Abstract
Phytosulfokine-α [PSK-α, Tyr(SO3H)-Ile-Tyr(SO3H)-Thr-Gln], a sulfated mitogenic peptide found in plants, strongly promotes proliferation of plant cells in culture at very low concentrations. Oryza sativa PSK (OsPSK) cDNA encoding a PSK-α precursor has been isolated. The cDNA is 725 base pairs long, and the 89-aa product, preprophytosulfokine, has a 22-aa hydrophobic region that resembles a cleavable leader peptide at its NH2 terminus. The PSK-α sequence occurs only once within the precursor, close to the COOH terminus. [Ser4]PSK-α was secreted by transgenic rice Oc cells harboring a mutated OsPSK cDNA, suggesting proteolytic processing from the larger precursor, a feature commonly found in animal systems. Whereas PSK-α in conditioned medium with sense transgenic Oc cells was 1.6 times as concentrated as in the control case, antisense transgenic Oc cells produced less than 60% of the control level. Preprophytosulfokine mRNA was detected at an elevated constitutive level in rice Oc culture cells on RNA blot analysis. Although PSK-α molecules have never been identified in any intact plant, reverse transcription–PCR analysis demonstrated that OsPSK is expressed in rice seedlings, indicating that PSK-α may be important for plant cell proliferation both in vitro and in vivo. DNA blot analysis demonstrated that OsPSK homologs may occur in dicot as well as monocot plants.
Keywords: peptide mitogen, plant cell proliferation, rice, sulfated protein
Although various hormones and growth factors in animal systems are polypeptides, none of the previous seven known plant hormone families is peptidal. However, the existence of peptidyl plant hormones has recently been indicated by the isolation of systemin that can initiate signal transduction to regulate the synthesis of defensive proteins in plants from tomato (1, 2).
Plant cells in low density culture usually display strictly low mitotic activity, which cannot be improved by supplementation with known plant hormones or defined nutrients. However, proliferation was activated by addition of conditioned medium (CM) derived from rapidly growing cells, suggesting that secreted mitogenic factor(s) exist (3). Various efforts have been made to characterize such factors in different culture systems during the last decade. Highly hydrophilic and neutral agents, highly hydrophilic and relatively heat-stable molecules, and a protease-resistant and relatively small compound were initially considered to be putative mitogenic factors existing in CM prepared from maize (4, 5), carrot (6), and Pinas radiata (7) cell cultures, respectively. However, no factor could be identified because the available assay methods were not sufficiently rapid or sensitive.
We previously established a highly sensitive bioassay system, isolated two mitogenic factors from the CM of asparagus mesophyll cell cultures, and determined their structures as the sulfated pentapeptide phytosulfokine-α (PSK-α) and the tetrapeptide PSK-β [Tyr(SO3H)-Ile-Tyr(SO3H)-Thr] (8). PSK-α was also identified in the CM of rice Oc culture cells (9) and was found to strongly stimulate proliferation of plant cells in low density cultures at concentrations as low as 1.0 nM (8–11). Truncated analogs of PSK-α without the first and second COOH-terminal amino acids retained 8 and 20% of the activity of the parent pentapeptide, respectively. In contrast, the NH2-terminal truncated analog and an unsulfated analog exhibited little or no activity, suggesting that the NH2-terminal tripeptide fragment is the active core (12). Substitution of isoleucine with valine ([Val2]PSK-α), both hydrophobic, resulted in a 20-fold decrease in the ED50. A similar decrease in activity was observed on replacing threonine with serine ([Ser4]PSK-α) indicating that these two amino acid residues do not simply serve as spacers and are involved in the mitogenic activity of PSK-α (12).
Binding assays in which 35S-labeled PSK-α is used demonstrated the existence of both high and low affinity specific saturable binding sites on the surfaces of rice cells. Analysis of [35S]PSK-α binding in differential centrifugation fractions suggested association of the binding with a plasma membrane-enriched fraction. Competition studies with [35S]PSK-α and several synthetic PSK-α analogs demonstrated that only peptides with mitogenic activity can effectively displace the radioligand, suggesting that PSK-α is involved in plant cell proliferation through a signal transduction pathway with similarities to those of mammalian peptide hormones and growth factors (9). Herein we describe the molecular cloning and characterization of a cDNA encoding a PSK-α precursor from rice.
Materials and Methods
Plant Materials.
The rice Oc cell line was subcultured at 25 ± 2°C in the dark at 120 rpm in Murashige and Skoog medium (13) supplemented with 1.0 mg/liter 2,4-dichlorophenoxyacetic acid at regular intervals of 2 weeks. Seeds of Oryza sativa L. (japonica cv. “Alborio J-1”) were surface disinfected and soaked for 48 h in a solution containing 2.5% Benomyl. The sterilized seeds were washed with water, sown on a moist basket set on a tray containing water, and kept at 28°C in the dark. Various organs of 8-day-old seedlings were sampled for total RNA extraction.
Construction and Screening of a cDNA Library.
