Abstract
Life expectancy of honey bees (Apis mellifera L.) is of general interest to gerontological research because its variability among different groups of bees is one of the most striking cases of natural plasticity of aging. Worker honey bees spend their first days of adult life working in the nest, then transition to foraging and die between 4 and 8 weeks of age. Foraging is believed to be primarily responsible for the early death of workers. Three large-scale experiments were performed to quantitatively assess the importance of flight activity, chronological age, extrinsic mortality factors and foraging specialization. Forager mortality was higher than in-hive bee mortality. Most importantly however, reducing the external mortality hazards and foraging activity did not lead to the expected strong extension of life. Most of the experimental effects were attributable to an earlier transition from hive tasks to foraging. This transition is accompanied by a significant mortality peak. The age at the onset of foraging is the central variable in worker life-history and behavioral state was found more important than chronological age for honey bee aging. However, mortality risk increased with age and the negative relation between preforaging and foraging lifespan indicate some senescence irrespective of behavioral state. Overall, honey bee workers exhibit a logistic mortality dynamic which is mainly caused by the age-dependent transition from a low mortality pre-foraging state to a higher mortality foraging state.
Keywords: Aging, Biodemography, Division of labor, Foraging, Life history, Social insects
1. Introduction
The evolutionary theory of aging predicts that a high extrinsic mortality rate in age-structured populations promotes rapid organismal aging and thus increases the intrinsic mortality. This argument is based on the notion that the force of natural selection opposing aging and senescence is directly related to the survivorship to any given age (Rose, 1991; Kirkwood and Austad, 2000). Important empirical support comes from social insects (Keller and Genoud, 1997; Chapuisat and Keller, 2002). Their social group living habits and the construction of sophisticated nests have reduced the external mortality rate for their reproductives and favored exceptionally long lives (Keller and Genoud, 1997). In general, the reproductives live an order of magnitude longer than their sterile helpers, never leave the safety of the nest center, and act simultaneously as stem cells and gonads of their colony “super-organism”.
The honey bee (Apis mellifera L.) is an emerging model for aging research for multiple reasons (Omholt and Amdam, 2004; Rueppell et al., 2004a). Honey bees have one queen per colony as the sole reproductive. In the queen’s presence the thousands of female workers are functionally sterile and perform all non-reproductive tasks in the colony. Worker/queen caste determination is based on nutrition. Queens receive high-quality food, develop in 16 days, and can live several years, producing up to 2000 eggs per day (Page and Peng, 2001). In contrast, the life expectancy of workers varies seasonally from only 3 to 4 weeks in the summer to over 6 months in the winter (Omholt and Amdam, 2004). Thus, aging in female honey bees is highly variable between and within castes but the proximate factors underlying this plasticity are not clear (Finch, 1990; Corona et al., 2005). This study addresses the proximate causes of worker mortality from a demographic perspective as a first step to understanding the proximate causes for the queen/worker aging plasticity.
The aging rate varies considerably within the honey bee worker caste and this variation is central for understanding the proximate causes of worker mortality (Omholt and Amdam, 2004). Honey bee workers display an unusual type-2 survivorship pattern (Sakagami and Fukuda, 1968) with low mortality rates as young hive bees and high mortality at older ages. The pronounced age-dependent mortality increase is believed to be intimately linked to the workers’ behavioral changes throughout life (Page and Peng, 2001). Honey bee workers display a largely age-based division of labor (Winston, 1987; Beshers and Fewell, 2001): Young bees perform in-hive tasks, such as nest maintenance, food processing, and brood care before a relatively sharp transition to foraging outside the hive. Until their death, foragers collect mainly nectar, pollen, water and plant resins, specializing to varying degrees on these resources (Winston, 1987). The significant increase in age-specific mortality has been regarded as evidence for a high mortality caused by foraging activity (Sakagami and Fukuda, 1968; Page and Peng, 2001) and one early report has estimated that up to 98% of workers die outside the hive (Lundie, 1925). However, these reports present data that confound the mortality effects of chronological aging, behavioral and physiological profile, and extrinsic mortality.
Several hypotheses to explain the peculiar worker mortality patterns have been suggested. Down-regulation of the workers’ free protein reserves at the onset of foraging is central for the elevated mortality in foragers (Amdam and Omholt, 2002; Omholt and Amdam, 2004). This hypothesis that foragers deplete themselves of protein in face of their high external mortality rate to preserve colony resources (Amdam et al., 2005) focuses on vitellogenin, a major hemolymph protein with immune (Amdam et al., 2005) and anti-oxidant functions (Seehuus et al., 2006). In addition, several other mechanistic hypotheses have been suggested to account for the high mortality rate of foraging workers. First, the external mortality hazards of foraging may be sufficient to explain the high mortality rate of foragers (Sakagami and Fukuda, 1968; Visscher and Dukas, 1997). Possible causes of death include predation, accidents, dehydration, or disorientation. Another influential hypothesis proposes that the limited glycogen reserves in foragers cannot be replenished at older ages, leading to death by exhaustion (Neukirch, 1982). Simple wear-and-tear can lead to the inability to fly and ultimately death (Page and Peng, 2001), or oxidative tissue damage may limit lifespan (Corona et al., 2005). While experimental data show that some of these processes take place, but with the exception of vitellogenin titers (Nelson et al., 2007; Amdam et al., 2007) they have not been linked quantitatively to mortality rates. It is difficult to distinguish between these alternative hypotheses because it is difficult to determine the cause of death for individual worker honey bees.
