Abstract
Women with a history of preeclampsia or eclampsia (seizure during preeclamptic pregnancy) are at increased risk for cardiovascular disease after pregnancy for reasons that remain unclear. Prospective studies during pregnancy suggest that inflammation, dyslipidemia, and insulin resistance are associated with increased risk of preeclampsia. Elevated serum C-reactive protein (CRP >3 mg/L) is an indicator of inflammation and cardiovascular risk. We hypothesized that Icelandic postmenopausal women with a history of eclampsia would manifest higher concentrations of serum CRP than Icelandic postmenopausal controls with a history of uncomplicated pregnancies. We also asked whether elevated CRP is associated with the dyslipidemia and insulin resistance previously identified in this cohort. CRP, measured by high-sensitivity enzyme-linked immunoassay, was higher in women with prior eclampsia (n=25) than controls (n=28) (median mg/L [interquartile range]: 9.0 [0.9 to 13.2] versus 2.0 [0.3 to 5.1]; P<0.03). This difference remained significant after adjustment for body mass index, smoking, hormone replacement, and current age. Women with prior eclampsia clustered into either high CRP (range 8.97 to 40.6 mg/L, n=13) or lower CRP (median 1.0, range 0.05 to 3.77, n=12) subsets. The prior eclampsia/high CRP subset displayed significantly elevated systolic blood pressures, lower high-density lipoprotein (HDL) cholesterol, higher apolipoprotein B, and higher fasting insulin and homeostasis model of insulin resistance (HOMA) values compared to controls, whereas the prior eclampsia/low CRP subset differed from controls only by marginally increased apolipoprotein B. The triad of inflammation, low HDL, and insulin resistance may elevate risk for both preeclampsia/eclampsia and cardiovascular disease in later life.
Keywords: preeclampsia, eclampsia, cardiovascular disease, C-reactive protein, insulin resistance, inflammation, HDL cholesterol
Preeclampsia is a human pregnancy-specific syndrome that adversely affects the mother (by vascular endothelial dysfunction) and often the fetus (by intrauterine growth restriction, early delivery, and a 5-fold increased risk of fetal demise).1,2 Eclampsia (seizure or coma unrelated to other cerebral conditions, usually heralded by signs and symptoms of preeclampsia) is a severe variant of the preeclampsia syndrome.3 Although the defining characteristics of preeclampsia—hypertension and proteinuria developing after 20 weeks of gestation—typically remit within a few days after delivery, women with a history of preeclampsia have a 2- to 4-fold increased risk of developing hypertension, coronary artery disease, or stroke in later years.4,5 The increase in mortality from cardiovascular disease generally becomes evident 2 to 3 decades after pregnancy in women with a history of nonrecurrent preeclampsia, substantially later than women who have had preeclampsia more than once.4 There are increasing indications that preeclampsia and later cardiovascular disease have a common pathogenesis involving vascular endothelial dysfunction promoted by underlying risk factors related to the insulin resistance (metabolic) syndrome, including hyperinsulinemia, dyslipidemia, chronic inflammation, obesity, and family history of cardiovascular disease.1,5-11
C-reactive protein (CRP) is an important component of the innate immune system. When measured by high-sensitivity assay, elevated serum CRP provides a sensitive biomarker of chronic systemic inflammation.12 Over 20 large-scale prospective studies show that CRP is an independent predictor of future cardiovascular events.12,13 Evidence also implicates CRP, and thus inflammation, as a useful clinical measure for identifying risk of developing the insulin resistance syndrome, particularly in women.14,15
Serum CRP concentrations correlate positively with abdominal obesity, hyperinsulinemia, and other components of the insulin resistance syndrome. Nevertheless, associations of CRP with insulin resistance independent of obesity suggest that divergent pathways linking inflammation and insulin resistance are of importance, especially among nonobese individuals.15,16 Further, prospective studies have shown that individuals meeting criteria for the insulin resistance syndrome who have elevated CRP concentrations (greater than 3 mg/L) are at higher risk for cardiovascular events than individuals with the insulin resistance syndrome who do not have elevated CRP.17,18
We previously examined Icelandic postmenopausal women who experienced eclampsia during their reproductive years.7 These women, 50 to 67 years old at time of reexamination, were matched for date of birth, age at pregnancy, and parity to Icelandic postmenopausal controls with only uncomplicated pregnancies. Compared to controls, women with a history of eclampsia had low-density lipoprotein (LDL) particles of smaller average diameter and higher plasma concentrations of apolipoprotein B, a dyslipidemic combination associated with an especially high cardiovascular risk.19 These differences persisted after adjustment for body mass index (BMI), smoking, hormone replacement therapy or use of thiazide diuretics, βadrenergic blockers, or cholesterol-lowering drugs. The subset of Icelandic women who experienced hypertension in at least 1 pregnancy additional to the eclamptic index pregnancy displayed additional adverse changes, including increased diastolic blood pressure and decreased high-density lipoprotein (HDL) cholesterol.7
With the advent of a reliable high-sensitivity assay for serum CRP,20 we further analyzed this population, testing the hypothesis that postmenopausal women with a history of eclampsia have higher concentrations of CRP than postmenopausal women with a history of only uncomplicated pregnancies. We secondarily investigated whether elevated CRP was associated with dyslipidemia and surrogate measures of insulin resistance in these women.