Poly(A)+ mRNA was purified with oligo(dT) columns from Oc cells cultured for 10 days, and a cDNA library was constructed with a ZAP-cDNA Synthesis Kit (Stratagene). All possible codon selections for the amino acid sequence of PSK-α were considered, and a mixture of 96 oligonucleotides [5′-TAT(C)ATC(A/T)TAC(T)ACC(A/G/T)CAA(G)-3′] was synthesized. These 15-mer oligonucleotides were labeled by [γ-32P]ATP with a Kination Kit (Toyobo, Osaka, Japan) and used for screening of the cDNA library by plaque hybridization at 25°C in a hybridization solution containing 6× saline sodium citrate (1× SSC = 150 mM NaCl/1.5 mM C6H5Na3O7⋅2H2O, pH 7.5), 20 mM NaH2PO4, 0.4% SDS, 5× Denhardt’s solution, and 500 μg/ml denatured, sonicated salmon sperm DNA. After hybridization, the filters were washed in several changes of 6× SSC and 0.1% SDS at 25°C for 1 h. The library was also screened with C26907, a partial cDNA the predicted incomplete ORF of which contains the PSK-α sequence. Hybridization was executed in a solution of 5× SSC, 0.5% SDS, 5× Denhardt’s solution, and 500 μg/ml salmon sperm DNA at 65°C by using the cDNA probe 32P-labeled with a Random Primed DNA Labeling Kit (Takara, Tokyo, Japan). Washing was performed three times with 2× SSC at 25°C for 15 min and then three times with 2× SSC containing 0.1% SDS at 65°C for 15 min.
Excision and DNA Sequencing.
pBluescript plasmids containing the positive inserts were excised from the positive phages and introduced into Escherichia coli strain SOLR with an in vivo protocol recommended by the manufacturer (Stratagene). The subcloned inserts were amplified with a BigDye Terminator Cycle Sequencing Kit (Applied Biosystems) and sequenced with an ABI PRISM 310 Genetic Analyzer (Applied Biosystems) in accordance with the manufacturer’s protocols.
Construction of Chimeric Genes.
A 22-mer primer (5′-CATCTTGGGAGTAGATATAATC-3′) was synthesized and used to obtain mutated cDNA with an LA PCR In Vitro Mutagenesis Kit (Takara). The mutant was designed to produce [Ser4]PSK-α instead of PSK-α. The pAct-nos/Hmz-harboring kanamycin- and hygromycin-resistant genes, which flank the foreign gene and are used for selection of transformants, was used as a binary vector for Oc cell transformation. The original or mutated cDNA was digested with SmaI and EcoRV and inserted into the SmaI site of the vector to construct chimeric genes harboring the cDNA in sense or antisense orientations. Expression of the chimeric genes was driven by the rice actin promoter incorporated within the binary vector.
Transformation of Rice Oc Culture Cells.
The constructs were transformed into Agrobacterium tumefaciens (LBA4404) by triparental mating (14), and the Agrobacterium-mediated transformation of Oc cells was effected as described (15). Rice Oc cells transformed with the binary vector alone served as controls. In the complementary test, PSK was added into the medium for antisense transformants at a final concentration of 100 nM. To detect the introduced genes, genomic DNA was isolated from transgenic cells according to the following protocol and digested with BamHI. The generated fragments were fractionated by agarose gel electrophoresis and hybridized with the labeled cDNA as described above.
Purification of PSKs from CM.
CM was prepared from 14-day-cultured Oc cells by filtration (Advantec no. 2) and stored at −20°C until use. Aliquots (40 ml) of each CM were buffered by adding Tris to a final concentration of 20 mM, adjusted to pH 8.0 with 6.0 N HCl, and then applied to a DEAE-Sephadex A-25 column (1.7 × 8 cm, Amersham Pharmacia) which was first equilibrated with 20 mM Tris⋅HCl buffer at pH 8.0. The column was washed with 50 ml of equilibration buffer and eluted successively with 50 ml of buffer containing 0, 400, 800, or 1200 mM KCl at a flow rate of 60 ml/h. Trifluoroacetic acid was added to a final concentration of 0.1% into the last two fractions containing PSKs, and then the samples were applied to a Sep-Pak Vac column (12 ml, C18, Millipore) after equilibration with 0.1% trifluoroacetic acid. The column was washed with 30 ml of the same buffer at a flow rate of 60 ml/h and eluted with 30 ml of 30% acetonitrile containing 0.1% trifluoroacetic acid. The fraction containing PSKs was collected and lyophilized for subsequent analyses.
Liquid Chromatography (LC)–MS Experiment.
Mass spectra were obtained by using a Fisons VG platform quadrupole mass spectrometer with electrospray ionization interfaced to a Jasco PU 980 HPLC system. The fraction containing PSKs was dissolved in 200 μl of water and separated on a reversed phase HPLC column (Develosil ODS-HG-5, 4.6 × 250 mm, Nomura Chemicals, Seto, Japan) with 10% acetonitrile containing 0.1% trifluoroacetic acid at 1.0 ml/min. The HPLC eluate was split 1:9 so that 100 μl/min flowed to the mass spectrometer during the separation. The pseudomolecular ions of PSKs were scanned every 1.9 s with selected ion monitoring at m/z 831 ([M—H]− of [Ser4]PSK-α), m/z 751 ([M—H—SO3]− of [Ser4]PSK-α), m/z 845 ([M—H]− of PSK-α), m/z 765 ([M—H—SO3]− of PSK-α), m/z 703 ([M—H]− of [Ser4]PSK-β), and m/z 717 ([M—H]− of PSK-β). The semiquantitative amounts of the PSK-α, -β, [Ser4]PSK-α and -β in CM were measured based on the peak heights without internal standard. The fractions containing [Ser4]PSK-α or [Ser4]PSK-β were collected separately, lyophilized, and dissolved in 20 μl of water for sequencing.
Amino Acid Sequence Analysis.
Amino acid sequences were determined by Edman degradation with a 490 Procise Protein Sequencing System (Applied Biosystems). Phenylthiohydantoin derivatives of amino acids obtained at each cycle were analyzed by reversed phase HPLC on an ABI Brownlee C-18 column.
