyocyte hypertrophy, and mTOR activation were directly inhibited by adenosine or CADO in isolated cultures of cardiac fibroblasts or cardiomyocytes, respectively. These results indicate that CD73 activity and endogenous extracellular adenosine play a significant role in protection against systolic overload induced ventricular hypertrophy, fibrosis and congestive heart failure.
Materials and methods
Mice and TAC procedure
CD73-KO mice (129 background) and control wild type mice were generated as previously described 16 . This study was approved by the Institutional Animal Care and Use Committee of University of Minnesota. TAC was performed using the minimally invasive suprasternal approach described16.
Echocardiography and Western blots were performed with methods as previously described17, 18. For details, please see the online data supplement available at http://hyper.ahajournals.org.
Neonatal rat cardiomyocyte (NVM) isolation and culture
NVM were isolated from 2-day-old Sprague-Dawley rats by enzymatic digestion19 and separated from non-muscle cells on a discontinuous Percoll gradient as previously described19. Detailed method is included in the online supplementary data section (http://hyper.ahajournals.org).
Results
CD73 Knockout exacerbates TAC-induced myocardial hypertrophy, fibrosis and dysfunction in the overloaded heart
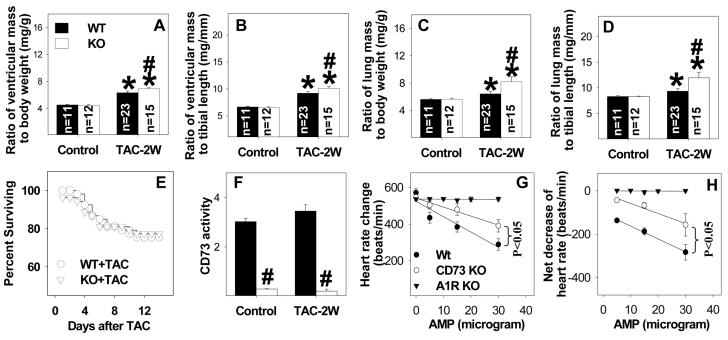
Under basal conditions, we observed no statistical differences in LV structure or function (Figures 1-3, Table 1) between CD73 KO mice and their wild type control littermates. To examine the role of extracellular adenosine in modulating the response to systolic overload, we exposed KO mice and WT mice to TAC. In response to TAC for 2 weeks, KO mice developed significantly greater increases of ventricular weight and the ratio of ventricular weight to body weight or tibia length than WT mice (Table 1, Figure 1A, 1B), indicating that KO exacerbated the TAC-induced myocardial hypertrophy. In addition, the ratio of lung weight to body weight or tibia length was significantly greater in KO mice as compared to WT mice 2 weeks after TAC, indicating more pulmonary congestion in the KO mice (Figure 1C, 1D, Table 1). During the 2 weeks of study, the mortality rate following TAC was not different between KO mice and WT mice (Figure 1E).
Figure 1.
CD73 KO exacerbates TAC-induced ventricular hypertrophy (A, B), and pulmonary congestion (C, D). KO had no effect on TAC induced mortality (E), but decreased CD73 activity (F). KO significantly attenuated the 5'-AMP induced decrease of heart rate (G, H), consistent with the diminished capacity of KO mice to produce extracellular adenosine from 5'-AMP. *P<0.05 compared to the corresponding control; #p<0.05 compared to wild type mice. CD73 activity was obtained from 4-5 mice per group (F). The decrease of heart rate in response to 5'-AMP infusion was obtained from KO (n=5), wild type littermates of KO mice (n=5), and adenosine A1 receptor KO mice (n=3). The rest of the data was obtained from 11- 23 mice per group as labeled.
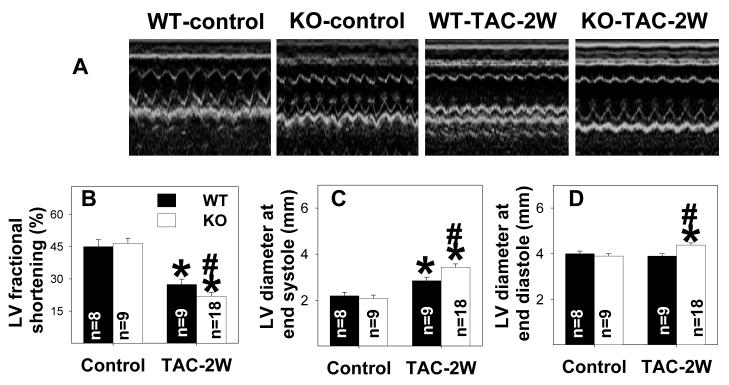
Figure 3.
CD73 KO exacerbates TAC-induced ventricular dysfunction (A, B) and dilation (C, D). *P<0.05 compared to the corresponding control; #p<0.05 compared to WT.
Table 1.