Methods
Patient Population
The study was approved by the ethics committee of the Ethical Committee of Landspitali University Hospital and the Icelandic Data Protection Authority. All women gave written informed consent. The initial groups consisted of 30 Icelandic women with prior eclampsia and 30 Icelandic controls with an uncomplicated reproductive history.7 Serum for CRP measurement was no longer available from 5 of 30 women with prior eclampsia and 2 of 30 controls with uncomplicated pregnancy history; these subjects were not included the present study. The inclusion/exclusion criteria, details of enrollment for reexamination and clinical characteristics of these groups have been described.7 Briefly, women who had had eclampsia (defined as seizure in hypertensive pregnancy, without prepregnancy history of epilepsy or other convulsive disorders) were identified from review of medical records for the years 1931 to 1996 at the National Hospital in Reykjavik. Gestational hypertension was defined as having maximum blood pressure of at least 90 mm Hg diastolic and 140 mm Hg systolic before labor. Women with a history of hypertension before week 20 of gestation were excluded. The case study group fulfilled the criteria of having prior eclampsia, being postmenopausal at the time of reexamination, and being without evidence of prepregnancy renal, convulsive, or hypertensive disease. Unrelated controls who had a history of only uncomplicated normotensive pregnancies were matched to cases for date of birth, age at pregnancy, and parity. None of the women had a history of gestational diabetes mellitus.
The pregnancy characteristics of the 25 eclampsia cases and 28 controls did not differ substantially from the original 30 cases and 30 controls.7 By definition, predelivery systolic and diastolic blood pressures were significantly elevated in the eclampsia group. The groups did not differ by mean years of age (eclampsia 24.4; control 24.5) or parity (percent multiparous: eclampsia 24%; control 25%) at index pregnancy. Mean diastolic blood pressure during the first half of pregnancy was slightly but significantly elevated, and fetal birthweight was significantly lower, in the eclampsia group as previously reported.
By design, women with prior eclampsia and controls did not differ at the time of reexamination with respect to years of age or the time interval from index pregnancy to reexamination (Table 1). The postmenopausal groups did not differ by mean BMI, frequency of cigarette smoking, or serum estrogen, luteinizing hormone (LH) or follicle-stimulating hormone (FSH) concentrations, or use of estrogenic replacement therapy (Table 1). Two women, both with a history of eclampsia, reported 3-hydroxy-3-methylglutaryl (HMG)-coenzyme A (CoA) reductase inhibitor (simvastatin) usage at the time of reexamination. The number of women with prior eclampsia according to type of antihypertensive medication was the same as previously reported (n=2 angiotensin-converting enzyme inhibitor; n=2 thiazide diuretic; n=2 beta adrenergic blocker; n=2 combined angiotensin-converting enzyme inhibitor and thiazide diuretic; n=1 combined beta blocker and thiazide; n=1 combined beta blocker and calcium channel blocker). One control was using a calcium channel blocker. One control who had been using a beta adrenergic blocker was excluded because of unavailability of serum.
Table 1.
Clinical Characteristics of Study Groups
| Characteristic | Prior Eclampsia (n=25) |
Control (n=28) |
P |
|---|---|---|---|
| Age, y | 57 (5) | 57 (6) | NS |
| Years after index pregnancy | 32.6 (8.4) | 32.2 (7.7) | NS |
| Systolic blood pressure, mm Hg | 143 (16) | 130 (15) | <0.01 |
| Diastolic blood pressure, mm Hg | 86.6 (11) | 81.5 (9) | NS |
| Body mass index (BMI), kg/m2 | 27.5 (4.2) | 25.7 (4.4) | NS |
| Antihypertensive medication use | 40% (10/25) | 4% (1/28) | <0.03 |
| Hormone replacement use | 36% (9/25) | 43% (12/28) | NS |
| Smoking, current or former | 44% (11/25) | 54% (15/28) | NS |
Continuous variables are reported as mean (SD). Categorical variables are given as percentages.
NS indicates not significantly different from controls.
Blood Collection and Analyses
Venous blood was obtained at the time of reexamination, after an overnight fast. Aliquots of serum and EDTA-plasma were stored at −70°C immediately after centrifugation until analysis. All analyses were performed blinded to the pregnancy diagnosis. Predominant LDL particle size (LDL diameter), total cholesterol, total triglyceride, apolipoprotein B, free (nonesterified) fatty acids, LDL cholesterol, HDL cholesterol, lipoprotein(a), insulin, and glucose had been measured as described.7 Homeostasis model assessment of insulin resistance (HOMA) scores were calculated [(insulin [microunits per milliliter]×glucose [millimoles per liter])/22.5].