RNA Isolation and RNA Blot Analysis.
Total RNAs were isolated from Oc cells cultured for 3, 7, 10, or 14 days according to Chomczynski’s protocol (16). Twenty micrograms was denatured at 65°C for 5 min in 50% formamide/1× Mops (200 mM Mops/10 mM EDTA/50 mM sodium acetate, pH 7.0)/1.5% formaldehyde. The RNAs were then fractionated by electrophoresis on a 1.2% (wt/vol) agarose gel containing 2.2 M formaldehyde and subsequently transferred to Biodyne nylon membranes (Pall, Port Washington, NY) in 20× SSC and then allowed to hybridize with 32P-labeled cDNA according to the manufacturer’s protocol.
Reverse Transcription–PCR (RT-PCR).
The first leaves, second leaves, shoot apexes, seminal roots, and crown roots were cut from rice seedlings 8 days after germination. Total RNAs (1.5 μg) were isolated from the samples as described previously and then individually used to synthesize first strand cDNA with the Moloney murine leukemia virus reverse transcriptase supplied in a First-strand cDNA Synthesis Kit (Pharmacia). The first strand cDNAs and a pair of 22-mer primers identical or complementary to target sequence (5′-GAATGGTGAATCCAGGAAGAAC-3′ and 5′-GCATGCTACTGGACTATTGTCA-3′) were then used to perform PCR. PCR was run for 30 cycles each of which consisted of denaturation at 95°C for 1 min, primer annealing at 55°C for 30 s, and polymerization at 72°C for 30 s in a Robocycler (Stratagene). The RT-PCR products were fractionated by agarose gel electrophoresis and hybridized with the labeled cDNA as described above.
Genomic DNA Extraction and DNA Blot Analysis.
Genomic DNA was extracted from rice Oc culture cells and seedlings of the other species tested by using the CATB method (17), and 10-μg aliquots were digested with restriction endonucleases, separated on 0.8% (wt/vol) agarose gels, and transferred to Biodyne nylon filters in alkaline transfer buffer (0.4 N NaOH/0.6 N NaCl). Hybridization was performed as described above.
Results
Molecular Cloning of Rice PSK cDNA.
Because rice Oc culture cells produce more PSK-α than asparagus mesophyll cells and maize culture cells (9), we chose this cell line to isolate PSK cDNA. A cDNA library was constructed by using polyadenylated mRNA purified from Oc cells cultured for 10 days. All possible codon selections were used to synthesize a mixture of 96 oligonucleotides from which the amino acid sequence of PSK-α can be deduced. Approximately 200,000 recombinants from the primary library were screened by plaque hybridization with a degenerate mixture of the 96 oligonucleotides. About 50 positive clones were obtained through first screening. Ten of the initial positive clones were rescreened, and three cDNA clones were hybridized with the probes. All three clones were sequenced on both strands; only one of them was found to encode the PSK-α within a larger protein, PP-PSK. This clone was designated as OsPSK cDNA. The PSK sequence was used to perform reiterative searches of the expressed sequence tags database by using the TBLASTN and BLASTN algorithms. Five rice expressed sequence tags were thereby identified, most containing multiple stop codons but no start codon before their predicted PSK sequences except C26907, with an incomplete ORF containing the PSK sequence. By using this partial cDNA as a probe, several clones identical with OsPSK cDNA were also isolated.
Structure of the PSK Precursor.
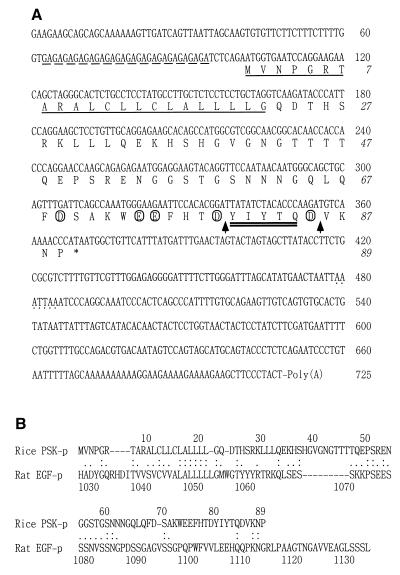
The cloned OsPSK cDNA is 725 bp long with 16 GA repeats in the 5′-untranslated region (Fig. 1A). The ORF starting with the first ATG available is 267 bp long, encoding an 89-aa PP-PSK with a predicted molecular mass of 9.8 kDa and an isoelectric point of 6.48. Amino acids 80–84 encode PSK-α (Fig. 1A). Interestingly, the PP-PSK has a 22-aa hydrophobic NH2-terminal signal sequence (residues 1–22) that resembles a cleavable leader peptide commonly found in animal bioactive peptide precursors (18), as shown in Fig. 1A, and consequently its predicted mature form is 67 aa long with a high percentage of charged amino acids (6% aspartic acid, 7.5% glutamic acid, and 6% lysine) making it hydrophilic. There is an aspartic acid residue immediately NH2-terminal to the first tyrosine of PSK-α in the −1 position, and two and three acidic residues are present between −5 and +5 around the first and second tyrosine residues, respectively (Fig. 1A). Putative processing sites bordering PSK-α conform to the consensus sequence for V8-peptidase (Fig. 1A), suggesting that PSK-α could be proteolytically processed from PP-PSK (19).
Figure 1.