Anatomic and functional data for wild type and CD73-KO mice.
| Parameter | Wild type control |
CD73 -KO control |
Wild type- TAC |
CD73-KO TAC |
|---|---|---|---|---|
| Body weight (g) | 25.8 ± 0.4 | 26.1 ± 0.6 | 25.8 ± 1.0 | 25.8 ± 0.7 |
| Ventricular mass (mg) | 116 ± 2.0 | 115 ± 2.4 | 161 ± 4.9* | 178 ± 5.7*,† |
| Ratio of ventricular mass to body weight (mg/g) |
4.52 ± 0.08 | 4.44 ± 0.10 | 6.33 ± 0.23† | 6.91 ± 0.14*,† |
| Lung mass (mg) | 145 ± 2.4 | 146 ± 1.6 | 164 ± 8.4* | 211 ± 18.5*,† |
| Ratio of lung mass to body weight (mg/g) |
5.63 ± 0.08 | 5.65 ± 0.15 | 6.43± 0.36* | 8.20 ± 0.73*,† |
| Tibia length (mm) | 17.5 ± 0.14 | 17.5 ± 0.14 | 17.5 ± 0.13 | 17.6 ± 0.12 |
| Heart rate (beats per minute) | 499 ± 37 | 511 ± 17 | 466 ± 15* | 444 ± 7.8* |
| LV end systolic diameter (mm) | 2.21 ± 0.15 | 2.09 ± 0.14 | 2.85 ± 0.16* | 3.44 ± 0.14*,† |
| LV end diastolic diameter (mm) | 4.01 ± 0.12 | 3.90 ± 0.12 | 3.91 ± 0.11* | 4.38 ± 0.10*,† |
| LV ejection fraction (%) | 82.2 ± 2.6 | 84.0 ± 2.2 | 60.6 ± 3.7* | 51.3 ± 3.0*,† |
| LV posterior wall thickness at end diastole (mm) |
0.69 ± 0.01 | 0.68 ± 0.03 | 0.97 ± 0.03* | 0.94 ± 0.03* |
| LV posterior wall thickness at end systole (mm) |
1.14 ± 0.03 | 1.09 ± 0.02 | 1.25 ± 0.02* |
1.26 ± 0.23* |
| Mean aortic pressure (mmHg) | 87.1 ± 5.9 | 85.5 ± 2.7 | 96 ± 3.3 | 92 ± 3.6 |
| Systolic LV pressure (mmHg) | 106 ± 4.6 | 103 ± 2.5 | 153 ± 3.5* | 148 ± 4.7*,† |
| LV end diastolic pressure (mmHg) |
9.5 ± 1.1.7 | 8.8 ± 1.2 | 20 ± 2.9* | 25 ± 4.4* |
| LV dP/dtmax (mmHg/s) | 8791 ± 855 | 7181 ± 390 | 7171 ± 286* | 5208 ± 350*,† |
| LV dP/dtmin (mmHg/s) | −7638 ± 743 |
−6526 ± 284 |
−7984 ± 271 | −6036 ± 496† |
p<0.05 as compared with corresponding control conditions
p<0.05 as compared with WT mice.
The anatomic data were obtained from all mice studied (11-23 mice/group). The hemodynamic data were obtained from 8-9 mice/group after TAC, and 5 mice/group under control conditions.
As anticipated, myocardial CD73 activity was abolished in KO mice as compared to WT mice under control conditions or after 2 weeks TAC (Figure 1F). In addition, KO significantly attenuated 5'-AMP induced bradycardia, indicating that KO significantly disrupted extracellular adenosine production from 5'-AMP (Figure 1G, 1H). Adenosine A1 receptor KO almost totally abolished 5'-AMP induced bradycardia (Figure 1G, 1H), consistent with the concept that extracellular adenosine caused bradycardia through activation of the adenosine A1 receptor.
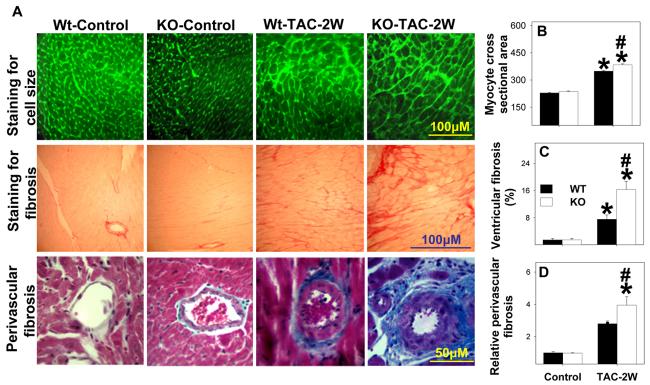
Histological analysis demonstrated that TAC resulted in more ventricular fibrosis (Figure 2), and a greater increase in cardiac myocyte cross-sectional area (Figure 2) in KO mice as compared with WT mice, indicating that the greater ventricular hypertrophy in the KO mice after TAC was due to both larger cardiomyocytes and an increase of fibrosis. The fibrosis after TAC in both wild type and KO mice was more apparent in the perivascular region. In comparison with WT mice, the relative increase in myocardial fibrosis following TAC in the KO mice was much greater than the relative increase in myocyte hypertrophy.
Figure 2.