CRP was measured by a high-sensitivity enzyme-linked immuno-assay (ELISA).20 In brief, microplate wells were coated overnight at 4°C with a rabbit antihuman CRP antibody (Dako Corp, Denmark). Potential nonspecific binding sites were blocked using 2% bovine serum albumin (BSA) and 0.05% Tween 20. Serum samples and recombinant CRP standards were diluted in physiological buffer (PBS) that contained 2% BSA. Diluted standards and samples were incubated in the microplate wells for 1 hour at room temperature and unbound proteins were washed with a PBS, 0.05% Tween 20 wash buffer. The wells were incubated with a polyclonal rabbit antihuman CRP antibody (1:500 dilution) conjugated to horseradish peroxidase (HRP) (Dako) for 1 hour at room temperature. After incubation, the microplate wells were washed again and incubated with a substrate for HRP. The amount of CRP was directly proportional to the amount of color in each well. The standard curve was linear from 0.2 to 10 ng/mL. The sensitivity of the assay was 0.2 ng/mL, and spike and recovery tests indicated 91% to 103% recovery. The intraassay variability was 3.9%, and interassay variability was 7.4%.
Statistical Analyses
Continuous variables are summarized for cases and controls by mean (standard deviation) for normally distributed variables or median (interquartile range) for skewed variables. Categorical variables are summarized by frequency (percentage) and were compared using Fisher exact test. In general, between-group univariate comparisons were conducted using 2-sample t or Mann-Whitney U tests, for parametric or nonparametric data, respectively. Linear regression was used to assess CRP as a continuous outcome variable, and a multivariable linear regression was performed considering BMI, smoking, hormone replacement, and current age as potential confounders. Because the CRP distribution was skewed, analyses were conducted after ln transformation to approximate normality, as confirmed by Shapiro-Wilk test, to meet criteria for linear regression. Nonparametric correlations of CRP with other blood variables, BMI, and blood pressure were evaluated by Spearman rho (rs) correlation coefficient.
Comparison of the 2 subgroups of women with prior eclampsia (eclampsia-high CRP; eclampsia-lower CRP) versus controls with a history of uncomplicated pregnancy were done by ANOVA with Bonferroni post test or by nonparametric Kruskal-Wallis test with Dunn post test as appropriate.
Results
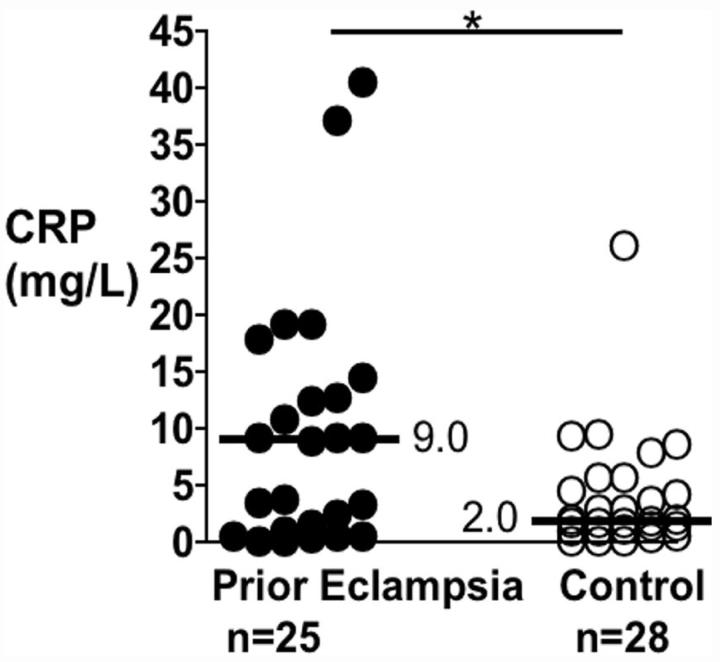
As shown in the Figure, serum CRP was significantly elevated in women with a history of eclampsia compared to controls (median mg/L [interquartile range]: 9.0 [0.9 to 13.2] versus 2.0 [0.3 to 5.1]; P<0.03); geometric means were 4.7 and 2.2 mg/L, respectively. This difference remained significant (P<0.04) after adjustment for current BMI, smoking, hormone replacement, and age. The difference also remained significant after removal of outliers (P<0.05). CRP did not correlate with BMI in either prior eclampsia (rs=0.11, P=0.6) or control (rs=0.15, P=0.4) groups.
Figure.
Scatterplot of serum C-reactive protein (CRP) concentrations in postmenopausal women with a history of eclampsia (prior eclampsia) or history of uncomplicated pregnancy (control). Each symbol corresponds to a different individual. Thick horizontal bars with adjacent numbers indicate median values for each group. *P<0.03.
Fasting insulin was significantly higher in women with a history of eclampsia than controls (median μU/mL [inter-quartile range]: prior eclampsia 8.6 [7.5 to 13.1], controls 7.0 [5.7 to 10.9]; P<0.04). These insulin values did not differ substantially from the initial 30 cases (median 8.6) and 30 controls (median 7.2) in which group differences approached but did not achieve significance.7 As shown in Table 2, HOMA insulin resistance index scores (not reported in our earlier study) were higher in women with a history of eclampsia, albeit marginally so (P=0.06).
Table 2.
