Abstract
Although much has been learned about the complication of fatigue during breast cancer treatment, the possibility that there are differences across treatment modalities in breast cancer patients’ experience of fatigue has not yet been established. In this study, fatigue was assessed in 134 women receiving chemotherapy and radiotherapy or radiotherapy only for early stage breast cancer. Comparisons of fatigue during initial treatment indicated that women who received chemotherapy reported greater fatigue severity and disruptiveness than women receiving radiotherapy. Women not pre-treated with chemotherapy experienced increased fatigue over the course of radiotherapy. Results confirmed predictions that fatigue in women with early stage breast cancer differs as a function of the type of treatment and sequencing of treatment. Findings indicating increases in fatigue during radiotherapy only among women not pretreated with chemotherapy suggest a response shift, or a change in internal standards, in women’s perceptions of fatigue as a function of prior chemotherapy treatment.
Keywords: Fatigue, breast cancer, chemotherapy, radiotherapy
Introduction
Increasingly, fatigue is recognized as one of the most distressing symptoms of breast cancer treatment.1–3 Although much has been learned about fatigue, the possibility that there are differences in breast cancer patients’ experience of fatigue during the active treatment period based on treatment modality has not yet been matter established. Studies investigating treatment differences in fatigue have focused primarily on women who have already completed treatment for breast cancer. Findings have been mixed, with some studies reporting significant differences in fatigue between women treated with and without chemotherapy4,5 and other studies reporting no differences.6,7
A limited number of longitudinal studies have examined the course of fatigue in women receiving chemotherapy or radiotherapy for breast cancer. Irvine and colleagues8 found that fatigue increased significantly over the course of radiotherapy for breast cancer. Greenberg and colleagues9 found that fatigue levels increased initially and then plateaued at Week 4 in a radiotherapy regimen that lasted 6 t o 9 weeks. Similarly, a study by Geinitz et al.10 of women who received radiotherapy following surgery for breast cancer found that fatigue increased significantly over the course of treatment until Week 4, then remained stable during the fifth and final week of treatment. With regard to chemotherapy, Jacobsen et al.11 found that fatigue increased significantly after the first cycle of chemotherapy and remained elevated during the following three cycles of treatment. Greene et al.12 assessed fatigue over two treatment cycles in women receiving chemotherapy for breast cancer and found that the severity of fatigue decreased between the second and fifth days after each treatment cycle. Berger13 evaluated women with breast cancer at the beginning and midpoint of their first three cycles of chemotherapy. Findings revealed a “roller coaster” pattern in which fatigue was significantly greater at the start of each cycle than at the midpoint.
In patients receiving multiple forms of cancer treatment (e.g., chemotherapy followed by radiotherapy), a “response shift” during the initial treatment may influence perceptions of fatigue during the second treatment. Response shift has been defined as a change in internal standards or values, or a reconceptualization of the quality of life brought about by a change in health status.14 In the context of cancer treatment, a response shift may be a normal adaptive psychological mechanism.15,16 As patients adapt to the side effects of treatment (i.e., fatigue), a change in one’s internal standards may occur such that perceptions of side effect severity are attenuated.
Two previous studies examined whether a response shift in fatigue occurs during the course of radiotherapy.17,18 In both studies, the “then test” was used to assess response shift. The then test involves having patients provide a renewed judgment of the severity of a target symptom (i.e., how they perceive themselves to have been at some point in the past).14 The comparison between the actual rating and renewed judgment for the same time point is presumed to reflect the amount and direction of a shift in the internal standard for rating the severity of the target symptom. Jansen and colleagues17 examined response shift in perceptions of fatigue among breast cancer patients treated with radiotherapy. Adaptation to an increased level of fatigue was reflected by the fact that patients’ mean then test scores were significantly lower than their mean pretest scores. Visser and colleagues18 also observed a response shift in fatigue among women with early stage breast cancer undergoing radiotherapy. Once again, patients’ mean then test scores were significantly lower than their actual mean pretest scores.