CD73 KO exacerbates TAC-induced cardiac myocyte hypertrophy (A, B), ventricular fibrosis (A, C) and perivascular fibrosis (A, D). *P<0.05 compared to the corresponding control; #p<0.05 compared to WT (n=4 mice/group).
Echocardiographic imaging of the heart 2 weeks after TAC (Figure 3A) demonstrated significant increases of LV wall thickness (Table 1) and LV end-diastolic diameter (Figure 3). TAC for 2 weeks resulted in significant impairment of LV systolic function in the KO mice, as demonstrated by a greater reduction of systolic fractional shortening (Figure 3B) and a significant increase in LV end-systolic diameter as compared to WT mice (Figure 3C). TAC for 2 weeks also resulted in significant impairment of LV contractility in the KO mice, as demonstrated by a greater reduction of LV dP/dtmax and LV dP/dtmin as compared to WT mice (Table 1). After TAC for 2 weeks, mean aortic pressure and LV systolic pressure were significantly lower in KO mice than WT mice (Table 1), consistent with the finding of more ventricular dysfunction in KO mice.
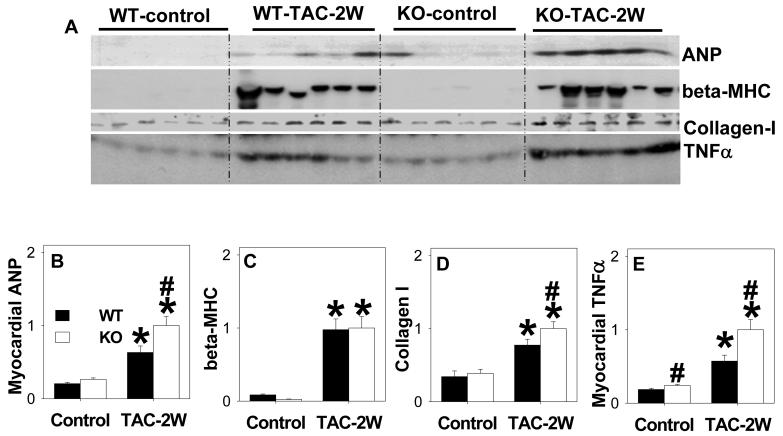
Consistent with increased hypertrophy in KO mice, myocardial ANP protein was significantly higher in KO mice than WT mice after TAC (Figure 4A, 4B). Consistent with the greater increase of LV fibrosis after TAC, KO mice hearts contained higher myocardial collagen-I content than WT mice. Myocardial TNFα levels were also significantly higher in the KO than WT mice after TAC (Figure 4a, 4e), suggesting an increased inflammatory response in the KO mice. This observation is in agreement with reports that adenosine can reduce cardiac TNFα expression20.
Figure 4.
CD73-KO significantly exacerbated TAC induced increase of myocardial ANP, β-MHC, Type I Collagen and TNFα *P<0.05 relative to sham; # P<0.05 compared to WT (n=6 samples/group).
CD73 KO enhances Akt- mTOR-p70S6K activation
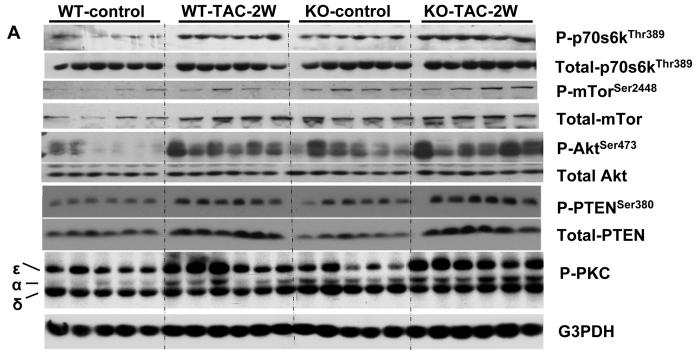
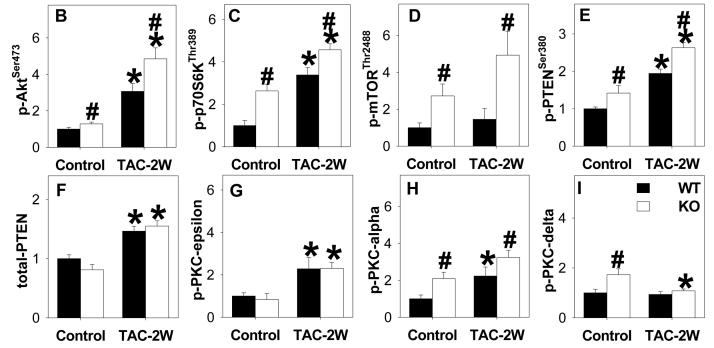
The PI-3 kinase/AKT signaling pathways target mTOR activity to increase translation of proteins important for cell growth21. Signaling from mTOR appears critical for cardiac hypertrophy, and also promotes the transition to heart failure during chronic pressure overload22. Akt can increase activation of mTOR indirectly by reducing TSC2 activity23, and it is also a direct target of mTOR kinase activity24. Interestingly, Western blot analysis revealed that the mTOR effector phosphorylation sites at AktSer473 and p70S6KThr389 were significantly increased in the KO mice above levels found in WT mice even under basal conditions. In WT mice, TAC increased levels of p-AktSer473 and p-70S6KThr389, and both were further elevated in the KO mice. Consistent with increased phosphorylation of mTOR targets in the KO mice, KO mice demonstrated higher levels of p-mTORSer2488 as compared with WT mice 2 weeks after TAC (Figure 5). The increased levels of p-mTORSer2488 in KO mice were the result of both increased mTOR expression, as well as increased phosphorylation relative to total levels. The lipid phosphatase known as Phosphatase and Tensin Homologue on Chromosome Ten (PTEN) can down regulate PI-3 kinase signaling by dephosphorylating phosphatidylinositol 