Blood Variables at Reexamination
| Blood Variable | Prior Eclampsia (n=25) |
Control (n=28) |
P |
|---|---|---|---|
| Median, IQR | |||
| C-reactive protein, mg/L | 9.0 (0.9 to 13.2) | 2.0 (0.2 to 5.1) | <0.03 |
| Insulin, μU/mL | 8.6 (7.5 to 13.1) | 7.0 (5.7 to 10.9) | <0.04 |
| Glucose, mmol/L | 4.9 (4.5 to 5.4) | 4.8 (4.6 to 5.1) | 0.95 |
| HOMA score | 1.72 (1.43 to 3.96) | 1.49 (1.18 to 2.54) | 0.06 |
| LDL cholesterol, mg/dL | 125 (109 to 170) | 117 (97 to 138) | 0.11 |
| Triglycerides, mg/dL | 110 (74 to 177) | 100 (73 to 158) | 0.53 |
| Mean, SD | |||
| HDL cholesterol, mg/dL | 57.6 (14.1) | 69.1 (16.7) | <0.02 |
| Apolipoprotein B, mg/dL | 125 (38) | 96 (29) | <0.01 |
| LDL particle diameter, Å | 263 (7) | 268 (7) | <0.04 |
| Total cholesterol, mg/dL | 227 (44) | 216 (36) | 0.16 |
| Free fatty acids, mmol/L | 0.60 (0.30) | 0.54 (0.26) | 0.62 |
| Lipoprotein(a), mg/dL | 12.0 (10.3) | 11.2 (10.2) | 0.69 |
Values are given as mean (standard deviation) or median (interquartile range) as indicated.
IQR indicates interquartile range; HOMA, homeostasis model of insulin resistance.
Systolic blood pressures were significantly higher in women with previous eclampsia compared to controls whereas diastolic blood pressures did not differ (Table 1). The percentage of women using antihypertensive medications at the time of reexamination was greater in the prior eclampsia group ([10/25 (40%)] versus 1/28 [4%], P<0.03). Apolipoprotein B concentrations were significantly elevated, whereas HDL cholesterol concentration and predominant low-density lipoprotein particle diameter were significantly lower, in the women with previous eclampsia compared to controls. Total cholesterol, LDL cholesterol, triglycerides, free fatty acids, lipoprotein(a), and glucose did not differ between the 2 groups (Table 2). These findings were in accordance with our previous report.7
Among the prior eclamptics, the following were significantly correlated with serum CRP: HOMA score (rs=0.56, P<0.01), fasting insulin (rs=0.53, P<0.01), systolic blood pressure (rs=0.55, P<0.01), and free fatty acids (rs=0.44, P<0.04). CRP was marginally correlated with triglyceride (rs=0.39, P=0.05) and marginally inversely correlated with HDL cholesterol (rs=−0.39, P=0.06). Among controls, CRP did not correlate with HOMA score, insulin, lipids or blood pressure.
From the scatterplot of CRP values (Figure) postmenopausal women with a history of eclampsia could be segregated into 2 evenly sized subgroups on the basis of very high risk (median 12.8 mg/L, range 9.0 to 40.6 mg/L, n=13) and lower risk (median 0.8 mg/L, range 0.05 to 3.8, n=12) CRP values. The eclampsia-high CRP and eclampsia-low CRP subgroups did not differ by BMI (Table 3). As shown in Table 3, however, the eclampsia-high CRP subgroup manifested several adverse lipid and insulin differences compared to controls with a history of normotensive pregnancy—higher fasting insulin and HOMA values, lower HDL cholesterol, and higher apolipoprotein B. In contrast, the eclampsia-low CRP subgroup did not show these differences from controls (except for marginally increased apolipoprotein B, P=0.055). Systolic blood pressure was significantly elevated in the eclampsia-high CRP subgroup compared to controls (mean mm Hg [SD]: 148.1 [16.5] versus 130.0 [14.7]; P<0.05) but not so in the eclampsia-low CRP subgroup (136.1 [13.9]). Diastolic blood pressures did not differ between the 3 groups. The percentage of women currently using antihypertensive medications was substantially higher in the eclampsia-high CRP subgroup [62% (8/13)] than the eclampsia–low CRP subgroup (17% 2/12; P<0.05). The number of smokers (current or former) was fairly evenly distributed between the eclampsia-high CRP (3 current, 2 former) and eclampsia–low CRP (2 current, 4 former) subgroups.
Table 3.
Prior Eclampsia With Either High CRP or Low CRP vs Controls
| Variable | Prior Eclampsia High CRP (n=13) |
Prior Eclampsia Low CRP (n=12) |
Control (n=28) |
|---|---|---|---|
| Median, IQR | |||
| CRP, mg/L | 12.8 (9.3 to 19.2) | 0.8 (0.4 to 2.9) | 2.0 (0.2 to 5.1) |
| Fasting Insulin, microU/mL | 11.3 (8.5 to 20)* | 7.5 (6.4 to 10.95) | 7.0 (5.7 to 10.9) |
| HOMA [(insulin×glucose)/22.5] | 2.53 (1.69 to 5.61)* | 1.49 (1.31 to 2.68) | 1.49 (1.18 to 2.54) |
| Mean (SD) | |||
| Body mass index (BMI), kg/m2 | 27.5 (3.8) | 27.4 (4.7) | 25.7 (4.4) |
| Systolic blood pressure, mm Hg | 148 (17)* | 136 (14) | 130 (15) |
| HDL cholesterol, mg/dL | 53.6 (14.4)* | 62.3 (12.8) | 69.1 (16.7) |
| Apo B, mg/dL | 125.4 (34)† | 124 (44) (P=0.055) | 95.5 (29) |
| LDL diameter, Å | 263 (9) | 264 (5) | 268 (7) |
P<0.01 vs controls
P<0.04 vs controls.