(A) Nucleic acid and deduced amino acid sequences of OsPSK cDNA. The GA repeats are indicated with a dashed underline; the most likely sequence for a polyadenylation signal is dotted. The potential NH2-terminal signal sequence is single underlined, and the PSK-α sequence is underscored with a double underline. The acidic residues near PSK-α are circled, and the putative processing sites bordering PSK-α are indicated by arrows. (B) Amino acid comparison of the rice PSK precursor with the precursor of rat EGF. The alignment was generated by using the FASTA program. Identical and functionally conserved amino acids are indicated by double and single dots, respectively.
Novel Protein Without Homolog in Plants.
A FASTA search (20) did not reveal any significant homology between the cDNA and other sequences in the DNA databases, except for two rice expressed sequence tags, C26907 and its sibling clone, with no known function. Interestingly, a comparison of the predicted PP-PSK amino acid sequence with sequences in current protein databases uncovered some similarity to the C-terminal part of the rat epidermal growth factor (EGF) precursor (21). The most similar region spans 62 aa (1–62) and is 29% identical (66% positive; identical and conserved substitutions) with the C-terminal part of rat EGF precursor, but only limited similarity was found throughout the remaining parts flanking the PSK-α (Fig. 1B).
OsPSK cDNA Is Involved in Cell Proliferation.
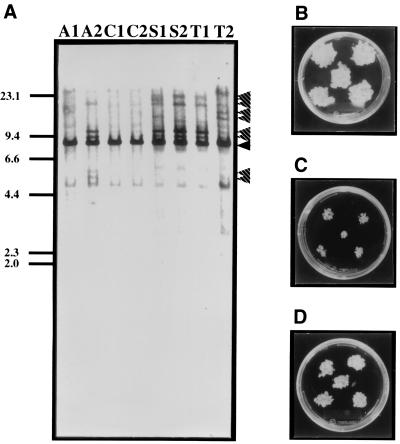
To investigate OsPSK action, we introduced the wild-type cDNA in sense and antisense orientations into Oc cells by using the binary vector. The presence of the introduced OsPSK cDNA in the transgenic Oc cells was confirmed by DNA blot analysis (Fig. 2A, lane A2, S1, S2, T1, and T2). Transgenic cell lines were obtained at frequencies of 16.0% for the sense construct and 8.5% for the antisense construct relative to the number of cell clusters that had been cocultivated with Agrobacterium. While checking the effects of supernumerary or suppressed expression of OsPSK on cell proliferation, we noted that the sense transgenic cells (Fig. 2B, line S2) divided about 2 times faster than the controls (Fig. 2D, line C1), whereas the antisense transgenic cells had decreased mitogenic activity (Fig. 2C, line A2). This was complemented in part (38–64%) by supplementation of PSK-α to the medium (Table 1). These data indicated that the OsPSK gene promotes plant cell division through its product, PSK-α.
Figure 2.
(A) Detection of introduced genes. Genomic DNAs (10 μg) isolated from the control (lane C1 and C2) and the transgenic Oc cells harboring the antisense (lane A1 and A2), sense (lane S1 and S2), or mutated OsPSK cDNA (lane T1 and T2) were digested with BamHI. The generated fragments were hybridized with the labeled OsPSK cDNA. Bands derived from the endogenous OsPSK gene and the introduced OsPSK cDNA are indicated by black and striped arrowheads, respectively. (B–D) Comparison of growth of control and transgenic Oc cells. Control or transgenic Oc cells were transplanted into fresh MS medium containing 1.0 mg/liter 2,4-dichlorophenoxyacetic acid and 0.2% Gelrite. Photographs were taken 2 weeks after culturing at 25°C under light. (B) S2 sense transformants. (C) A2 antisense transformants. (D) C1 control Oc cells.
Table 1.
Accumulation of PSKs in media conditioned by control or transgenic Oc cells
| Cell line | Fresh weight | PSK-α | PSK-β | [Ser4]PSK-α | [Ser4]PSK-β |
|---|---|---|---|---|---|
| C1 | 9.6 ± 1.1 | 12.6 ± 1.1 | 332.7 ± 20.1 | 0 | 0 |
| A2 | 5.1 ± 0.9 | 7.3 ± 0.4 | 175.8 ± 15.4 | 0 | 0 |
| A2 + PSK | 7.9 ± 1.5 | UD | UD | UD | UD |
| S2 | 16.2 ± 2.7 | 21.0 ± 1.9 | 555.7 ± 31.1 | 0 | 0 |
| T1 | 12.3 ± 1.9 | 11.5 ± 0.9 | 302.5 ± 15.8 | 3.1 ± 0.3 | 105.2 ± 8.9 |
Control (C1) or transformed Oc cells (0.8 g) were transplanted into 100 ml fresh Musashige and Skoog liquid medium supplemented with 1.0 mg/l 2,4-dichlorophenoxyacetic acid and incubated at 25°C in the dark with rotary shaking at 120 rpm. In the complementary test (A2 + PSK), PSK was added into the medium at a final concentration of 100 nM. After 2 weeks of culture, fresh weights of cells were measured, and PSK-α and its analog concentrations in the CM were purified and quantified by LC/MS. Fresh weights are presented in gram and PSK concentrations as nanomolar. Averages of three independent experiments are given with the standard deviation. C1 is the transgenic cells harboring vector alone. A2, S2, and T1 are the transgenic cell lines harboring antisense, sense, or [Ser4]-mutated construct, respectively. UD, undetermined.
OsPSK cDNA Is Indeed Translated with the ORF in Cells.