Demographic techniques cannot directly establish individual causes of death but actuarial analysis of the mortality patterns can test general mortality models and aid in the discrimination of competing hypotheses on major mortality factors (Carey, 2001). We performed three large-scale demographic experiments to analyze worker mortality patterns and better understand aging in honey bees. Specifically, we assessed (1) the importance of extrinsic risk on worker mortality, (2) how foraging is quantitatively related to mortality, (3) how variation in life history between two selected strains correlates with mortality, and (4) how chronological age affects mortality.
The first experiment was designed to eliminate most extrinsic mortality factors by training bees to forage within a flight cage. We predicted the cage-restricted workers to have a significantly lower mortality, particularly as foragers, if extrinsic mortality factors such as predation, disorientation, or other accidents (Visscher and Dukas, 1997) played a major role in limiting honey bee worker lifespan.
The second experiment addressed the quantitative aspect of foraging by varying the amount of foraging within flight cages by restricting access to food. We predicted significantly lower worker mortality, particularly as foragers, in the limited colony if worker mortality is quantitatively related to foraging effort (Neukirch, 1982).
In the third experiment, we compared worker mortality between two honey bee strains (high and low pollen-hoarding strains; Page and Fondrk, 1995) to assess how their lifehistory differences, including life expectancy (Amdam et al., 2007), relate to age-specific mortality during the inhive and foraging state. Compared to the low pollen-hoarding strain, the high strain workers specialize more on pollen foraging (Page et al., 1995), initiate foraging earlier (Pankiw and Page, 2001; Rueppell et al., 2004b), and have larger and more active ovaries (Amdam et al., 2006). When young, the high pollen-hoarding bees have higher levels of vitellogenin. However, vitellogenin levels drop faster in adult high pollen-hoarding bees causing an earlier initiation of foraging (Amdam et al., 2007). Thus we predicted that the worker mortality is higher in the high pollen-hoarding strain then in the low strain specifically at intermediate ages when they exhibit lower vitellogenin titers and earlier foraging.
2. Methods
2.1. Experiments
We studied focal cohorts of honey bees (Apis mellifera L.) in colonies of a natural age composition. Honey bee queens in the source colonies were induced to lay eggs in empty combs. These combs were brought into a humidity-and temperature-controlled incubator (33 °C/60% Rel. Humid.) 1 day prior to emergence of the focal cohort bees. Within 12 h of emergence, worker bees were marked with individually numbered color-tags (BeeWorks, Canada) and introduced into an unrelated host colony. The host colonies were maintained in 4-frame observation hives in a dark, temperature-controlled room with immediate access to the outside (either flight cage or natural habitat).
During the experiments, resource and brood levels were maintained equal between the respective experimental groups by exchanging selected frames and additional feeding if necessary. We maintained approximately 1-frame of nectar, half a frame of stored pollen and 1-frame of brood in each 4-frame observation unit. The flight cages were cleaned of spider webs and flowering plants daily before any observations of foraging activity. The entrance of each hive was observed for incoming, tagged bees during the peak of foraging activity. Bees with observable pollen loads were classified as pollen foragers, all others as non-pollen foragers, assumed to be nectar foragers. Survival was assessed by nightly censuses of all bees in the observation hives. Observations were directly read into a laptop computer via an advanced microphone (Radioshack, TX) and Microsoft Office Speech© voice recognition software. This required prolonged software training sessions by individual observers and data clean-up with Visual Basic© macros, but ultimately increased observer efficiency and accuracy.
2.1.1. Experiment 1
In the first experiment, we compared the life-histories of workers that were free-flying to those workers that were confined to foraging in a flight cage in which food (30% sucrose solution and ground, dried pollen) was offered from 10:00 am to 12:00 am daily. We used two simultaneous replicates of the following paired design. Two equal colony halves were established (ca. 4000 workers each) from a source colony, stocked with a queen, and introduced into a 4-frame observation hive. The two observation hives were connected at the back through a mesh-wire screen to permit food exchange between colony halves. For one hive the hive entrance opened into the natural foraging environment, for the other hive it led into a semi-circular flight cage (11 m long, 6.5 m wide, 3.3 m high, 60% shade cloth) with one sucrose and one pollen feeder located 5 m from the hive entrance.
At the beginning of the experiment 960 newly emerged, individually tagged workers were introduced into each colony half. Daily foraging observations and nightly survival censuses began the following day. Bees that died during the first 5 days were excluded from the analyses because the handling and marking can artificially increase mortality. Foraging activity of both colony halves was observed for 30 min each during the feeding period. All incoming bees were recorded to obtain an estimate of total foraging activity along with specific foraging data on the tagged bees to verify the experimental treatment. Overall foraging activity during the feeding interval was not higher in the caged colonies #2 (534 ± 275) and #4 (337 ± 174) then in the free-flying colonies #1 (579 ± 223) and #3 (528 ± 184) and significantly reduced prior and after the feeding interval (#2: t(24,4) = 26.7, p = 0.0238; #4, t(24,4) = 20.39, p = 0.0312), in contrast to the free-flying colonies (colony 1: t(24,4) = 4.15, p = 0.1506; colony 3: t(24,4) = 0.62, p = 0.6485).
2.1.2. Experiment 2
In the second experiment, we assessed the quantitative effect of foraging into flight cages. Worker mortality was compared between cohorts that had access to pollen and nectar sources in the flight cages either ad-libitum or for only 1 h per day. Each cohort was introduced into a separate host colony, controlled for levels of brood and food. In the ad-libitum treatment, three pollen and three nectar feeders were available throughout the day. The other group of bees only had access to one pollen and one nectar feeder from 10:00 am to 11:00 am. During feeding, foraging activity was not significantly lower in the limited colony than in the unlimited colony (t(4,4) = 1.87, p = 0.110; limited colony: 112.50 ± 43.73; unlimited colony 155.98 ± 15.66) but it was significantly reduced when no food was available (t(4,4) = 4.01, p = 0.007; limited colony without food: 21.67 ± 12.06).