IQR indicates interquartile range; HOMA, homeostasis model of insulin resistance.
The 12 women with a history of recurrent hypertension in pregnancy (hypertension in at least 1 pregnancy in addition to the eclamptic pregnancy) were equally represented within the high CRP (46% recurrent 6/13) and lower CRP (50% 6/12) subgroups. None of the historical gestational characteristics differed between the high and lower CRP subgroups.
Discussion
We report that Icelandic postmenopausal women with a history of eclampsia have higher median serum CRP values than Icelandic women with a history of uncomplicated pregnancies. These data are consistent with the hypothesis that the link between preeclampsia, including eclampsia as a severe variant, and later-life cardiovascular disease, consistently observed in contemporary epidemiological studies,5 involves chronic inflammation.
By design, the groups in our study did not differ by age or parity at the time of index pregnancy or date of birth; nor did they differ by body mass index (BMI), percentage of smokers, or percentage using hormone replacement therapy use at the time of reexamination. The group difference in CRP remained significant after adjustment for BMI, smoking, hormone replacement, and age. CRP concentrations and BMI are highly correlated in the general population, suggesting that the 2 variables are on the same causal pathway involving inflammation.13 BMI and CRP surprisingly did not correlate in our Icelandic women; however most of these women were nonobese (BMI <30).
One limitation of our study is that we did not have urinary protein levels, and thus could not confirm significant proteinuria, for several of the eclampsia cases. An appreciable percentage of women who develop eclampsia, however, do so without abnormal proteinuria before the abrupt onset of convulsions, despite having ruled out other neurological causes of convulsions. In such cases, eclampsia is sometimes heralded by other disturbances such as headache or visual disturbances.3
Because eclampsia is a severe form of the preeclampsia syndrome, it is possible that there are differences in risk profile of women who have had eclampsia compared to other preeclampsia subtypes. A previous study found an elevated ratio of plasma interleukin (IL)-10 to IL-12, an index of proinflammatory cytokine status, in women 20 years after preeclamptic first pregnancy compared to women with a history of normal first pregnancy, independent of body mass index (BMI), smoking, or menopause status. Mean plasma CRP concentrations were nearly double in the group with prior preeclampsia in that study but the difference was not statistically significant.21
Several, but not all, studies found a heightened inflammatory response as evidence by increased serum CRP22-26 or proinflammatory cytokine concentrations27 during the first or second trimester of pregnancy, before clinically evident preeclampsia. In many of the studies showing an association of CRP with risk of developing preeclampsia, the association was considerably attenuated after adjustment for prepregnancy BMI.22,24 Studies that matched on prepregnancy BMI have been inconsistent with regard to CRP and preeclampsia risk.25,26 The elevation in CRP attributable to pregnancy might transiently mask underlying CRP differences among women destined to develop preeclampsia compared to those not destined to do so. It is noteworthy, however, that elevated CRP (≥4.9 mg/L) during the first trimester of pregnancy was independently associated with a 2.5-fold increased risk of developing preeclampsia in lean women (BMI <25 kg/m2), but elevated CRP was not associated with a marked increase of preeclampsia risk in overweight women.22 Taken together, these data suggest that pathways of low-grade, chronic systemic inflammation, some independent of obesity, are involved in the pathogenesis of preeclampsia/eclampsia and persist or reappear decades postpartum.
The association of serum CRP with cardiovascular risk in the general population is linear across a full range of high-sensitivity CRP assay values in a manner analogous to that of LDL cholesterol.13 Concentrations of CRP between 5 and 60 mg/L define individuals at very high risk of future vascular events, whereas levels of <1 mg/L and 1 to 3 mg/L generally define low risk and moderate risk, respectively.13,17 Given that Icelandic postmenopausal women with history of eclampsia could be divided into 2 subgroups on the biological basis of very high-risk versus low/moderate-risk CRP values, we asked whether these subgroups differed on the basis of clinical characteristics, atherogenic blood lipids and surrogate measures of insulin resistance. We found that the eclampsia subgroup with high-risk serum CRP concentrations evidenced elevated systolic blood pressures (and pulse pressures), greater frequency of use of antihypertensive medications, reduced HDL cholesterol, and elevated fasting insulin and HOMA scores compared to controls with a history of uncomplicated pregnancy. The subgroup with low/moderate CRP values, however, showed none of these differences compared to controls. Furthermore, CRP was significantly correlated with insulin, HOMA score, and atherogenic lipids among prior eclamptics but not controls. Thus, inflammation appears to be closely linked to elements of the insulin resistance syndrome in postmenopausal women with previous eclampsia.