3,4,5-trisphosphate, and mutations that inhibit PTEN or cardiac specific deletion of PTEN result in constitutive Akt activity25. Under basal conditions, phosphorylation at serine 380 (Serine 380 phosphorylation of PTEN involved in reducing membrane association and activity) was significantly increased, while total PTEN levels were slightly, but not significantly (p=0.06) lower in KO mice. TAC increased expression of PTEN to similar levels in both WT and KO mice, while phospho-PTENSer380 was raised to significantly higher levels in KO than WT, suggesting that PI-3 kinase signaling may be particularly enhanced in these mice via down regulation of PTEN activity. In addition, TAC for 2 weeks resulted in greater increases of p-PKCα in KO mice as compared with WT mice (Figure 5).
Figure 5.
Systolic overload produced by TAC for 2 weeks increased phosphorylated AKTser473, p70S6 kinasethr 389 and mTORser2448 in heart lysates as measured by western blot and scanning densitometry. Phosphorylation of these sites in KO was significantly elevated above levels WT animals. The results indicate that KO increased mTOR signaling after TAC. KO also increased PKCα under both control conditions and after TAC. *P<0.05 relative to sham; # P<0.05 compared to WT (n= 4 -6 samples/group).
Adenosine or adenosine analogue attenuates cardiac myocyte hypertrophy and activation of mTOR and p70S6K
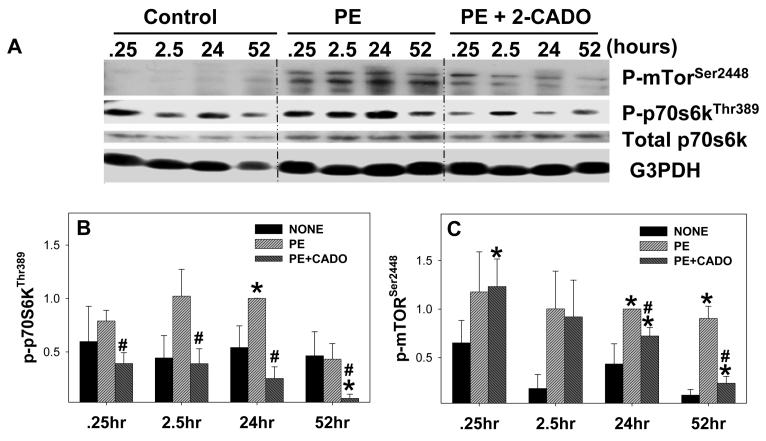
Because in vivo adenosine can increase blood flow26, reduce inflammatory responses20, inhibit norepinephrine release from nerve endings27, and decrease ET-1 production28, it was important to determine whether the amplified mTOR/p70S6 signaling in the KO mice was the result of indirect effects of adenosine that caused paracrine regulation of these signaling pathways, or a direct effect of adenosine on cardiomyocytes. Therefore, we examined the effect of the adenosine analogue CADO on phenylephrine (PE)-induced hypertrophy and activation of p-mTORSer2488 and p70S6KThr389 in isolated neonatal cardiomyocytes. PE significantly increased the size of the cardiac myocytes and expression of the hypertrophy marker ANP, while CADO significantly attenuated the PE-induced increase in cell size and reduced ANP expression (Figure S1, http://hyper.ahajournals.org). PE treatment also significantly increased phosphorylation of mTORSer2448 and p-70S6KThr389, and this activation was dramatically reduced by CADO (Figure 6). Similarly, adenosine attenuated the PE-induced increase of cardiac myocyte size and activation of p-70S6KThr389 and p-mTORSer2488 (data not shown).
Figure 6.
2-chloroadenosine (2-CADO, 5μM) time-dependently reduced phenylephrine (PE) induced phosphorylation of mTOR and p70S6 kinase in cultured neonatal cardiomyocytes(A,B,C). Cultured neonatal cardiomyocytes were treated with PE in the presence or absence of 2-CADO. At the indicated time points, lysates were collected and analyzed by western blot for phosphorylation of p70S6 kinasethr 389(B), and mTORser2448(C). Glyceraldehyde 3-phosphate dehydrogenase and total p70S6 kinase are shown as controls. Each bar represents average from 3-7 individual experiments. *P<0.05 relative to untreated; # P<0.05 compared to PE treated.