Despite the fact that fatigue is a common symptom of breast cancer treatment, we are unaware of any research that has directly compared the characteristics and course of fatigue related to chemotherapy and radiotherapy during the active treatment period. Based on prior research on women’s experience of fatigue following the completion of breast cancer treatment,4,5 we hypothesized that fatigue would be more severe and more disruptive during initial treatment in women treated with chemotherapy plus radiotherapy than in women treated with radiotherapy only. That is, patients who received chemotherapy as their initial treatment were expected to report greater fatigue severity and disruptiveness than patients who received radiotherapy as their initial treatment. We also hypothesized that there would be differences in the course of fatigue during radiotherapy between the two treatment groups. Specifically, we hypothesized that fatigue would increase during radiotherapy only among patients who had not been treated previously with chemotherapy. Compared to women with no experience of chemotherapy-related fatigue, women who had already experienced chemotherapy-related fatigue might perceive radiotherapy to be less fatigue-inducing.
Methods
Participants
Participants were women with early stage breast cancer scheduled for chemotherapy followed by radiotherapy (CT+RT group) or radiotherapy only (RT group) at the Moffitt Cancer Center at the University of South Florida or at the Comprehensive Breast Care Center of the Lucille Parker Markey Cancer Center at the University of Kentucky. Eligibility criteria were that participants: a) be at least 18 years of age, b) be diagnosed with Stage 0, 1 or 2 breast cancer, c) have no history of another cancer other than basal cell skin carcinoma, d) be scheduled to receive a minimum of 4 cycles of chemotherapy and then radiotherapy following surgery (CT+RT group), or be scheduled to receive only radiotherapy following surgery (RT group), e) have no prior history of treatment with either chemotherapy or radiotherapy, f) have no other chronic or life-threatening diseases in which fatigue is a prominent symptom (e.g., multiple sclerosis or chronic fatigue syndrome), and g) provide written informed consent.
Procedure
Eligibility was determined by chart review and consultation with the attending physician. Those women who provided informed consent were instructed to complete a pre-treatment questionnaire on the day of their first clinic visit for chemotherapy (CT+RT group) or radiotherapy (RT group). Additional assessments were conducted with the CT+RT group at the start of their first, third, and final cycles of chemotherapy and at their first, fifteenth, and final visits for radiotherapy, and with the RT group at their first, fifteenth, and final visits for radiotherapy.
Of 167 women considered eligible and asked to participate, 159 accepted. Following consent, 13 women (8%) became ineligible (due to change in treatment plans or decision to receive treatment elsewhere) and 12 (8%) elected to discontinue participation prior to completing all assessments. There were no significant (P <0.05) demographic (i.e., age, race, marital status, education, and income) or clinical (i.e., menopausal status, disease stage, and surgery type) differences between these 25 women and those women who completed participation. All subsequent analyses were based on the 134 women who provided baseline and follow-up data on fatigue.
Fatigue was assessed using the Fatigue Symptom Inventory19 (FSI), a 14-item measure that assesses the frequency and severity of fatigue as well as its perceived disruptiveness. Analyses focused on items assessing fatigue severity and fatigue disruptiveness. The 11-point scale (0 = not at all fatigued, 10 = as fatigued as I could be) that asks respondents to identify their level of fatigue on the day they felt most fatigued during the past week was used to assess fatigue severity. Perceived disruptiveness was measured using seven separate 11-point scales (0 = no interference, 10 = extreme interference) that ask respondents to identify the degree to which fatigue interfered with general level of activity, ability to bathe and dress, normal work activity, ability to concentrate, relations with others, enjoyment of life, and mood during the past week. These seven ratings were summed to yield a total disruptiveness score. Previous research has demonstrated the reliability and validity of the FSI in women diagnosed with breast cancer.19,20 The perceived disruptiveness scale demonstrated adequate internal consistency reliability at each assessment (alpha = 0.92 to 0.95).
Results
Demographic and Clinical Characteristics
Among patients treated with chemotherapy, all but one of the women received a regimen that included four cycles of cyclophosphamide and/or doxorubicin. One woman received six cycles of the two agents. Total cumulative radiation doses ranged from 3,000 to 8,000 cGy with a mean of 5,685 cGy (SD = 677). Preliminary analyses were conducted to compare demographic and clinical characteristics of the two treatment groups (see Table 1). Results indicated that at study entry, women in the RT group were significantly older (M = 58, SD = 10) than women in the CT+RT group (M = 51, SD = 9; t (132) = 4.3, P < 0.0001) and more likely to be postmenopausal (P = 0.02). As expected, women in the RT group were significantly more likely to have undergone lumpectomy than mastectomy (P = 0.003) and to have earlier stage disease (P = 0.001) than women in the CT+RT group.