A focal cohort of 480 workers was introduced into both colonies. In contrast to the first experiment, these were initially installed in small hive boxes and only transferred to the 4-frame observation hives at the onset of the observations (5 days after the introduction of the focal bees). Overall foraging activity was assessed during 6 min entrance scans, but individual foraging data was collected by directly observing the feeders (between 20 and 40 min daily). Individual survival was additionally monitored by nightly censuses, as in the first experiment.
2.1.3. Experiment 3
The third experiment compared the mortality between the workers from the bidirectionally selected high and low pollen-hoarding strains (Page and Fondrk, 1995). One host colony received 350 high and 530 low pollen-hoarding bees, the second host colony received 250 of each as focal cohorts. As in the second experiment, the colonies were transferred to observation hives 5 days after the introduction of the focal cohorts, just before the beginning of the observations. Both colonies foraged into the natural environment but their resource and brood levels were maintain at comparable levels.
2.2. Analyses
Experiments were performed until all bees had died (Experiment 1), or the number of censused survivors was insignificant for the overall test statistics (Experiment 2: 2/480 and 8/480; Experiment 3: 2/500 and 0/880). After separate analyses, hive replicates were pooled for most analyses because the replicates did not differ significantly.
2.2.1. Lifespan and its components
In each experiment, the lifespan of all bees was determined from the combined foraging and census data. For bees that were observed foraging, the age of first foraging (AFF), the lifespan as forager (flightspan), and the proportion of foraging observations that involved pollen collection (pollen specialization) were computed. Kaplan–Meier survival analyses were used to estimate the average lifespan, AFF, and flightspan (±95% confidence interval). The main treatment effects on lifespan of all bees were assessed by Mantel–Cox log rank tests. The overall treatment effects were separately assessed for bees that had been observed foraging or not. Cox regressions were used to assess treatment and covariate effects on AFF, flightspan, and lifespan of bees that were observed foraging. For the analysis of flightspan and lifespan, we used AFF and pollen specialization as covariates, for the analysis of AFF only pollen specialization. When multiple colonies were used (Experiments 1 and 3), the colony effect was also incorporated into the model. We used bivariate, parametric estimation to directly assess the relation between AFF and flightspan.
2.2.2. Mortality dynamics
For all instances with five or more bees in a cohort, we calculated age-specific mortality rate for all bees, non-foraging bees and foragers. For foragers, we also calculated the mortality rate per day of observed foraging activity. Age-specific mortality was smoothed (5-day sliding window) for all graphical representations. In addition, we investigated the mortality dynamics with the computer program WinModest (Pletcher, 1999) conducting maximum likelihood searches for the best fit of the data to Gompertz, Gompertz–Makeham, logistic, and logistic-Makeham mortality models.
3. Results
3.1. Experiment 1
Experiment 1 compared the age-specific mortality between workers that foraged into protected flight cages with naturally foraging controls, while maintaining similar internal colony conditions. The initial survival (during the first 5 days) of the 960 marked and introduced workers ranged from 74% to 85% and left 816 and 736 focal individuals in the two freely-foraging colonies and 714 and 774 in the two caged colonies for the subsequent analyses.
3.1.1. Lifespan and its components
Overall, the average lifespan was higher in the two caged colonies (#2 and #4) than in the two free-flying ones (χ2 = 177.9 df = 1, p < 0.001; Table 1). This result held true when restricting the analysis to foragers (χ2 = 138.9 df = 1, p < 0.001) or bees that were never observed foraging (χ2 = 179.4 df = 1, p < 0.001). Cox regression analysis showed that lifespan was shortened by an earlier AFF, while being positively influenced by the cage treatment and specialization on pollen foraging (Table 2 The cage treatment and foraging for pollen likewise positively influenced the pre-foraging lifespan (AFF) and the foraging lifespan (flightspan) and AFF had a negative effect on flightspan (Table 2). Bivariate regression analyses suggested that flightspan was decreased by 0.2 days per day of delayed onset of foraging in the free-flying workers (B = −0.19, F(1,621) = 44.7, p < 0.001) and by 0.3 days in the caged workers (B = −0.27, F(1,356) = 80.0, p < 0.001; Fig. 1, orange lines).
Table 1.