Adenosine attenuates collagen synthesis and fibroblast proliferation
We also determined the effect of adenosine on cardiac fibroblast proliferation and collagen production, and the results showed that adenosine significantly reduced cardiac fibroblast proliferation and collagen production (Figure S2, see the online data supplement available at http://hyper.ahajournals.org), which is in agreement with previous reports9.
Discussion
The major finding in this study is that deletion of CD73 significantly exacerbates LV hypertrophy, dilation and dysfunction in the TAC model of LV pressure overload. These results suggest that conversion of extracellular AMP to adenosine plays a significant role in modulating maladaptive tissue remodeling and hypertrophic signaling pathways activated in response to systolic overload. The greater ventricular hypertrophy in CD73 KO mice after TAC was the result of both increased fibrosis and greater cardiomyocyte hypertrophy. The more prominent hypertrophy and dysfunction in the KO hearts was associated with increased activation of the mTOR signaling pathway. Moreover, the demonstration that adenosine or the stable adenosine analogue CADO attenuated phenylephrine-induced cardiomyocyte mTOR/p70S6 kinase signaling and myocyte hypertrophy, as well as fibroblast collagen production, suggests that the in vivo effects of CD73 deletion can be attributed to loss of adenosine production. Taken together, these findings provide the first direct evidence that endogenous extracellular adenosine exerts a protective effect against ventricular tissue remodeling and cardiac myocyte hypertrophic signaling pathways during chronic systolic overload.
Although no previous reports have directly examined the effect of CD73 on pressure overload-induced ventricular remodeling, there is evidence that increased endogenous adenosine can attenuate the development of cardiovascular disease29. Adenosine is known to inhibit norepinephrine release from presynaptic vesicles27, reduce production of ET-128, and reduce TNF-α production20. Recently we also found that while 8-SPT significantly increased myocardial oxygen consumption in dogs with failing hearts, it had no effect on oxygen consumption in normal dogs26, suggesting that endogenous adenosine may help reduce myocardial oxygen demand particularly in the failing heart. Studies in rats in which extracellular adenosine was increased by blockade of adenosine uptake with dipyridamole7 also reported attenuation of pressure overload induced myocardial hypertrophy. Interestingly, a mutation of the adenosine monophosphate deaminase 1 gene (which results in increased adenosine production) predicted a better clinical outcome in patients after myocardial infarction30, implying that increased endogenous adenosine levels can exert a protective effect on the diseased human heart. Myocardial adenosine concentrations increase during the compensated phase of ventricular hypertrophy, but then decrease when there is evidence of decompensation10, 11, suggesting that a decrease of extracellular adenosine levels might be a contributing factor in the transition to heart failure. Our finding that a genetically engineered loss of CD73 activity exacerbated TAC-induced ventricular hypertrophy and dysfunction provide further evidence that endogenous adenosine production protects against progression to heart failure under conditions of pressure overload.
Adenosine Effects on Cardiomyocyte Hypertrophic Signaling
The present data identify a major hypertrophic signaling pathway targeted by extracellular adenosine. KO mice demonstrated increased phosphorylation of p-mTORSer2488, p-70S6KThr389 and pAKTSer473, suggesting that adenosine normally acts to down-regulate these signaling pathways. Activation of mTOR and its downstream targets results in increased cell size and is commonly associated with cardiac hypertrophy. Furthermore, over-expression of p70s6 kinase resulted in cardiac hypertrophy31, while inhibition of mTOR signaling with rapamycin attenuated the development of ventricular hypertrophy in mice exposed to ascending aortic constriction22. The finding that phospho-PTENSer380 was increased under basal conditions and after TAC suggests that PI-3 kinase signaling may be particularly enhanced in KO mice by the increase of phospho-PTENSer380, and may explain increased levels of mTOR and AKT activation25. The direct effect of CADO on inhibition of cardiac myocyte hypertrophy and mTOR/p70S6 kinase activation in isolated cardiomyocytes confirms that adenosine regulates mTOR signaling. Interestingly, PKCα phosphorylation within the activation loop was also significantly increased in CD73 KO mice compared to WT mice under both basal conditions and during TAC. Increased PKCα activity may contribute to the reduced contractility found in CD73 KO mice during pressure overload, as PKCα deletion has been shown to increase contractility and protect against heart failure from pressure overload32.