Table 1.
Demographic and Clinical Variables
| Treatment Group
|
|||
|---|---|---|---|
| Variable | CT+RT n (%) | RT n (%) | P valuea |
| Race | |||
| White | 51 (38%) | 74 (55%) | 0.10 |
| Non-white | 6 (5%) | 3 (2%) | |
| Marital Status | |||
| Married | 48 (36%) | 60 (45%) | |
| Not married | 9 (7%) | 1 7 (13%) | 0.50 |
| Education | |||
| College graduate | 24 (18%) | 30 (22%) | |
| Non-college graduate | 33 (25%) | 47 (35%) | 0.70 |
| Household Income | |||
| Over $40,000 | 36 (31%) | 48 (41%) | |
| Under $40,000 | 16 (14%) | 18 (15%) | 0.70 |
| Menopausal Status | |||
| Pre- or peri- | 25 (19%) | 19 (14%) | |
| Post | 32 (24%) | 58 (43%) | 0.02 |
| Surgery Type | |||
| Lumpectomy Only | 46 (34%) | 77 (57%) | |
| Mastectomy | 11 (8%) | 0 (0%) | 0.003 |
| Disease Stage | |||
| Stage 0 or 1 | 16 (12%) | 73 (54%) | |
| Stage 2 | 41 (31%) | 4 (3%) | 0.001 |
Sixteen participants declined to provide information about income.
Differences between groups analyzed using χ2 analysis.
Correlational analyses were conducted to examine the relation of selected demographic and clinical variables to fatigue severity and disruptiveness. Among women who received chemotherapy as their initial treatment (CT+RT group), neither age, menopausal status, disease stage, nor surgery type was significantly related to fatigue severity or disruptiveness averaged across the three chemotherapy assessments (P values > 0.05). Among women who received radiotherapy as their initial treatment (RT group), neither age, menopausal status, disease stage, nor surgery type was significantly related to fatigue severity and disruptiveness averaged across the radiotherapy assessments (P values > 0.05). For the entire sample (i.e., both CT+RT group and RT group) there also were no significant relationships between these demographic and clinical variables and fatigue severity and disruptiveness (P values > 0.05).
Course of Fatigue in Women Receiving Chemotherapy Followed by Radiotherapy
Figure 1 illustrates the course of fatigue severity among women treated with chemotherapy followed by radiotherapy. Analysis of the effect of time on fatigue severity yielded a significant effect (F (2,95) = 10.16, P < 0.0001). Pairwise comparisons revealed that fatigue severity increased significantly from the start of chemotherapy to the middle of chemotherapy (F (1,56) = 5.73, P < 0.05) but did not change significantly from the middle of chemotherapy to the end of chemotherapy (F (1,56) = 1.13, P = 0.3). However, fatigue severity did decrease significantly during the period of time (M = 31 days, SD = 18 days) between the end of chemotherapy and the start of radiotherapy (F (1,56) = 13.52, P < 0.0005). Fatigue severity at the start of radiotherapy among these women was statistically equivalent to their fatigue severity at the start of chemotherapy (F (1,56) = 0.0, P = 1). There were no significant changes in fatigue severity between the start of radiotherapy and the middle of radiotherapy (F (1,56) = 0.6, P = 0.5) or between the middle of radiotherapy or the end of radiotherapy (F (1,56) = 2.5, P = 0.1) in this group. Fatigue severity at the end of radiotherapy was statistically equivalent to fatigue severity reported at the start of chemotherapy (F (1,56) = 0.14, P = 0.7). Analysis of fatigue disruptiveness data also yielded a significant effect of time (F (2,95) = 8.47, P < 0.0004) and an identical pattern of pairwise comparisons.
Fig. 1.