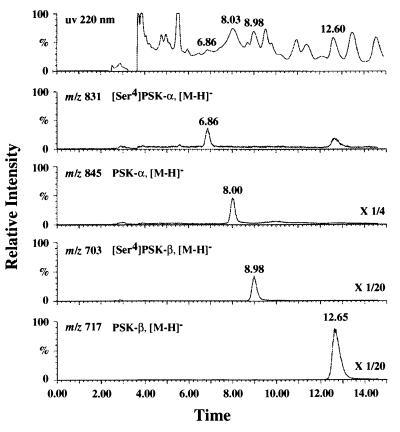
To confirm that the OsPSK cDNA is indeed coding for PSK-α, we transformed rice Oc cells with a mutated cDNA in the sense orientation designed to produce [Ser4]PSK-α instead of PSK-α. The chimeric gene was placed under the control of the constitutive rice actin promoter incorporated within the binary vector pAct-nos/Hmz. Thirty-seven kanamycin- and hygromycin-resistant cell clusters were obtained at a transformation efficiency of 28.5%. We purified PSK-α and its analogs produced and released into the CM of the T1 transformants, and we performed LC-MS analysis. [Ser4]PSK-α and [Ser4]PSK-β peaks were detected in the elutes derived from the CM of the transgenic Oc cells harboring the mutated cDNA (Fig. 3). Furthermore, sequencing of the peptides contained in the corresponding fractions confirmed that they indeed were [Ser4]PSK-α and -β, respectively, proving that OsPSK cDNA is undoubtedly translated with the ORF in the transgenic cells.
Figure 3.
Mass chromatograph from LC-MS analysis of peptides derived from the CM of T1 transformant with the mutated cDNA. The T1 transgenic Oc cells were transplanted into fresh MS liquid medium and incubated for 2 weeks to prepare CM. PSK-α and its analogs containing in the CM were concentrated by two steps of column chromatography and subjected to LC-MS analysis with selected ion monitoring at m/z 831 ([M—H]− of [Ser4]PSK-α), m/z 845 ([M—H]− of PSK-α), m/z 703 ([M—H]− of [Ser4]PSK-β), and m/z 717 ([M—H]− of PSK-β). The peaks eluting at 6.86, 8.00, 8.98, and 12.65 min were [Ser4]PSK-α, PSK-α, [Ser4]PSK-β, and PSK-β, respectively.
Quantification of PSK-α and Its Analogs Secreted into CM.
Oc cells transformed with the sense (line S2), antisense (line A2), or mutated cDNA (line T1) were quantified for their secretion of PSK-α and its analogs into CM. Two weeks after the transformed cells had been transplanted into fresh medium, the semiquantitative amounts of the PSK-α and -β and [Ser4]PSK-α and -β in CM were measured by LC-MS based on peak heights without internal standard. Rice Oc cells transformed with the binary vector alone (line C1) served as controls. PSK-α and -β accumulated in the CM of the sense transformant at 1.6 times the control concentration (Table 1). On the other hand, PSK-α and -β production in the antisense transformant CM was <60% of the mean control level (Table 1). The transgenic Oc cells with the mutated cDNA produced [Ser4]PSK-α and -β at a ratio similar to that of PSK-α and -β. However, the total amount of [Ser4]PSK-α and -β produced by the introduced cDNA was only ≈34% of the PSK-α and -β produced by the endogenous gene in the transgenic Oc cells with the mutated cDNA (Table 1).
Expression of the OsPSK Gene.
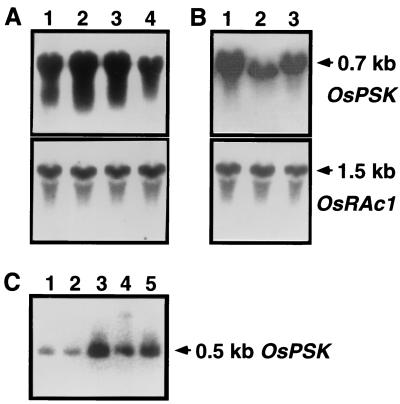
RNA blot analysis with RNA extracted from Oc suspension culture cells revealed that the OsPSK gene was continuously expressed and most abundantly 7–10 days after transplanting (Fig. 4A), suggesting provision of a continuous supply of PSK, which allows the cells to proliferate rapidly. Amounts of PP-PSK mRNA were higher in the sense but lower in the antisense transformants than that in the controls (Fig. 4B), consistent with the results of PSK quantification.
Figure 4.
Expression of the OsPSK gene. (A) The steady state levels of OsPSK transcripts in rice Oc culture cells. Total RNAs (20 μg) extracted from Oc cells cultured for 3 (lane 1), 7 (lane 2), 10 (lane 3), or 14 days (lane 4) were separated by electrophoresis and hybridized with labeled cDNA (OsPSK) at 60°C. The blot was reprobed with the labeled rice actin cDNA (OsRAc1) to indicate equal loading of RNA. (B) OsPSK transcripts in transgenic Oc cells. Total RNAs (20 μg) were extracted from S2 sense (lane 1), A2 antisense (lane 2), or C1 control (lane 3) Oc cells cultured for 3 days and used to perform RNA blot analysis as described in A. (C) Expression of the OsPSK gene in rice seedlings. Total RNAs (1.5 μg) isolated from the first leaves (lane 1), second leaves (lane 2), shoot apexes (lane 3), crown roots (lane 4), and seminal roots (lane 5) of rice seedlings 8 days after germination were individually used to perform RT-PCR and visualized by hybridization as described above. A 0.5-kb band was predictably proliferated and hybridized with the labeled OsPSK cDNA at 65°C.