Summary of demographic and foraging data (for explanation of variables see text)a
| Experiment 1
|
Experiment 2
|
Experiment 3
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Free-flying
|
Caged (2 h)
|
Caged (24 h food) | Caged (1 h food) | Low pollen
|
High pollen
|
|||||
| Col. 1 | Col. 3 | Col. 2 | Col. 4 | North | South | North | South | |||
| All bees (n) | 816 | 736 | 714 | 774 | 426 | 443 | 234 | 406 | 240 | 315 |
| Lifespan (days) | 21.5 (21.0–22.0) | 22.1 (21.6–22.7) | 26.4 (25.7–27.1) | 26.0 (25.2–26.7) | 23.1 (22.3–23.9) | 23.1 (22.2–24.1) | 23.1 (22.3–24.0) | 24.0 (23.4–24.6) | 21.7 (21.0–22.4) | 21.7 (21.0–22.3) |
| Non-foraging bees (n) | 528 | 401 | 531 | 599 | 313 | 383 | 103 | 160 | 75 | 147 |
| Lifespan (days) | 17.6 (16.9–18.2) | 17.5 (16.8–18.3) | 21.3 (20.4–22.3) | 20.3 (19.4–21.2) | 20.4 (19.6–21.2) | 21.0 (20.1–21.9) | 26.7 (25.9–27.5) | 26.5 (25.9–27.1) | 23.4 (22.6–24.1) | 23.2 (22.3–24.1) |
| Foragers (n) | 288 | 335 | 183 | 175 | 113 | 60 | 131 | 246 | 165 | 168 |
| Lifespan | 26.3 (25.6–27.0) | 25.6 (24.8–26.3) | 30.7 (29.6–31.9) | 32.9 (31.7–34.1) | 30.5 (29.2–31.8) | 36.8 (34.6–39.1) | 26.7 (25.9–27.5) | 26.5 (25.9–27.1) | 23.4 (22.6–24.1) | 23.2 (22.3–24.1) |
| AFF (days) | 23.9 (23.3–24.5) | 21.7 (21.0–22.4) | 26.4 (25.2–27.7) | 29.2 (27.9–30.5) | 24.3 (23.2–25.3) | 27.0 (25.5–28.5) | 24.1 (23.1–25.1) | 23.9 (23.1–24.7) | 21.1 (20.3–21.9) | 18.2 (17.2–19.2) |
| Flightspan (days) | 3.3 (2.9–3.8) | 4.9 (4.4–5.4) | 5.3 (4.4–6.1) | 4.7 (3.9–5.5) | 7.3 (6.2–8.4) | 11.3 (9.2–13.5) | 3.6 (3.0–4.1) | 3.6 (3.1–4.1) | 3.3 (2.8–3.7) | 6.1 (5.3–6.7) |
| Pollen-proportion | 0.19 (0.15–0.23) | 0.17 (0.13–0.21) | 0.02 (0.00–0.05) | 0.12 (0.08–0.17) | 0.43 (0.35–0.51) | 0.67 (0.57–0.78) | 0.12 (0.08–0.16) | 0.13 (0.09–0.16) | 0.26 (0.21–0.31) | 0.14 (0.10–0.19) |
Means with 95% confidence intervals are reported.
Table 2.
Cox regression analysis results for Experiment 1
| Dependent variable | Predictor | Hazard ratio | Wald statistics |
|---|---|---|---|
| Lifespan | AFF | 0.903 (0.895–0.912) | 461.1, df = 1, p < 0.001 |
| Cage | 0.590 (0.514–0.677) | 56.5, df = 1, p < 0.001 | |
| Pollen foraging | 0.669 (0.540–0.828) | 13.6, df = 1, p < 0.001 | |
| AFF | Cage | 0.482 (0.420–0.554) | 105.8, df = 1, p < 0.001 |
| Pollen foraging | 0.740 (0.611–0.896) | 9.5, df = 1, p = 0.002 | |
| Flightspan | AFF | 1.044 (1.035–1.054) | 91.8, df = 1, p < 0.001 |
| Cage | 0.692 (0.603–0.795) | 27.0, df = 1, p < 0.001 | |
| Pollen foraging | 0.670 (0.543–0.827) | 13.9, df = 1, p < 0.001 |
Fig. 1.
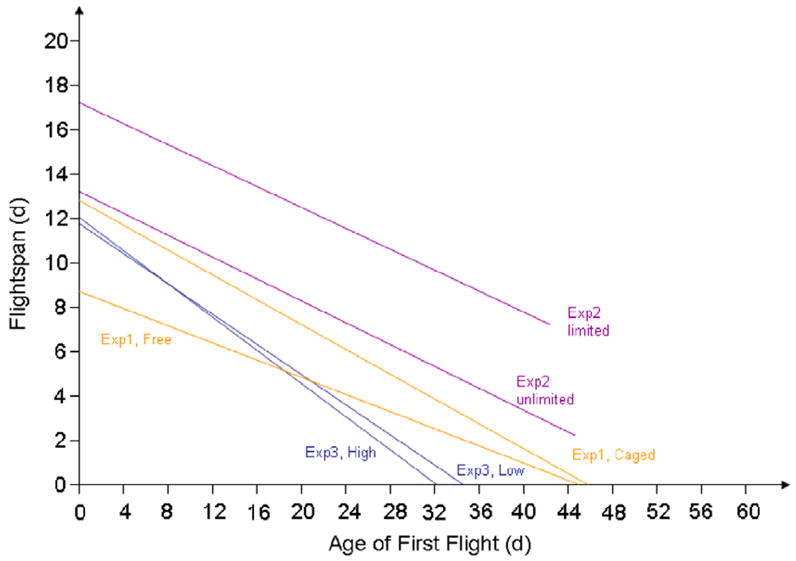
Regression of the foraging period (flightspan) on the pre-foraging period (AFF) from all experiments, illustrating the central trade-off in honey bee worker life-history.
3.1.2. Mortality dynamics
The general age-specific mortality rate increased in both treatment groups from approximately 2% to over 25% at the oldest ages. The mortality rate was higher in the freely-flying cohorts until 34 days of age (Fig. 2) and the mortality dynamics appeared to be delayed in the caged workers by approximately 10 days. Maximum likelihood comparisons suggested that the mortality patterns in all four cohorts best fit a logistic model (Table 3) because the simpler Gompertz models could be rejected (χ2 = 11.1–112.0, df = 1, p < 0.001) and logistic-Makeham models did not result in a significantly better fit.
Fig. 2.