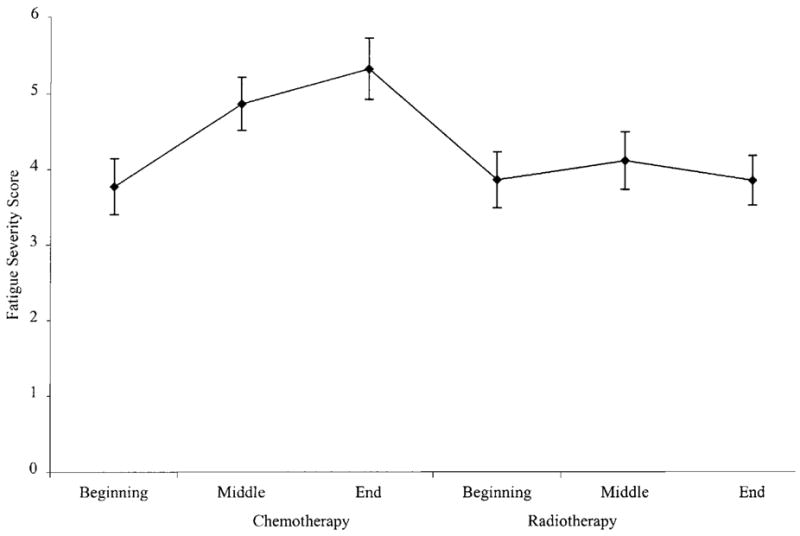
Fatigue severity among women treated with chemotherapy followed by radiotherapy.
Comparison of Fatigue During Radiotherapy Between Women Pre-Treated and Not Pre-Treated with Chemotherapy
Figure 2 illustrates the course of fatigue severity during radiotherapy for women who were and were not pre-treated with chemotherapy. The specific effects modeled in this analysis were the main effects of treatment and time and their interaction. Results yielded a significant treatment × time interaction effect (F (1,155) = 14.67, P < 0.0002). To further characterize this interaction, simple effects analyses were performed within each treatment group across the various times of assessment and between each treatment group at the same time of assessment. The within group simple effect analyses indicated that among women not pre-treated with chemotherapy (i.e., RT group), fatigue severity increased significantly over the course of radiotherapy (F (2,75) = 18.67, P < 0.0001). Fatigue severity increased from the beginning to the middle of radiotherapy (F (1,76) = 28.48, P < 0.0001), but not between the middle and end of radiotherapy (F (1,76) = 2.0, P = 0.2). In contrast, there were no significant changes in fatigue severity over the course of radiotherapy among women pre-treated with chemotherapy (i.e., CT+RT group) (F (2,55) = 1.24, P = 0.3). The between group simple effects analyses revealed that, compared to women not pre-treated with chemotherapy, women pre-treated with chemotherapy were significantly more fatigued at the beginning of radiotherapy (F (1,132) = 5.60, P < 0.02) but not the middle of radiotherapy (F (1,132) = 0.0, P = 0.9). The difference in fatigue severity at the end of radiotherapy was marginally significant (F (1,132) = 3.56, P = 0.06), with women pre-treated with chemotherapy tending to report less fatigue.
Fig. 2.
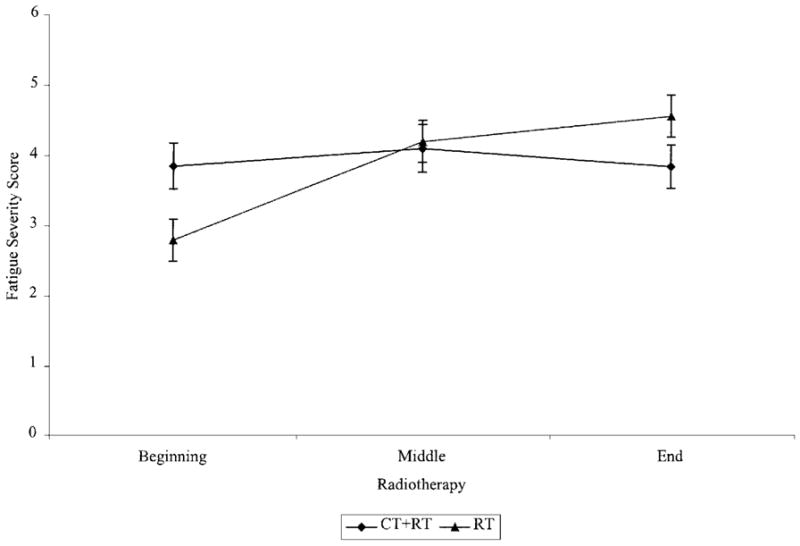
Fatigue severity during radiotherapy for women pre-treated and not pre-treated with chemotherapy.