Fasting apolipoprotein B concentration, which provides an estimate of the number of circulating atherogenic lipoprotein particles, did not correlate with CRP and was the only blood variable elevated in both high CRP and lower CRP eclampsia subgroups compared to controls. Mean apolipoprotein B concentrations were greater than 120 mg/dL in both subgroups, a threshold predictive of ischemic heart disease even in the face of normal cholesterol concentrations.19,28 Further work is needed to determine the relative risk conferred by elevated apolipoprotein B in women with a history of eclampsia.
Studies of nonpregnant individuals have shown powerful relationships between measures of low-grade inflammation, including high-sensitivity CRP, and measures of insulin resistance in both seemingly healthy subjects and individuals with coronary heart disease.15,16 Although the causal pathways are uncertain, insulin resistance and dyslipidemia could represent adverse consequences of proinflammatory cytokines on insulin signaling and lipid metabolizing pathways.29
We chose CRP over other inflammatory markers partly because of the relatively high within-person consistency of CRP levels over time (despite the fact that CRP can increase acutely due to ischemic stress or infection)13 and because of its stability in frozen serum samples. Controversy exists over whether CRP plays a direct causal role in development of cardiovascular disease30 but this has little bearing on its robustness as a marker for cardiovascular risk. Nevertheless, we cannot rule out the possibility that other biomarkers of inflammation would have yielded different results.
The effects of different antihypertensive medication classes on serum CRP concentrations are not well characterized but data from the Multi-Ethnic Study of Atherosclerosis (MESA) indicates slight CRP-lowering effects exerted by βblockers, angiotensin-converting enzyme inhibitors, and angiotensin II type I receptor blockers, whereas none of the other major antihypertensive classes showed statistically significant effects on CRP.31 Therefore, it is unlikely that elevated CRP is caused by antihypertensive medications. Similarly, statins can reduce CRP levels,32 but any such effects in the 2 women (both previously eclamptic) reporting statin use would only have lessened group differences.
There is no indication that Icelandic women have remarkably different rates of cardiovascular disease or eclampsia from what is observed in other Western populations.33,34 The seemingly high prevalence of smoking, 44% and 54% in cases and controls, respectively, merits comment. We could not obtain information about the exact prevalence of smoking in the general population during the time period of our study. However, it compares reasonably well with the reported prevalence of 41% among Icelandic women in the Reykjavik Study, a large-scale epidemiological study of cardiovascular and other chronic diseases that started in 1967.33
Our study cannot address the issue of risk status of women with a history of either normal pregnancy or eclampsia in relation to the general female population. Indeed, several studies indicate a decreased frequency of adverse cardiovascular events in women with an uncomplicated reproductive history compared to the overall female population.35 Passing the normal pregnancy “stress test” may identify a population more representative of a healthy phenotype than the population at-large. Nevertheless, the high CRP-prior eclampsia group had a remarkably adverse profile (69% with systolic blood pressure >140, 62% with HDL cholesterol <50 mg/dL, 62% using antihypertensive medications).
Finally, we cannot exclude the possibility that eclampsia was the cause of postpartum differences rather than a consequence of metabolic problems that antedated pregnancy. This distinction may be less important, however, if further studies were to confirm that subgroups of formerly preeclamptic women with elevated CRP and insulin resistance stand to benefit most from early and aggressive cardiovascular risk factor modification.
Perspectives
Examining postpartum women who have had preeclampsia offers a unique approach to investigation of the maternal contributions to preeclampsia and mechanisms of cardiovascular disease. These women appear especially prone to developing the insulin resistance syndrome later in life, and are at increased risk to develop hypertension, coronary artery disease, or stroke. On the basis of such data, it has been proposed that preeclampsia, including the severe variant eclampsia, be considered a ‘red flag’ identifying women who should have regular cardiovascular risk assessment and counseling about cardiovascular disease prevention beginning before menopause, and be offered treatment to reduce morbidity and mortality.6,36 Further study is needed to determine the extent to which early screening, not only for traditional risk factors such as cholesterol but also inflammation-related markers such as CRP, perhaps in combination with surrogate measures of insulin resistance, might facilitate preventative interventions in these women to reduce both risk for morbidity in subsequent pregnancies and risk of cardiovascular disease later in life.
Acknowledgments
We thank the clinical and technical staffs at Landspitali University Hospital, Reykjavik, and Magee-Womens Research Institute, Pittsburgh, for their invaluable assistance. We gratefully acknowledge Dr Reynir T. Geirsson, Department of Obstetrics and Gynaecology, Landspitali University Hospital, for assistance with design and implementation of the study.
Sources of Funding
This work was supported by the Icelandic Heart Association Research Fund (R.A.) and the National University Hospital Research Fund (R.A.), and by grants HL64144 and HD049453 (C.A.H.) from the National Institutes of Health.
Footnotes
Disclosures
None.