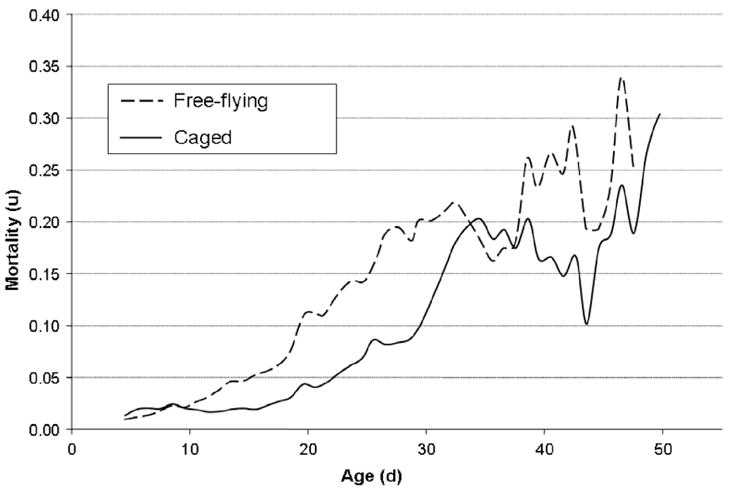
Age-specific mortality rates of workers in free-flying and caged colonies in the first experiment. The workers in the flight cages experienced a postponed mortality increase which is largely due to their delayed onset of foraging.
Table 3.
Summary of demographic model parameters to the mortality patterns of the different experimental groups
| Experiment 1
|
Experiment 2
|
Experiment 3
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Free-flying
|
Caged (2 h)
|
Caged (1 h) | Caged (24 h) | Low pollen
|
High pollen
|
|||||
| Col. 1 | Col. 3 | Col. 2 | Col. 4 | North | South | North | South | |||
| Modela | Logistic | Logistic | Logistic | Logistic | Logistic | Logistic | Gompertz | Gompertz | Logistic | Logistic |
| λ × 10−3 | 2.11 | 1.14 | 2.65 | 4.66 | 5.84 | 1.51 | 2.01 | 1.81 | 0.62 | 1.20 |
| γ | 0.209 | 0.232 | 0.140 | 0.109 | 0.137 | 0.225 | 0.169 | 0.177 | 0.271 | 0.253 |
| s | 0.778 | 1.025 | 0.416 | 0.215 | 0.757 | 1.222 | – | – | 0.635 | 0.733 |
λ, mortality at birth; γ, exponent of mortality increase; s, mortality deceleration at advanced ages.
In both treatment groups, age-specific mortality rate of foragers was higher than that of non-foraging bees at any given age (Fig. 3). The mortality rate of non-foraging bees increased slowly and gradually until age 29 in the free foraging and age 35 in the caged bees when mortality exceeded the initial mortality rate by eight and five fold, respectively. Subsequently, mortality declined in both groups for 9 days before a sharp, final increase. A mortality cross-over was observed on the 11th day. Age-specific mortality rate of foragers fluctuated more but a twofold increase was apparent under both experimental conditions from day 10 to day 33 (Fig. 3) after which mortality rate leveled.
Fig. 3.
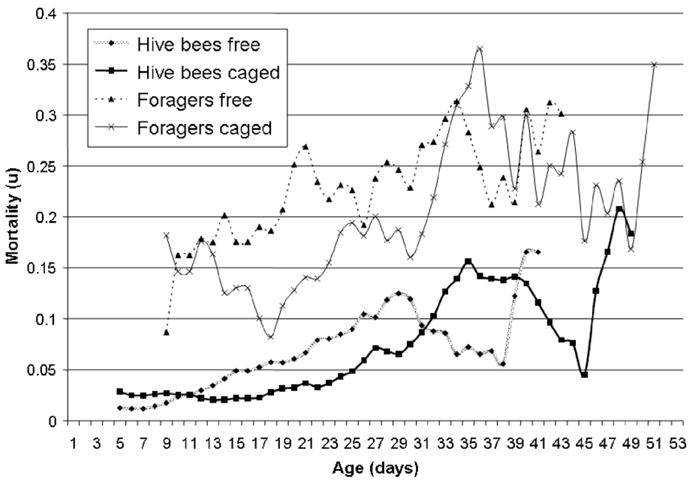
Age-specific mortality rate of foraging and non-foraging worker bees in the first experiment. Throughout the experiment foragers experienced a higher mortality rate and for both groups the mortality decrease through caging was most apparent at intermediate ages.
Mortality rate of foragers relative to foraging experience declined for the first 4 days of foraging in both treatment groups. The subsequent mortality rate increase was slower under cage conditions but mortality rate after 2 weeks of foraging was similar (Fig. 4, orange lines).
Fig. 4.
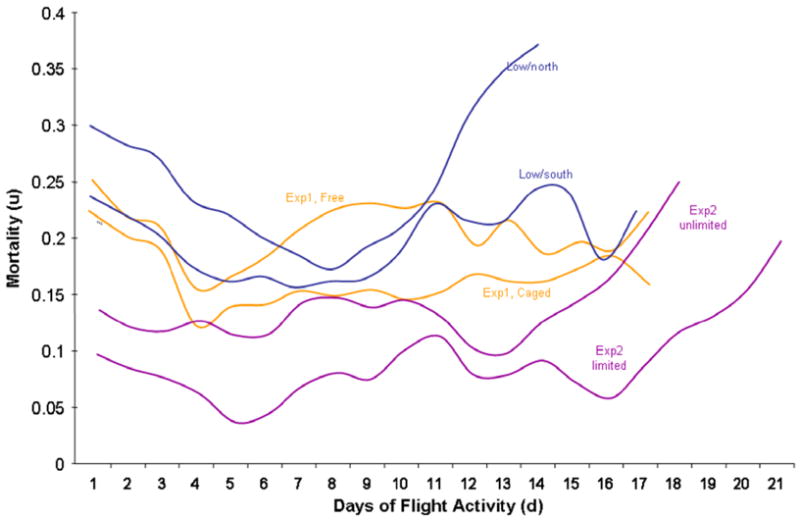
Mortality rate of foragers relative to the number of days that they were observed foraging from all experiments. All groups show an initial mortality peak during the first days of foraging and slowly rising mortality rates with increasing foraging exposure.