Next, to determine whether the OsPSK gene product is also important in vivo, we investigated the possibility that the OsPSK gene is expressed in intact plants. Rice seedlings 8 days after germination were used as materials. Because the OsPSK transcript could not be detected by RNA blot analysis in the seedlings, it is possible that OsPSK gene is expressed only in limited tissues and/or stages, so that intact plants contain a small amount of PP-PSK mRNA. Therefore, we performed RT-PCR analysis to examine the amount of PP-PSK mRNA in the seedlings. The results demonstrated that the OsPSK transcripts accumulated throughout the seedling, whereas the mRNA was most abundant in fragments containing shoot or root apexes where cells proliferate vigorously (Fig. 4C).
DNA Blot Analysis of the OsPSK Gene.
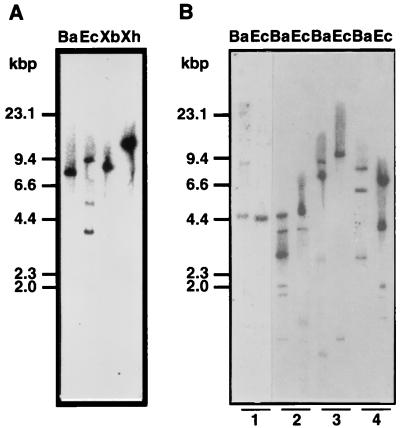
DNA blot analysis was performed by using the full-length cDNA as a probe. The results suggested that the OsPSK gene might belong to a small multigene family (Fig. 5A). However, the multiple hybridizing bands derived from EcoRI digestion could be attributed to the restriction sites found in OsPSK cDNA. To verify this notion, we reprobed the blot by using a 300-bp fragment from the 5′ terminus of OsPSK cDNA. As expected, only one band of 3.6 kbp was hybridized (data not shown). Therefore, PP-PSK is likely to be encoded by a single gene.
Figure 5.
DNA blot analyses of the OsPSK gene. (A) Genomic DNA (10 μg) isolated from rice Oc cells was digested with either BamHI (lane Ba), EcoRI (lane Ec), XbaI (lane Xb), or XhoI (lane Xh) and probed with the labeled full length cDNA at 65°C. (B) DNA blot analysis of the species distribution of OsPSK homologs. Genomic DNAs (10 μg) from Arabidopsis (1), asparagus (2), carrot (3), and zinnia (4) were digested with BamHI (lane Ba) or EcoRI (lane Ec) and hybridized with the same probe at 55°C.
To determine whether OsPSK homologs also occur in other plant species, we performed DNA blot analyses of genomic DNA from four other species, Asparagus officinalis, Arabidopsis thaliana, Daucus carota, and Zinnia elegana. OsPSK homologs were detected in all four (Fig. 5B), suggesting conservation in both monocot and dicot plants.
Discussion
We have described the molecular cloning and characterization of the OsPSK gene encoding the precursor of PSK-α, a peptide factor that can initiate signal transduction to activate plant cell division. Identification of the initiating methionine codon was made on the basis of three findings: (i) the ATG is proximal to the 5′ end of OsPSK cDNA (Fig. 1A); (ii) an adjacent sequence similar to the plant consensus sequence for translational initiation (22) is conserved (Fig. 1A); and (iii) the ORF is indeed functional in the transgenic Oc cells (Figs. 2 and 3 and Table 1). Possibly the long 3′-untranslated region has the ability to stimulate expression of the OsPSK gene or plays a role in mRNA stabilization, but a more probable explanation is that it is a translational regulating sequence, as reported in Drosophila (23).
By a reverse genetics approach, it could be demonstrated that the OsPSK cDNA indeed encodes PSK-α. Accumulation of [Ser4]PSK-α in the CM of the transgenic Oc cells with the mutated cDNA (Fig. 3 and Table 1) demonstrated that the OsPSK cDNA is in fact translated with the 89-aa ORF starting with the first ATG available and the precursor is successively processed to produce PSK-α. Simultaneously, the finding of [Ser4]PSK-β in the CM (Fig. 3 and Table 1) confirmed that PSK-β is an enzymatic degradation of product of PSK-α (8) and suggested that the posttranslational enzymatic events include not only limited endoproteolysis but also exopeptidase digestion (24). The fact that the amount of PSKs produced by the introduced cDNA was less than that produced by the endogenous gene (Table 1) might have resulted from a lower level of expression of the foreign gene (Fig. 4B). On the other hand, the total amount of PSKs found in the Oc cells containing mutated cDNA was less than that found in the sense-transgenic Oc cells (Table 1), suggesting that the amino acid replacement may decrease processing and/or modification efficiency.
The remarkable amount of PSK-α in Oc cells in comparison with asparagus mesophyll cells and maize culture cells (9) is in line with the notable level of OsPSK transcripts (Fig. 4A). The expression pattern of OsPSK gene in Oc culture cells corresponds to the accumulation curve of PSK secreted into CM and the proliferation pattern, with maximum rates of division observed 7–10 days after transplanting. Hitherto, we have not been able to identify a PSK-α peptide in any intact plant with chemical and immunological methods. Here we succeeded in detecting OsPSK transcripts in rice seedlings by using RT-PCR. Moreover, the OsPSK transcripts accumulated at a much higher level in the root and shoot apexes than in the other parts of rice seedlings (Fig. 4C). These findings indicate that the PSK-α molecule is also produced and has a physiological function in intact plants.
Recently, we also found PSK-α in CM derived from several dicot cells including Arabidopsis, carrot and zinnia (unpublished data). DNA blot analysis further showed that OsPSK homologs may exist in these three plants as well as in asparagus and rice (Fig. 5A), suggesting that PSK-α and its gene may be universally distributed in the plant kingdom. The OsPSK gene mode of action may be resolved in more detail by reverse genetic manipulation such as studying plants in which it is knocked out.