Analysis of fatigue disruptiveness data also revealed a significant time × treatment interaction (F (1,139) =11.52, P < 0.0009). Simple effects analyses performed within each treatment group indicated that, among women not pre-treated with chemotherapy (i.e., RT group), fatigue disruptiveness increased significantly over the course of radiotherapy (F (2,73) = 6.28, P < 0.003) (see Figure 3). Fatigue disruptiveness increased significantly from the beginning to the middle of radiotherapy (F (1,74) =5.63, P < 0.05) and from the middle to the end of radiotherapy (F (1,74) =5.92, P < 0.05). There were no significant changes in disruptiveness over the course of radiotherapy among women pre-treated with chemotherapy (i.e., CT+RT group) (F (2,64) =0.91, P = 0.4). The between group simple effects analyses revealed a pattern of significant results identical to that for fatigue severity.
Fig. 3.
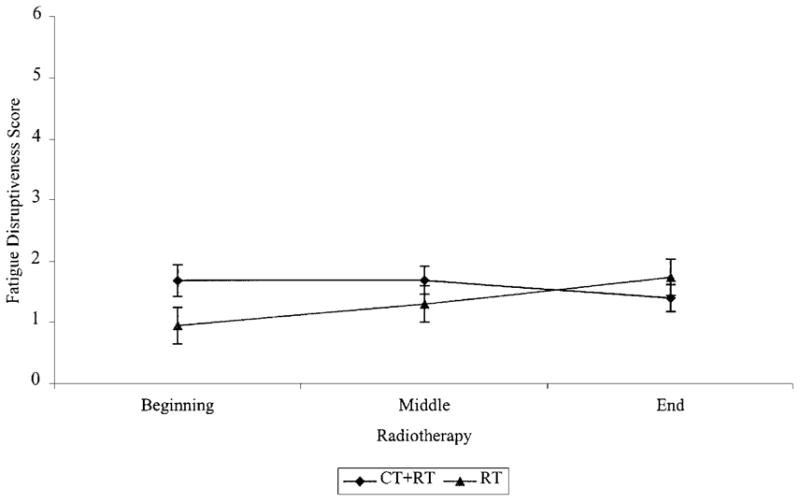
Fatigue disruptiveness during radiotherapy for women pre-treated and not pre-treated with chemotherapy.
Comparison of Fatigue During Initial Type of Treatment
Figure 4 illustrates the course of fatigue severity for each treatment group during their initial type of treatment. As in the previous analysis, the specific effects modeled were the main effects of treatment and time and their interaction. Results yielded significant effects for time (F (1,179) = 48.91, P < 0.0001) and treatment (F (1,179) = 4.90, P < 0.05) and a nonsignificant interaction effect (F (1,179) = 0.47, P = 0.5). Pairwise comparisons across time for the sample as a whole revealed a significant increase in fatigue severity between the start of treatment and the middle of treatment independent of treatment type (F (1,133) = 26.02, P < 0.0001). Fatigue severity did not increase significantly from the middle to the end of treatment (F (1,133) = 2.87, P = 0.1). To further characterize the treatment effect, pairwise comparisons were conducted between treatment groups at the beginning, middle, and end of the initial type of treatment. These comparisons revealed that, compared to those women who received radiotherapy as their initial type of treatment, women who received chemotherapy were significantly more fatigued at the beginning of treatment (F (1,132) = 5.0, P < 0.05). Differences between the two treatment groups at the middle (F (1,132) = 2.87, P = 0.1) and at the end of their initial treatment (F (1,132) = 2.09, P = 0.2) were not significant.
Fig. 4.