References
- 1.Ilekis J, Reddy UM, Roberts JM. Review article: Preeclampsia-a pressing problem: An executive summary of a National Institute of Child Health and Human Development workshop. Reproductive Sciences. 2007;14:508–523. doi: 10.1177/1933719107306232. [DOI] [PubMed] [Google Scholar]
- 2.Ness RB, Roberts JM. Heterogeneous causes constituting the single syndrome of preeclampsia: A hypothesis and its implications. Am J Obstet Gynecol. 1996;175:1365–1370. doi: 10.1016/s0002-9378(96)70056-x. [DOI] [PubMed] [Google Scholar]
- 3.Sibai B. Eclampsia. VI. Maternal-perinatal outcome in 254 consecutive cases. Am J Obstet Gynecol. 1990;163:1049–1054. doi: 10.1016/0002-9378(90)91123-t. [DOI] [PubMed] [Google Scholar]
- 4.Funai EF, Friedlander Y, Paltiel O, Tiram E, Xue X, Deutsch L, Harlap S. Long-term mortality after preeclampsia. Epidemiology. 2005;16:206–215. doi: 10.1097/01.ede.0000152912.02042.cd. [DOI] [PubMed] [Google Scholar]
- 5.Bellamy L, Casas J-P, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ness RB, Hubel CA. Risk for coronary artery disease and morbid preeclampsia: A commentary. Ann Epidemiol. 2005;15:726–733. doi: 10.1016/j.annepidem.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Hubel CA, Snaedal S, Ness RB, Weissfeld LA, Geirsson RT, Roberts JM, Arngrímsson R. Dyslipoproteinemia in postmenopausal women with a history of eclampsia. Br J Obstet Gynaecol. 2000;107:776–784. doi: 10.1111/j.1471-0528.2000.tb13340.x. [DOI] [PubMed] [Google Scholar]
- 8.Bodnar LM, Ness RB, Harger GF, Roberts JM. Inflammation and triglycerides partially mediate the effect of prepregnancy body mass index on the risk of preeclampsia. Am J Epidemiol. 2005;162:1198–1206. doi: 10.1093/aje/kwi334. [DOI] [PubMed] [Google Scholar]
- 9.Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet. 2005;366:1797–1803. doi: 10.1016/S0140-6736(05)67726-4. [DOI] [PubMed] [Google Scholar]
- 10.Wolf M, Hubel CA, Lam C, Sampson M, Ecker JL, Ness RB, Rajakumar A, Daftary A, Shakir AS, Seely EW, Roberts JM, Sukhatme VP, Karumanchi SA, Thadhani R. Preeclampsia and future cardiovascular disease: Potential role of altered angiogenesis and insulin resistance. J Clin Endocrinol Metab. 2004;89:6239–6243. doi: 10.1210/jc.2004-0548. [DOI] [PubMed] [Google Scholar]
- 11.Walsh S. Obesity: A risk factor for preeclampsia. Trends Endocrinol Metab. 2007;10:365–370. doi: 10.1016/j.tem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk. Moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol. 2007;49:2129–2138. doi: 10.1016/j.jacc.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 14.Yeh ET. High-sensitivity C-reactive protein as a risk assessment tool for cardiovascular disease. Clin Cardiol. 2005;28:408–412. doi: 10.1002/clc.4960280905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meng Y-X, Quarshie A, Gibbons GH, Ford ES, Li C, Al-Mahmoud AM, Giles W, Strayhorn G. Association of C-reactive protein with surogate measures of insulin resistance among nondiabetic us adults: Findings from the national health and nutrition examination survey 1999–2002. Clin Chem. 2007;53:2152–2159. doi: 10.1373/clinchem.2007.088930. [DOI] [PubMed] [Google Scholar]
- 16.Yudkin JS, Juhan-Vague I, Hawe E, Humphries SE, Di Minno G, Margaglione M, Tremoli E, Kooistra T, Morange PE, Lundman P, Mohamed-Ali V, Hamsten A. Low-grade inflammmation may play a role in the etiology of the metabolic syndrome in patients with coronary heart disease: The HIFMECH study. Metabolism. 2004;53:852–857. doi: 10.1016/j.metabol.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM. C-reactive protein, inflammation, and cardiovascular disease. Tex Heart Inst J. 2005;32:384–386. [PMC free article] [PubMed] [Google Scholar]
- 18.Ndumele CE, Pradhan AD, Ridker PM. Interrelationships between inflammation, C-reactive protein, and insulin resistance. J Cardiometab Syndr. 2006;1:190–196. doi: 10.1111/j.1559-4564.2006.05538.x. [DOI] [PubMed] [Google Scholar]
- 19.Lamarche B, Tchernof A, Mauriege P, Cantin B, Dagenais GR, Lupien PJ, Despres JP. Fasting insulin and apolipoprotein B levels and low-density lipoprotein particle size as risk factors for ischemic heart disease. JAMA. 1998;279:1955–1961. doi: 10.1001/jama.279.24.1955. [DOI] [PubMed] [Google Scholar]
- 20.Catov JM, Bodnar LM, Ness R, Barron SJ, Roberts JM. Inflammation and dyslipidemia related to risk of spontaneous preterm birth. Am J Epidemiol. 2007;166:1312–1319. doi: 10.1093/aje/kwm273. [DOI] [PubMed] [Google Scholar]