3.2. Experiment 2
The second experiment compared the mortality patterns of workers that foraged into protected flight cages with access to food only for one hour or the whole day. The survival before the first census was 92% in the colony that was limited to 1 h of cage foraging and 89% in the colony that could forage in the cage for the whole day (unlimited), resulting in 443 and 426 bees for the subsequent analyses.
3.2.1. Lifespan and its components
Overall lifespan between the two treatments was not significantly different (χ2 = 2.3 df = 1, p = 0.128; Table 1). However, when analyzed separately, forager lifespan (χ2 26.2 df = 1, p < 0.001) and non-forager lifespan (χ2 = 5.4 df = 1, p = 0.020) were significantly longer under limited foraging conditions. Cox regression analysis of lifespan showed that lifespan was simultaneously increased by limited foraging conditions, increased AFF, and pollen specialization (Table 4). The foragers’ AFF was higher in the limited than in the unlimited colony but not significantly affected by pollen specialization. Their flightspan was significantly higher in the limited colony (Table 1) and positively influenced by pollen specialization, while AFF had a negative impact on flightspan (Table 4).
Table 4.
Cox regression analysis results for Experiment 2
| Dependent variable | Predictor | Hazard ratio | Wald statistics |
|---|---|---|---|
| Lifespan | AFF | 0.912 (0.885–0.940) | 35.7, df = 1, p < 0.001 |
| Limited foraging | 0.514 (0.357–0.739) | 12.9, df = 1, p < 0.001 | |
| Pollen foraging | 0.524 (0.353–0.778) | 10.3, df = 1, p = 0.001 | |
| AFF | Limited foraging | 0.615 (0.440–0.859) | 8.1, df = 1, p = 0.004 |
| Flightspan | AFF | 1.030 (1.001–1.061) | 4.1, df = 1, p = 0.043 |
| Limited foraging | 0.523 (0.364–0.751) | 12.0, df = 1, p < 0.001 | |
| Pollen foraging | 0.562 (0.380–0.831) | 8.3, df = 1, p = 0.004 |
Again, the negative dependency of the flightspan on AFF was quantified by bivariate regressions. Workers in both treatment groups “traded” 1 day of foraging per 4 days of pre-foraging life (Fig. 1, purple lines) but the relation was not significant in the limited treatment (unlimited: B = −0.25, F(1,111) = 7.5, p = 0.007; limited: B = −0.25, F(1,58) = 2.0, p = 0.168).
3.2.2. Mortality dynamics
After remaining relative constant for the first 7 days of the experiment (bee age: 7–14 days) the age-specific mortality rate increased from 3% to over 25% (Fig. 5). A mortality cross-over was observed between the two colonies: the mortality rate was higher in the limited colony until age 14 days, and it was lower after age 20. Maximum likelihood estimation supported a logistic model of overall mortality in both colonies (Table 3). Gompertz models were a significantly worse fit to the data of the limited colony (χ2 = 10.2, df = 1, p = 0.001) and unlimited colony (χ2 = 62.4, df = 1, p < 0.001). Makeham extensions did not improve the fit of the model to the data.
Fig. 5.
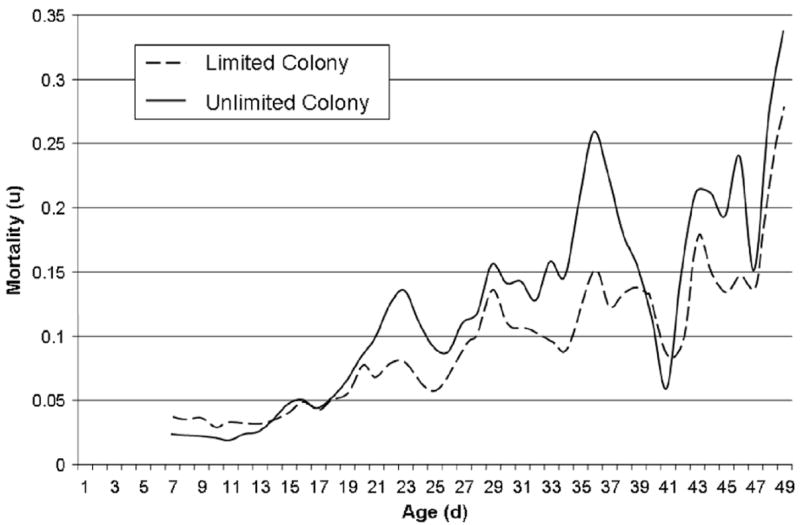
Age-specific mortality of all bees in the second experiment. For the first week mortality was higher in the colony with limited opportunity to forage. After day 20, it experienced consistently lower mortality. This mortality cross-over resulted in a similar mean lifespans of both treatments.
The age-specific mortality rate of non-foraging bees was in both groups comparable to the mortality pattern of non-foraging bees in the first experiment, with a low initial rate (2–3.5%), a slow increase over the first 2 weeks, and a final mortality rate of 15–20% (Fig. 6). The mortality rate among foragers in the unlimited colony also resembled the forager cohorts in the first experiment with an erratic increase from 5% to 35%. The forager mortality rate in the limited colony remained relatively constant with chronological age and fluctuated between 5% and 15% percent. In both groups, the mortality of older non-foragers exceeded the mortality in young foragers.
Fig. 6.