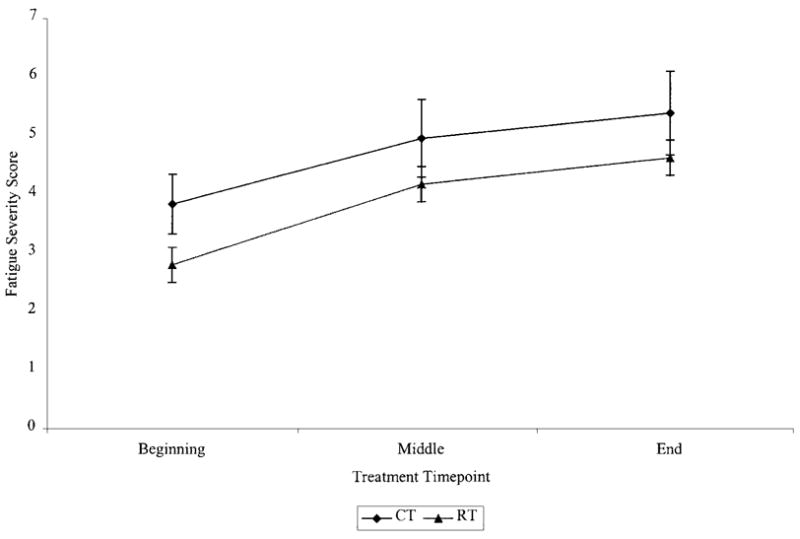
Fatigue severity during initial type of therapy.
In contrast to results for fatigue severity, analysis of fatigue disruptiveness data yielded a significant time × treatment interaction effect (F (1,155) = 13.76, P < 0.0003) (see Figure 5). To further characterize this interaction, simple effects analyses were performed within each treatment group across the various times of assessment and between each treatment group at the same time of assessment. The within group simple effect analyses indicated that among women receiving chemotherapy, fatigue disruptiveness increased significantly over the course of chemotherapy (F (2,55) = 4.62, P < 0.05). Fatigue disruptiveness increased significantly between the beginning and middle of treatment (F (1,56) = 9.24, P < 0.05), but did not change significantly between the middle and end of treatment (F (1,56) =0.74, P = 0.4). Likewise, among women receiving radiotherapy, fatigue disruptiveness increased significantly from the beginning to the middle of treatment (F (1,74) = 5.63, P < 0.05) and from the middle to the end of treatment (F (1,74) = 5.92, P < 0.05). The between group simple effects analyses revealed that compared to women treated with radiotherapy, women treated with chemotherapy experienced significantly greater disruptiveness at the beginning of treatment (F (1,130) = 4.40, P < 0.05) and at the middle of treatment (F (1,130) = 7.66, P < 0.007), but not at the end of treatment (F (1,130) = 1.88, P = 0.2).
Fig. 5.
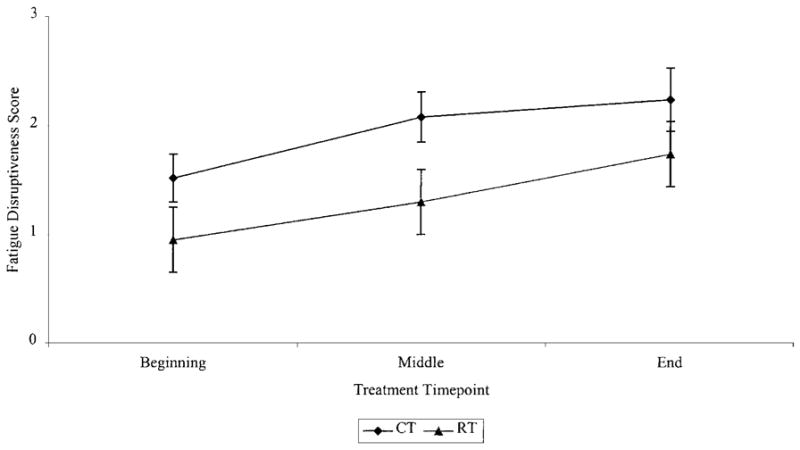
Fatigue disruptiveness during initial type of therapy.
Discussion
Results from the current study supported the hypothesis that women who received chemotherapy as their initial type of treatment for early stage breast cancer would report greater fatigue during the active treatment period than women who received radiotherapy as their initial treatment. Results also supported the hypothesis that women’s experience of fatigue during radiotherapy would differ based on whether radiotherapy was the first or second treatment they received. Women not pre-treated with chemotherapy experienced increased fatigue over the course of radiotherapy, whereas women pre-treated with chemotherapy did not.
To the best of our knowledge, this study provides the first evidence that, among women with early stage breast cancer, chemotherapy is associated with more severe fatigue than radiotherapy. We were somewhat surprised that even before the start of their initial treatment, women in the CT+RT group reported significantly more fatigue, both in terms of severity and disruptiveness, than women in the RT group. Women in the CT+RT group were younger, had relatively more advanced disease, and had undergone more extensive surgical resection of their disease than women in the RT group. However, there were no significant relationships between fatigue and age, disease stage, and type of surgery, an indication that these variables did not account for the observed differences in fatigue. Previous research has demonstrated significant relationships between emotional distress and increased fatigue. Thus, it may be the case that certain psychological factors, such as distress, depression, and anxiety, though not the focus of our study, may have accounted for these differences in fatigue at baseline.