Extracellular Adenosine Protection against Systolic Overload
The specific roles of adenosine receptor subtypes in protection against pathologic hypertrophy have not been well defined. While an A1 receptor agonist has been shown to reduce hypertrophy and heart failure in response to pressure overload, and also reduces hypertrophy of isolated cardiomyocytes6, transgenic over-expression of A1 or A3 receptors in the heart actually promotes cardiac hypertrophy and dilation33, 34. The role of the A2b receptor is slightly more well defined, as most published data suggests a role in reducing cardiac fibroblast proliferation and collagen synthesis8, 9. The A2b receptor also plays a role in ameliorating pathological LV tissue remodeling after infarct15. Activation of type 2A adenosine receptors, which are highly expressed in the coronary vasculature, can down regulate v-cam expression35 to reduce monocyte adhesion to endothelial cells and vascular inflammation. The increased vascular inflammation and fibrosis in the CD73 KO mice after TAC suggests that A2A and A2b receptors may not be adequately activated in absence of CD73 dependent adenosine production. In addition to increased vascular inflammation, CD73 KO has been reported to cause a 15% decrease in basal coronary flow36. While this modest decrease in coronary flow would not likely affect cardiac function under basal conditions, abnormalities of coronary flow might impair oxygen delivery during pressure overload, when oxygen demand is increased and diffusion distances are increased by perivascular fibrosis. It is therefore probable that the protective effects of extracellular adenosine against the TAC induced ventricular hypertrophy and dysfunction are not mediated by activation of any individual adenosine receptor subtype alone, but more likely involves complimentary effects of multiple adenosine receptor subtypes on multiple cardiac cell types. Additional studies will be needed to distinguish the specific adenosine receptors and cell types which mediate the protective effects of adenosine in the pressure overloaded heart.
Limitations
Although we demonstrated that CD73-KO abolished myocardial CD73 activity, and a previous study using the same mouse strain demonstrated that KO abolished extracellular adenosine production in other tissues, we were not able to collect extracellular fluid from the mouse heart for adenosine analysis due to the small size of the heart. Secondly, as all of the adenosine receptors are expressed in the heart, future studies will be needed to determine the specific adenosine receptor(s) responsible for the protective effect against pressure overload induced ventricular hypertrophy.
Perspectives
Previous studies have demonstrated that adenosine analogues and selective adenosine A1 or A3 receptor agonists protect the heart from ischemia/reperfusion induced myocardial damage. However, the effect of endogenous extracellular adenosine on chronic pressure overload induced ventricular hypertrophy and heart failure has not been previously studied. Here we demonstrated that loss of CD73 activity exacerbates the ventricular hypertrophy, fibrosis and dysfunction that occur in the heart exposed to chronic hemodynamic overload. This study also identifies, for the first time, a specific hypertrophic signaling pathway (mTOR-p70S6K) that is targeted by adenosine and which may explain the anti-hypertrophic effects of adenosine. These findings provide the first direct evidence that endogenous extracellular adenosine plays an important role in regulating pressure overload induced ventricular remodeling, indicating that increasing extracellular adenosine production or activation of specific adenosine receptors may be a therapeutic approach for treating the pressure overloaded heart.
Supplementary Material
Acknowledgments
Sources of Funding: This study was supported by NHLBI Grants HL71790 (YC) and HL21872 (RJB) from the NIH, and a Scientist Development Grant 0730451N (JF) and a Postdoctoral Fellowship 0725795Z (XX) from the American Heart Association.
Footnotes
Disclosures: None
References
- 1.Peart JN, Headrick JP. Adenosinergic cardioprotection: multiple receptors, multiple pathways. Pharmacol Ther. 2007;114:208–221. doi: 10.1016/j.pharmthera.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Ashton KJ, Peart JN, Morrison RR, Matherne GP, Blackburn MR, Headrick JP. Genetic modulation of adenosine receptor function and adenosine handling in murine hearts: insights and issues. J Mol Cell Cardiol. 2007;42:693–705. doi: 10.1016/j.yjmcc.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Donato M, Gelpi RJ. Adenosine and cardioprotection during reperfusion--an overview. Mol Cell Biochem. 2003;251:153–159. [PubMed] [Google Scholar]