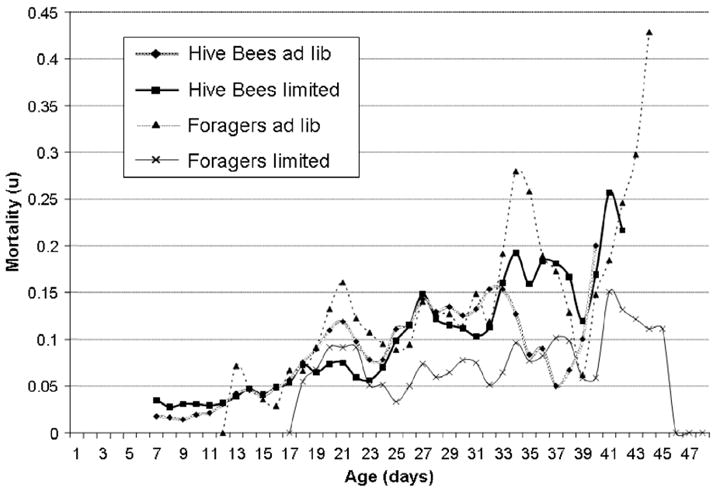
Age-specific mortality of foraging and hive worker bees in the second experiment. In contrast to the other experiments, foragers that were limited to 1 h of foraging experienced a lower mortality than that of non-foraging bees.
The mortality rate of foragers relative to foraging experience showed an initial drop but remained relatively constant in the unlimited and in the limited treatment until an increase after 14 and 18 flight days, respectively. It was consistently lower under limited than under unlimited foraging opportunity (Fig. 4, purple lines).
3.3. Experiment 3
The third experiment addressed mortality in honey bee strains that were selected for high and low pollen-hoarding. Initial survival of the high pollen-hoarding strain workers was 90% in colony “south” and 96% in colony “north”, leaving 315 and 240 workers for the subsequent analyses. The low pollen-hoarding bees were reduced to 406 and 234 (initial survival 77% and 94%), respectively.
3.3.1. Lifespan and its components
Low pollen-hoarding bees lived longer than the high pollen-hoarding bees (χ2 = 40.0, df = 1, p < 0.001; Table 1) and lifespan in the two colonies was marginally different (χ2 = 3.8, df = 1, p = 0.052). Among foragers, similar results were obtained (strain: χ2 = 49.8, df = 1, p < 0.001; colony: χ2 = 3.2, df = 1, p = 0.075) but non-foraging bees died at similar rates from both strains (χ2 = 1.9, df = 1, p = 0.163). In the Cox regression analysis of lifespan including the effects of strain, colony, AFF, and pollen specialization, only the colony and AFF were significant predictors of lifespan (Table 5). AFF itself was significantly lower in the high strain bees but not related to pollen specialization or colony. Flightspan was affected by colony and AFF but not by strain or pollen specialization (Table 5). The high strain bees specialized more on pollen in the one colony (F(1,294) = 18.6, p < 0.001; Table 1) but not in the other (F(1,411) = 0.04, p = 0.834).
Table 5.
Cox regression analysis results for Experiment 3
| Dependent variable | Predictor | Hazard ratio | Wald statistics |
|---|---|---|---|
| Lifespan | AFF | 0.884 (0.873–0.894) | 415.4, df = 1, p < 0.001 |
| Colony | 1.412 (1.210–1.648) | 19.1, df = 1, p < 0.001 | |
| AFF | High strain | 1.813 (1.560–2.107) | 60.3, df = 1, p < 0.001 |
| Flightspan | AFF | 1.100 (1.085–1.116) | 186.9, df = 1, p < 0.001 |
| Colony | 1.231 (1.057–1.433) | 7.1, df = 1, p = 0.007 |
The relationship between AFF and flightspan was not significantly different between strains (Fig. 1, blue lines). The regressions suggested that flightspan was shortened in both high strain and low strain bees by a little under 0.3 day per day of delayed AFF (high: B = −0.38, F(1,331) = 115.0, p < 0.001; low: B =−0.35, F(1,375) = 197.3, p < 0.001).
3.3.2. Mortality dynamics
The mortality rates increased similarly in both strains (Fig. 7) from negligible levels to over 25%. Bees from the high pollen-hoarding strain experienced higher mortality rates between the age of 12 and 29 days. Maximum likelihood estimation indicated a Gompertz mortality dynamics for low pollen-hoarding bees in both colonies (Table 3) because none of the more complex models results in a significantly better fit to the data. However, the mortality in high pollen-hoarding bees was best described by a logistic model in both colonies (Table 3) because Gompertz models provided a significantly worse fit to these data (χ2 = 23.04, df = 1, p < 0.001 and χ2 = 18.80, df = 1, p < 0.001, respectively), and Makeham extension did not improve the fit of the models to the data.
Fig. 7.
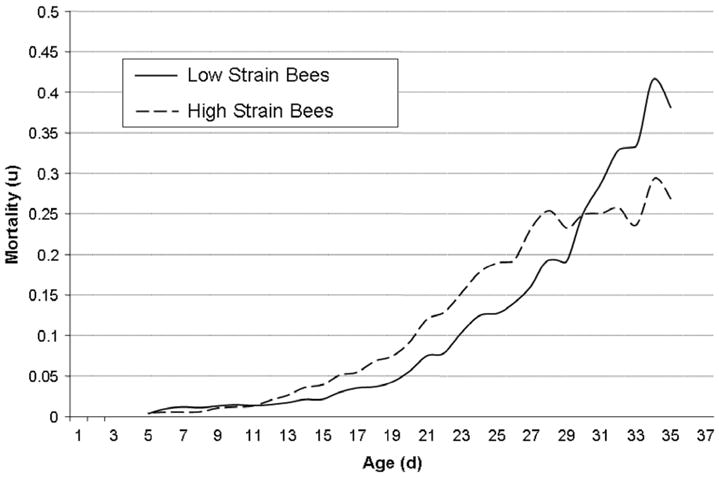
Age-specific mortality rates of high and low pollen-hoarding strain workers under free-flying conditions in the third experiment. High strain bees experienced a higher mortality at intermediate ages, presumably due to their earlier onset of foraging.