- 21.Freeman DJ, McManus F, Brown EA, Cherry L, Norrie J, Ramsay JE, Clark P, Walker ID, Sattar N, Greer IA. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension. 2004;44:708–714. doi: 10.1161/01.HYP.0000143849.67254.ca. [DOI] [PubMed] [Google Scholar]
- 22.Qiu C, Luthy DA, Zhang C, Walsh SW, Leisenring WM, Williams MA. A prospective study of maternal serum C-reactive protein concentrations and risk of preeclampsia. Am J Hypertens. 2004;17:154–160. doi: 10.1016/j.amjhyper.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Tjoa ML, van Vugt JM, Go AT, Blankenstein MA, Oudejans CB, van Wijk IJ. Elevated C-reactive protein levels during first trimester of pregnancy are indicative of preeclampsia and intrauterine growth restriction. J Reprod Immunol. 2003;59:29–37. doi: 10.1016/s0165-0378(02)00085-2. [DOI] [PubMed] [Google Scholar]
- 24.Wolf M, Kettyle E, Sandler L, Ecker JL, Roberts J, Thadhani R. Obesity and preeclampsia: The potential role of inflammation. Obstet Gynecol. 2001;98:757–762. doi: 10.1016/s0029-7844(01)01551-4. [DOI] [PubMed] [Google Scholar]
- 25.Garcia RG, Celedon J, Sierra-Laguado J, Alarcon MA, Luengas C, Silva F, Arenas-Mantilla M, Lopez-Jaramillo P. Raised C-reactive protein and impaired flow-mediated vasodilation precede the development of preeclampsia. Am J Hypertens. 2007;20:98–103. doi: 10.1016/j.amjhyper.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Djurovic S, Clausen T, Wergeland R, Brosstad F, Berg K, Henriksen T. Absence of enhanced systemic maternal inflammatory response at 18 weeks of gestation in women with subsequent preeclamsia. Br J Obstet Gynaecol. 2002;109:759–764. doi: 10.1111/j.1471-0528.2002.01330.x. [DOI] [PubMed] [Google Scholar]
- 27.Williams MA, Farrand A, Mittendorf R, Sorensen TK, O'Reilly GC, King IB, Zebelman AM, Luthy DA. Maternal second trimester serum soluble tumor necrosis factor-alpha-soluble receptor p55 (sTNFp55) and subsequent risk of preeclampsia. Am J Epidemiol. 1999;149:323–329. doi: 10.1093/oxfordjournals.aje.a009816. [DOI] [PubMed] [Google Scholar]
- 28.Wagner AM, Perez A, Calvo F, Bonet R, Castellvi A, Ordonez J. Apolipoprotein(B) identifies dyslipidemic phenotypes associated with cardiovascular risk in normocholesterolemic type 2 diabetic patients. Diabetes Care. 1999;22:812–817. doi: 10.2337/diacare.22.5.812. [DOI] [PubMed] [Google Scholar]
- 29.Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo J-L, Naugler W, Grivennikov S, Wynshaw-Boris A, Scadeng M, Olefsky JM, Karin M. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Hirschfield GM, Gallimore JR, Kahan MC, Hutchinson WL, Sabin CA, Benson GM, Dhillon AP, Tennent GA, Pepys MB. Transgenic human C-reactive protein is not proatherogenic in apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A. 2005;102:8309–8314. doi: 10.1073/pnas.0503202102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmas W, Mac S, Psaty B, Goff D,C, Jr., Darwin C, Barr RG. Antihypertensive medications and C-reactive protein in the multi-ethnic study of atherosclerosis. Am J Hypertens. 2007;20:233–241. doi: 10.1016/j.amjhyper.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Eiriksdottir G, Aspelund T, Bjarnadottir K, Olafsdottir E, Gudnason V. Apolipopprotein E genotype and statins affect CRP levels through independent and different mechanisms: AGES-Reykjavik Study. Atherosclerosis. 2006;186:222–224. doi: 10.1016/j.atherosclerosis.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Aspelund T, Thorgeirsson G, Sigurdsson G, Gudnason V. Estimation of 10-year risk of fatal cardiovascular disease and coronary disease in Iceland with results comparable with those of the Systematic Coronary Risk Evaluation project. Eur J Cardiovasc Prev Rehabil. 2007;14:761–768. doi: 10.1097/HJR.0b013e32825fea6d. [DOI] [PubMed] [Google Scholar]
- 34.Geirsson RT, Arngrimsson R, Apalset E, Einarsson A, Snaedal G. Falling population incidence of eclampsia. A case-control study of short term outcome. Acta Obstet Gynecol Scand. 1994;73:465–467. doi: 10.3109/00016349409013432. [DOI] [PubMed] [Google Scholar]
- 35.Fisher KA, Lluger A, Spargo BH, Lindheimer MD. Hypertension in pregnancy: Clinical-pathological correlations and remote prognosis. Medicine. 1981;60:267–276. [PubMed] [Google Scholar]
- 36.Magee LA, von Dadelszen P. Pre-eclampsia and increased cardiovascular risk. BMJ. 2007;335:945–946. doi: 10.1136/bmj.39337.427500.80. [DOI] [PMC free article] [PubMed] [Google Scholar]