To the best of our knowledge, this study also provides the first evidence that the experience of fatigue among women undergoing radiotherapy for early stage breast cancer can be influenced by prior treatment with chemotherapy. We believe the finding that fatigue increased during radiotherapy only among women not pretreated with chemotherapy is consistent with research suggesting a response shift in perceptions of fatigue as a consequence of cancer treatment. In the present study, it may be the case that women’s perceptions of their fatigue during radiotherapy were altered as a consequence of their preceding experience of chemotherapy-related fatigue. That is, reports of fatigue severity during subsequent radiotherapy may have been influenced by the perception that it was “not as bad” or “no greater” than the fatigue experienced during chemotherapy. In contrast, radiotherapy-only patients could not have been influenced by prior experience with chemotherapy-related fatigue. At least one alternative explanation can be ruled out. The amount of radiotherapy each group received did not differ.
The current study suggests two directions for future research. Previous studies suggest that fatigue may persist for months and even years after the completion of breast cancer treatment.4,7,20 Whether heightened fatigue during the active treatment period is a risk factor for persistent fatigue following treatment completion is not yet known. Thus, one direction for future research would be to document women’s experience of fatigue as they complete treatment and move into the post-treatment period. A second direction for future research involves achieving a more complete understanding of possible response shifts in perceptions of fatigue both during and after completion of active treatment. Had the then test been used in the current study, it may have been possible to obtain direct evidence to support the view that differences in the course of fatigue during radiotherapy between women who were and were not pretreated with chemotherapy reflect a response shift in fatigue among the women pretreated with chemotherapy. Findings from such research would have important methodological implications. If patients routinely accommodate to their fatigue over time, then longitudinal studies that did not include a consideration of response shift might fail to capture an important change in patients’ perception of this symptom. In addition, there are implications for clinical care. Evidence of a response shift suggests that some individuals perceive and are responding to symptoms differently over time. Those who do not have the ability to shift their perceptions of symptoms over time may benefit from psychological interventions designed specifically to alter their perceptions.
Limitations of the current study should be noted. The FSI19 is a well-known measure of fatigue in cancer patients. Although our measure of fatigue severity consisted of just one of 14 FSI items, we essentially replicated our findings with the measure of fatigue disruptiveness comprised of seven FSI items. It is also important to note that the women in our sample were predominately Caucasian, married, and had annual household incomes over $40,000. Whether our findings generalize to a more diverse population of women with early stage breast cancer is not known. Underlying biological mechanisms of fatigue, including anemia and infection, were not the focus of the current study. Nevertheless, that we did not examine potential correctable etiologies for patients’ fatigue is a limitation of this study. A strength of the current study is its longitudinal design and the repeated assessments during the course of active treatment. However, we assessed fatigue only three times during chemotherapy and during radiotherapy, with several days and sometimes weeks elapsing between assessments. It is possible that this assessment schedule may have obscured more meaningful day-to-day fluctuations in fatigue.
Acknowledgments
This research was supported in part by National Cancer Institute Grant R01 CA82822.