- 4.Baxter GF, Marber MS, Patel VC, Yellon DM. Adenosine receptor involvement in a delayed phase of myocardial protection 24 hours after ischemic preconditioning. Circulation. 1994;90:2993–3000. doi: 10.1161/01.cir.90.6.2993. [DOI] [PubMed] [Google Scholar]
- 5.Wakeno M, Minamino T, Seguchi O, Okazaki H, Tsukamoto O, Okada K, Hirata A, Fujita M, Asanuma H, Kim J, Komamura K, Takashima S, Mochizuki N, Kitakaze M. Long-term stimulation of adenosine A2b receptors begun after myocardial infarction prevents cardiac remodeling in rats. Circulation. 2006;114:1923–1932. doi: 10.1161/CIRCULATIONAHA.106.630087. [DOI] [PubMed] [Google Scholar]
- 6.Liao Y, Takashima S, Asano Y, Asakura M, Ogai A, Shintani Y, Minamino T, Asanuma H, Sanada S, Kim J, Ogita H, Tomoike H, Hori M, Kitakaze M. Activation of adenosine A1 receptor attenuates cardiac hypertrophy and prevents heart failure in murine left ventricular pressure-overload model. Circ Res. 2003;93:759–766. doi: 10.1161/01.RES.0000094744.88220.62. [DOI] [PubMed] [Google Scholar]
- 7.Chung ES, Perlini S, Aurigemma GP, Fenton RA, Dobson JG, Jr., Meyer TE. Effects of chronic adenosine uptake blockade on adrenergic responsiveness and left ventricular chamber function in pressure overload hypertrophy in the rat. J Hypertens. 1998;16:1813–1822. doi: 10.1097/00004872-199816120-00015. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Epperson S, Makhsudova L, Ito B, Suarez J, Dillmann W, Villarreal F. Functional effects of enhancing or silencing adenosine A2b receptors in cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2004;287:H2478–2486. doi: 10.1152/ajpheart.00217.2004. [DOI] [PubMed] [Google Scholar]
- 9.Dubey RK, Gillespie DG, Jackson EK. Adenosine inhibits collagen and protein synthesis in cardiac fibroblasts: role of A2B receptors. Hypertension. 1998;31:943–948. doi: 10.1161/01.hyp.31.4.943. [DOI] [PubMed] [Google Scholar]
- 10.Meyer TE, Chung ES, Perlini S, Norton GR, Woodiwiss AJ, Lorbar M, Fenton RA, Dobson JG., Jr. Antiadrenergic effects of adenosine in pressure overload hypertrophy. Hypertension. 2001;37:862–868. doi: 10.1161/01.hyp.37.3.862. [DOI] [PubMed] [Google Scholar]
- 11.Funakoshi H, Zacharia LC, Tang Z, Zhang J, Lee LL, Good JC, Herrmann DE, Higuchi Y, Koch WJ, Jackson EK, Chan TO, Feldman AM. A1 adenosine receptor upregulation accompanies decreasing myocardial adenosine levels in mice with left ventricular dysfunction. Circulation. 2007;115:2307–2315. doi: 10.1161/CIRCULATIONAHA.107.694596. [DOI] [PubMed] [Google Scholar]
- 12.Castrop H, Huang Y, Hashimoto S, Mizel D, Hansen P, Theilig F, Bachmann S, Deng C, Briggs J, Schnermann J. Impairment of tubuloglomerular feedback regulation of GFR in ecto-5'-nucleotidase/CD73-deficient mice. J Clin Invest. 2004;114:634–642. doi: 10.1172/JCI21851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darvish A, Pomerantz RW, Zografides PG, Metting PJ. Contribution of cytosolic and membrane-bound 5'-nucleotidases to cardiac adenosine production. Am J Physiol. 1996;271:H2162–2167. doi: 10.1152/ajpheart.1996.271.5.H2162. [DOI] [PubMed] [Google Scholar]
- 14.Kitakaze M, Hori M, Morioka T, Minamino T, Takashima S, Okazaki Y, Node K, Komamura K, Iwakura K, Itoh T, Inoue M, Kamada T. Alpha 1-adrenoceptor activation increases ecto-5'-nucleotidase activity and adenosine release in rat cardiomyocytes by activating protein kinase C. Circulation. 1995;91:2226–2234. doi: 10.1161/01.cir.91.8.2226. [DOI] [PubMed] [Google Scholar]
- 15.Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK. Cardioprotection by ecto-5'-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 16.Hu P, Zhang D, Swenson L, Chakrabarti G, Abel ED, Litwin SE. Minimally invasive aortic banding in mice: effects of altered cardiomyocyte insulin signaling during pressure overload. Am J Physiol Heart Circ Physiol. 2003;285:H1261–1269. doi: 10.1152/ajpheart.00108.2003. [DOI] [PubMed] [Google Scholar]
- 17.Zhang P, Xu X, Hu X, van Deel ED, Zhu G, Chen Y. Inducible nitric oxide synthase deficiency protects the heart from systolic overload-induced ventricular hypertrophy and congestive heart failure. Circ Res. 2007;100:1089–1098. doi: 10.1161/01.RES.0000264081.78659.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu Z, Xu X, Hu X, Zhu G, Zhang P, van Deel ED, French JP, Fassett JT, Oury TD, Bache RJ, Chen Y. Extracellular superoxide dismutase deficiency exacerbates pressure overload-induced left ventricular hypertrophy and dysfunction. Hypertension. 2008;51:19–25. doi: 10.1161/HYPERTENSIONAHA.107.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang W, Anger T, Su J, Hao J, Xu X, Zhu M, Gach A, Cui L, Liao R, Mende U. Selective loss of fine tuning of Gq/11 signaling by RGS2 protein exacerbates cardiomyocyte hypertrophy. J Biol Chem. 2006;281:5811–5820. doi: 10.1074/jbc.M507871200. [DOI] [PubMed] [Google Scholar]