The term “protein sulfation” describes two types of modification of proteins by covalent binding of sulfate. The first features linkage to carbohydrate moieties of glycoproteins and proteoglycans, whereas in the second binding is to amino acid residues, i.e., primary modification of the polypeptide chain itself. Only one amino acid, tyrosine, has thus far been shown to undergo this modification. And many secretory and membrane proteins have been reported to contain tyrosine sulfate (25). Protein tyrosine sulfation is a ubiquitous posttranslational modification in animal cells, although PSK-α is the only peptide possessing a sulfated tyrosine reported in higher plants.
Tyrosine sulfation has been shown to affect the biological activity of proteins, as demonstrated in the case of cholecystokin (26). Our previous demonstration that unsulfated PSK-α has no activity indicates that sulfation is of vital importance for its activation. Deletion of the sulfate groups of Tyr1 and Tyr3 resulted in compounds with 0.6 and 4% of the activity of PSK-α, indicating that the former to be more important for its activity (12).
Sulfated tyrosines are usually located within acidic regions of secretory proteins such as cholecystokinin and all sites that have been characterized in animals have aspartic and glutamic residues near the sulfated tyrosine. Three or more acidic residues are usually found between −5 and +5 around the sulfated tyrosine (27). PSK-α is located within the acidic region of the precursor (Fig. 1A). And the existence of an aspartic acid at −1, a glutamic acid at −5, and an aspartic acid at position +5 relative to the first tyrosine, as well as aspartic acids at −3 and +5 positions around the second tyrosine residue in the PSK-α precursor (Fig. 1A), suggests that the two tyrosine residues could be sulfated by a tyrosylprotein sulfotransferase with a similar mechanism to that operating in the animal system (28, 29). Thus, OsPSK cDNA is the first one encoding a putative sulfated protein identified from plants.
Generally, peptide–hormones and other biologically active peptides are synthesized as inactive higher molecular weight precursors that must undergo a variety of posttranslational processing steps to yield the active peptides in animals (24). In plants, systemin is formed as a precursor without a signal peptide (2). We have shown here that PSK-α is also derived from a larger precursor. Notably, the PSK precursor has a 22-aa prepeptide, a feature commonly found in animal-active peptides and hormones. With the prohormones studied, the posttranslational enzymatic events include limited endoproteolysis and may include other modifications of the generated peptide such as limited exopeptidase digestion (24). The secretory vesicle hypothesis, considered of major significance regarding processing, states that the initial endoproteolytic event occurs on formation of the secretory vesicle. Although the processing procedure of PSK precursor is under investigation, it is likely that the hydrophobic pre- or signal peptide of the PSK-α precursor mediates translocation across membranes of the endoplasmic reticulum during its synthesis and allows secretion into the extracellular space as in animal systems (18).
It is generally established that the primary processing recognition site in prohormone precursor proteins often contains a monobasic amino acid or a strongly polar residue in close sequence proximity to the doublet of basic residues that brackets the peptide hormone in animal systems (24). The putative processing sites bordering systemin (2) or PSK-α do not conform to the consensus sequence for endoproteolytic processing sites flanking bioactive peptides within animal prohormone precursors (24). The consensus sequence for V8 peptidase (19) was, however, found twice in PP-PSK at amino acid residues 78 to 79 and 85 to 87, flanking on PSK-α both sides. Judging from the available data, plants and animals appear to have different endoproteolytic processing enzymes.
EGF was the first animal growth factor to be described and regulates various biological responses in a wide variety of mammalian cell types. EGF-like peptides are also found in other species (30–32), pointing to their conservation throughout evolution and probably a function of general significance in nature. Although the exact interrelationship between PP-PSK and the EGF precursor is not known to this point, it is likely that both might be derived from a common ancestor. Perhaps, and most importantly, EGF-like peptides may exist in plant cells and regulate various responses, but they have yet to be identified (33).
In the last 50 years, great progress has been made in plant research and no more so than in the field of plant growth controls. The introduction of molecular biological, genetic, and cell biological methodologies has made an enormous impact on research on plant growth substances and resulted in the discovery of plant polypeptide factors such as systemin and PSK-α. Our report is the first describing a cDNA for a plant sulfated protein, providing evidence that peptide signals are involved in plant growth and cell division as well as in plant defense, where systemin, another signaling polypeptide, is involved (1, 2). Because of its universality and the very low concentrations at which it is active, PSK-α may be a very important growth factor in plants.
Acknowledgments
We thank Dr. K. Syono for supplying the rice Oc cells, Dr. S. Imanishi for assistance with the cDNA library construction, Dr. T. Sasaki for providing the rice expressed sequence tag clone C26907, Dr. M. Matsuoka for the gift of the pAct-nos/Hmz vector, Mr. K. Maeo for help in triparental mating, and Ms. T. Asano for preparing rice seedlings. This research was supported by the Program for the Promotion of Basic Research Activities for Innovative Biosciences, Bio-oriented Technology Research Advancement Institution of Japan.
Abbreviations
- CM
conditioned medium
- LC
liquid chromatography
- OsPSK
Oryza sativa phytosulfokine gene
- PSK
phytosulfokine
- PP-PSK
preprophytosulfokine
- RT
reverse transcription
- EGF
epidermal growth factor
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank, EMBL, and DDBJ nucleotide sequence databases (accession no. AB020505).