References
- 1.Winningham ML, Nail LM, Burke MB, et al. Fatigue and the cancer experience: The state of the knowledge. Oncol Nurs Forum. 1994;21:23–36. [PubMed] [Google Scholar]
- 2.Richardson A, Ream E. The experience of fatigue and other symptoms in patients receiving chemotherapy. Eur J Cancer Care. 1996;5(suppl 2):24–30. doi: 10.1111/j.1365-2354.1996.tb00248.x. [DOI] [PubMed] [Google Scholar]
- 3.Vogelzang NJ, Breitbart W, Cella D, et al. Patient, caregiver, and oncologist perceptions of cancer-related fatigue: Results of a tripart assessment survey. Semin Hematol. 1997;34(suppl 2):4–12. [PubMed] [Google Scholar]
- 4.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in breast cancer survivors: Occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 5.Woo B, Dibble SL, Piper BF, et al. Differences in fatigue by treatment methods in women with breast cancer. Oncol Nurs Forum. 1998;25:915–920. [PubMed] [Google Scholar]
- 6.Andrykowski MA, Curran SL, Lightner R. Off-treatment fatigue in breast cancer survivors: A controlled comparison. J Beh Med. 1998;21:1–18. doi: 10.1023/a:1018700303959. [DOI] [PubMed] [Google Scholar]
- 7.Berglund G, Bolund C, Fornander T, et al. Late effects of adjuvant chemotherapy and postoperative radiotherapy on quality life among breast cancer patients. Eur J Cancer. 1991;27:1075–1081. doi: 10.1016/0277-5379(91)90295-o. [DOI] [PubMed] [Google Scholar]
- 8.Irvine DM, Vincent L, Graydon JE, et al. Fatigue in women with breast cancer receiving radiation therapy. Cancer Nurs. 1998;21:127–135. doi: 10.1097/00002820-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg DB, Sawicka J, Eisenthal S, et al. Fatigue syndrome due to localized radiation. J Pain Symptom Manage. 1992;7:38–45. doi: 10.1016/0885-3924(92)90106-r. [DOI] [PubMed] [Google Scholar]
- 10.Geinitz H, Zimmerman FB, Stoll P, et al. Fatigue, serum cytokine levels, and blood cell counts during radiotherapy of patients with breast cancer. Int J Radiat Oncol Biol Phys. 2001;51:691–698. doi: 10.1016/s0360-3016(01)01657-1. [DOI] [PubMed] [Google Scholar]
- 11.Jacobsen PB, Hann DM, Azzarello LM, et al. Fatigue in women receiving adjuvant chemotherapy for breast cancer: Characteristics, course, and correlates. J Pain Symptom Manage. 1999;18:233–242. doi: 10.1016/s0885-3924(99)00082-2. [DOI] [PubMed] [Google Scholar]
- 12.Greene D, Nail LM, Fieler VK, et al. A comparison of patient-reported side effects among three chemotherapy regimens for breast cancer. Cancer Prac. 1994;2:57–62. [PubMed] [Google Scholar]
- 13.Berger AM. Patterns of fatigue and activity and rest during adjuvant breast cancer chemotherapy. Oncol Nurs Forum. 1998;25:51–62. [PubMed] [Google Scholar]
- 14.Schwartz CE, Sprangers MAG. Methodological approaches for assessing response shift in longitudinal health-related quality-of-life research. Soc Sci Med. 1999;48:1531–1548. doi: 10.1016/s0277-9536(99)00047-7. [DOI] [PubMed] [Google Scholar]
- 15.Wilson IB. Clinical understanding and clinical implications of response shift. Soc Sci Med. 1999;48:1577–1588. doi: 10.1016/s0277-9536(99)00050-7. [DOI] [PubMed] [Google Scholar]
- 16.Sprangers MAG. Response-shift bias: A challenge to the assessment of patients’ quality of life in cancer clinical trials. Cancer Treat Rev. 1996;22(Suppl A):55–62. doi: 10.1016/s0305-7372(96)90064-x. [DOI] [PubMed] [Google Scholar]
- 17.Jansen SJT, Stiggelbout AM, Nooij MA, et al. Qual Life Res. 2000;9:603–615. doi: 10.1023/a:1008928617014. [DOI] [PubMed] [Google Scholar]
- 18.Visser MRM, Smets EMA, Sprangers MAG, et al. How response shift may affect the measurement of fatigue. J Pain Symptom Manage. 2000;20:12–18. doi: 10.1016/s0885-3924(00)00148-2. [DOI] [PubMed] [Google Scholar]
- 19.Hann DM, Jacobsen PB, Azzarello LM, et al. Measurement of fatigue in cancer patients: Development and validation of the Fatigue Symptom Inventory. Qual Life Res. 1998;7:301–310. doi: 10.1023/a:1024929829627. [DOI] [PubMed] [Google Scholar]
- 20.Broeckel JA, Jacobsen PB, Horton J, et al. Characteristics and correlates of fatigue after adjuvant chemotherapy for breast cancer. J Clin Oncol. 1998;16:1689–1696. doi: 10.1200/JCO.1998.16.5.1689. [DOI] [PubMed] [Google Scholar]