- 20.Wagner DR, Combes A, McTiernan C, Sanders VJ, Lemster B, Feldman AM. Adenosine inhibits lipopolysaccharide-induced cardiac expression of tumor necrosis factor-alpha. Circ Res. 1998;82:47–56. doi: 10.1161/01.res.82.1.47. [DOI] [PubMed] [Google Scholar]
- 21.Proud CG. Ras, PI3-kinase and mTOR signaling in cardiac hypertrophy. Cardiovasc Res. 2004;63:403–413. doi: 10.1016/j.cardiores.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 22.McMullen JR, Sherwood MC, Tarnavski O, Zhang L, Dorfman AL, Shioi T, Izumo S. Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation. 2004;109:3050–3055. doi: 10.1161/01.CIR.0000130641.08705.45. [DOI] [PubMed] [Google Scholar]
- 23.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 24.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 25.Crackower MA, Oudit GY, Kozieradzki I, Sarao R, Sun H, Sasaki T, Hirsch E, Suzuki A, Shioi T, Irie-Sasaki J, Sah R, Cheng HY, Rybin VO, Lembo G, Fratta L, Oliveira-dos-Santos AJ, Benovic JL, Kahn CR, Izumo S, Steinberg SF, Wymann MP, Backx PH, Penninger JM. Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell. 2002;110:737–749. doi: 10.1016/s0092-8674(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 26.Traverse JH, Chen Y, Hou M, Li Y, Bache RJ. Effect of K+ATP channel and adenosine receptor blockade during rest and exercise in congestive heart failure. Circ Res. 2007;100:1643–1649. doi: 10.1161/CIRCRESAHA.107.150219. [DOI] [PubMed] [Google Scholar]
- 27.Rongen GA, Lenders JW, Lambrou J, Willemsen JJ, Van Belle H, Thien T, Smits P. Presynaptic inhibition of norepinephrine release from sympathetic nerve endings by endogenous adenosine. Hypertension. 1996;27:933–938. doi: 10.1161/01.hyp.27.4.933. [DOI] [PubMed] [Google Scholar]
- 28.Pelletier S, Dube J, Villeneuve A, Gobeil F, Jr., Bernier SG, Battistini B, Guillemette G, Sirois P. Adenosine induces cyclic-AMP formation and inhibits endothelin-1 production/secretion in guinea-pig tracheal epithelial cells through A(2B) adenosine receptors. Br J Pharmacol. 2000;129:243–250. doi: 10.1038/sj.bjp.0702999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Bache RJ. Adenosine: a modulator of the cardiac response to stress. Circ Res. 2003;93:691–693. doi: 10.1161/01.RES.0000097920.18551.36. [DOI] [PubMed] [Google Scholar]
- 30.Loh E, Rebbeck TR, Mahoney PD, DeNofrio D, Swain JL, Holmes EW. Common variant in AMPD1 gene predicts improved clinical outcome in patients with heart failure. Circulation. 1999;99:1422–1425. doi: 10.1161/01.cir.99.11.1422. [DOI] [PubMed] [Google Scholar]
- 31.McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Dorfman AL, Longnus S, Pende M, Martin KA, Blenis J, Thomas G, Izumo S. Deletion of ribosomal S6 kinases does not attenuate pathological, physiological, or insulin-like growth factor 1 receptor-phosphoinositide 3-kinase-induced cardiac hypertrophy. Mol Cell Biol. 2004;24:6231–6240. doi: 10.1128/MCB.24.14.6231-6240.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, Kimball TF, Lorenz JN, Nairn AC, Liggett SB, Bodi I, Wang S, Schwartz A, Lakatta EG, DePaoli-Roach AA, Robbins J, Hewett TE, Bibb JA, Westfall MV, Kranias EG, Molkentin JD. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med. 2004;10:248–254. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- 33.Funakoshi H, Chan TO, Good JC, Libonati JR, Piuhola J, Chen X, MacDonnell SM, Lee LL, Herrmann DE, Zhang J, Martini J, Palmer TM, Sanbe A, Robbins J, Houser SR, Koch WJ, Feldman AM. Regulated overexpression of the A1-adenosine receptor in mice results in adverse but reversible changes in cardiac morphology and function. Circulation. 2006;114:2240–2250. doi: 10.1161/CIRCULATIONAHA.106.620211. [DOI] [PubMed] [Google Scholar]
- 34.Black RG, Jr., Guo Y, Ge ZD, Murphree SS, Prabhu SD, Jones WK, Bolli R, Auchampach JA. Gene dosage-dependent effects of cardiac-specific overexpression of the A3 adenosine receptor. Circ Res. 2002;91:165–172. doi: 10.1161/01.res.0000028007.91385.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zernecke A, Bidzhekov K, Ozuyaman B, Fraemohs L, Liehn EA, Luscher-Firzlaff JM, Luscher B, Schrader J, Weber C. CD73/ecto-5′-nucleotidase protects against vascular inflammation and neointima formation. Circulation. 2006;113:2120–2127. doi: 10.1161/CIRCULATIONAHA.105.595249. [DOI] [PubMed] [Google Scholar]
- 36.Koszalka P, Ozuyaman B, Huo Y, Zernecke A, Flogel U, Braun N, Buchheiser A, Decking UK, Smith ML, Sevigny J, Gear A, Weber AA, Molojavyi A, Ding Z, Weber C, Ley K, Zimmermann H, Godecke A, Schrader J. Targeted disruption of cd73/ecto-5′-nucleotidase alters thromboregulation and augments vascular inflammatory response. Circ Res. 2004;95:814–821. doi: 10.1161/01.RES.0000144796.82787.6f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.