References
- 1.Pearce G, Strydom D, Johnson S, Ryan C A. Science. 1991;253:895–898. doi: 10.1126/science.253.5022.895. [DOI] [PubMed] [Google Scholar]
- 2.McGurl B, Pearce G, Orozco-Cardenas M, Ryan C A. Science. 1992;255:1570–1573. doi: 10.1126/science.1549783. [DOI] [PubMed] [Google Scholar]
- 3.Stuart R, Street H E. J Exp Bot. 1969;20:556–571. [Google Scholar]
- 4.Birnberg P R, Somers D A, Brenner M L. J Plant Physiol. 1988;132:316–321. [Google Scholar]
- 5.Somers D A, Birnberg P R, Petersen W L, Brenner M L. Plant Sci. 1987;53:249–256. [Google Scholar]
- 6.Bellincampi D, Morpurgo G. Plant Sci. 1987;51:83–91. [Google Scholar]
- 7.Teasdale R D, Richards D K. Plant Cell Tissue Organ Cult. 1991;26:53–59. [Google Scholar]
- 8.Matsubayashi Y, Sakagami Y. Proc Natl Acad Sci USA. 1996;93:7623–7627. doi: 10.1073/pnas.93.15.7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsubayashi Y, Takagi L, Sakagami Y. Proc Natl Acad Sci USA. 1997;94:13357–13362. doi: 10.1073/pnas.94.24.13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsubayashi Y, Sakagami Y. Plant Cell Rep. 1998;17:368–372. doi: 10.1007/s002990050408. [DOI] [PubMed] [Google Scholar]
- 11.Matsubayashi Y, Morita A, Matsunaga E, Furuya A, Hanai N, Sakagami Y. Planta. 1999;207:559–565. [Google Scholar]
- 12.Matsubayashi Y, Hanai H, Hara O, Sakagami Y. Biochem Biophys Res Commun. 1996;225:209–214. doi: 10.1006/bbrc.1996.1155. [DOI] [PubMed] [Google Scholar]
- 13.Murashige T, Skoog F. Physiol Plant. 1962;15:473–479. [Google Scholar]
- 14.Van Haute E, Joos H, Maes S, Warren G, Van Montagu M, Schell J. EMBO J. 1983;2:411–418. doi: 10.1002/j.1460-2075.1983.tb01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiei Y, Ohta S, Komari T, Kumashiro Plant J. 1994;6:271–282. doi: 10.1046/j.1365-313x.1994.6020271.x. [DOI] [PubMed] [Google Scholar]
- 16.Chomczynski P. BioTechniques. 1993;15:532–536. [PubMed] [Google Scholar]
- 17.Murry M G, Thompson W F. Nucleic Acids Res. 1980;8:4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Douglass J, Civelli O, Herbert E. Annu Rev Biochem. 1984;53:665–715. doi: 10.1146/annurev.bi.53.070184.003313. [DOI] [PubMed] [Google Scholar]
- 19.Houmard J, Drapeau G R. Proc Natl Acad Sci USA. 1972;69:3506–3509. doi: 10.1073/pnas.69.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearson W R, Lipman D J. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simpson R J, Smith J A, Moritz R L, O’Hare M J, Rudland P S, Morrison J R, Lloyd C J, Grego B, Burgess A W, Nice E C. Eur J Biochem. 1985;153:629–637. doi: 10.1111/j.1432-1033.1985.tb09346.x. [DOI] [PubMed] [Google Scholar]
- 22.Lutcke H A, Chow K C, Mickel F S, Moss K A, Kern H F, Scheele G A. EMBO J. 1987;6:43–38. doi: 10.1002/j.1460-2075.1987.tb04716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubnau J, Struhl G. Nature (London) 1996;379:694–699. doi: 10.1038/379694a0. [DOI] [PubMed] [Google Scholar]
- 24.Harris R. Arch Biochem Biophys. 1989;275:315–333. doi: 10.1016/0003-9861(89)90379-2. [DOI] [PubMed] [Google Scholar]
- 25.Huttner W B. Methods Enzymol. 1984;107:200–223. doi: 10.1016/0076-6879(84)07013-0. [DOI] [PubMed] [Google Scholar]
- 26.Bodanszky M, Martinez J, Priestley G P, Gardener J D, Mutt V J. Med Chem. 1978;21:1030–1035. doi: 10.1021/jm00208a006. [DOI] [PubMed] [Google Scholar]
- 27.Dorner A J, Kaufman R J. Methods Enzymol. 1990;185:577–598. doi: 10.1016/0076-6879(90)85046-q. [DOI] [PubMed] [Google Scholar]
- 28.Niehrs C, Huttner W B. EMBO J. 1990;9:35–42. doi: 10.1002/j.1460-2075.1990.tb08077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ouyang Y B, Lane W S, Moore K L. Proc Natl Acad Sci USA. 1998;95:2896–2901. doi: 10.1073/pnas.95.6.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singson A, Mercer K B, L’Hernault S W. Cell. 1998;93:71–79. doi: 10.1016/s0092-8674(00)81147-2. [DOI] [PubMed] [Google Scholar]
- 31.Knust E, Dietrich U, Bremer K A, Weigel D, Vassin H, Campos-Ortega J A. EMBO J. 1987;6:761–766. doi: 10.1002/j.1460-2075.1987.tb04818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Q, Angerer L M, Angerer R C. Science. 1989;246:806–808. doi: 10.1126/science.2814501. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed S U, Bar-Peled M, Raikhel N V. Plant Physiol. 1997;114:325–336. doi: 10.1104/pp.114.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]