The mortality rate for non-foraging bees was lower than for foragers at all ages and it increased gradually from negligible levels to 10%. Mortality rate of foragers increased consistently with age from negligible levels to over 50% mortality and showed a mortality cross-over between high and low pollen-hoarding bees (Fig. 8). High strain worker mortality was higher from the age of 8 to 21 days and low strain worker mortality was higher after 28 days of age. Experience-based forager mortality rate (per flight day) decreased significantly in all colonies for the first 4–9 days of foraging activity and subsequently increased again in three of the four groups with increasing foraging experience (Fig. 4, blue lines).
Fig. 8.
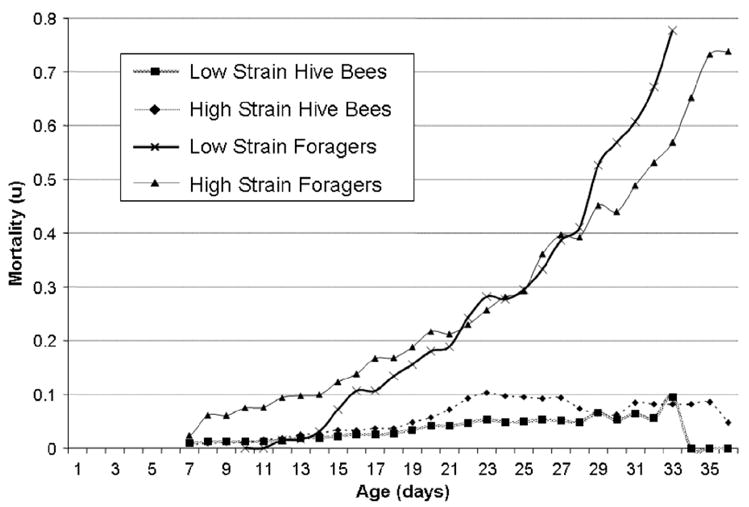
While no significant differences in hive bee mortality were detected between the high and low pollen-hoarding strains, the age-specific forager mortality showed a mortality cross-over with a lower initial risk for the low strain bees.
4. Discussion
4.1. The age of first foraging
The transition from pre-foraging hive activities to foraging behavior (age of first foraging: AFF) emerged as the key determinant of honey bee worker lifespan. In all three experiments the AFF showed a significant positive influence on overall lifespan. This pattern is consistent with previous reports (Sakagami and Fukuda, 1968; Neukirch, 1982; Guzmán-Novoa et al., 1994). The average AFF coincides well with the maximal increase of mortality across all 10 experimental cohorts. The majority of our experimental treatment effects can be explained by a direct effect on the AFF, indirectly affecting mortality and lifespan.
The AFF is a central life-history variable for honey bee workers (Guzmán-Novoa et al., 1994). It is a complex trait that is controlled by a variety of genetic, physiological, and external factors (Schulz et al., 1998; Pankiw and Page, 2001; Robinson, 2002) and regulated at the colony level (Huang and Robinson, 1996; Robinson, 2002). The reduced foraging opportunities in the first and second experiment presumably lead to less recruitment by successful returning foragers (Seeley, 1995). This experimental effect could not be controlled for and is most likely responsible for the observed delay in the AFF. The recruitment mechanism provides a direct control to reduce the number of foragers (experiencing high mortality) when resources are rare. This control of colony foraging effort (resource allocation of the “super-organism”) thus may be a main benefit of the honey bee dance (Dornhaus and Chittka, 2004). On the other hand, genetic differences in AFF between the high and low pollen-hoarding strains (Pankiw and Page, 2001; Rueppell et al., 2004b), demonstrate the evolutionary flexibility of the AFF and thus life expectancy.
The AFF was negatively correlated to the remaining lifespan after foraging initiation (flightspan) in all experimental groups, which indicates pre-foraging senescence (Guzmán-Novoa et al., 1994). Hive activities, such as brood rearing may be energetically costly and specifically deplete workers of protein (Amdam and Omholt, 2002). The negative relation between pre-foraging and foraging life span describes a central trade-off in honey bee biology because pre-foragers fulfill essential hive tasks while foragers supply essential resources to the nest (Winston, 1987; Seeley, 1995).
The strength of the correlation between the AFF and flightspan is a measure of how deterministic lifespan can be expressed in terms of the two behavioral states (pre-foraging bee and forager), while the slope can be interpreted as the longevity cost of either state relative to each other. Strength and slope were highest in the high and low pollen- hoarding strain cohorts in the third experiment, suggesting that genetic homogeneity may strengthen the negative association between pre-foraging and foraging lifespan.
4.2. Forager mortality
The observed positive relationship between the AFF and lifespan demonstrates that foraging bees die faster than pre-foraging bees. Our prediction that forager mortality was higher than that of non-foraging bees irrespective of age was confirmed in all free-flying groups. Thus, behavioral status is more important than chronological age (Omholt and Amdam, 2004; Rueppell et al., 2004a), although our data show a slight increase of mortality rate with chronological age (see also Rueppell et al., 2007). Flight activity varied strongly across treatments and experiments without strong differences in life expectancy. This result is not compatible with the wear-and-tear hypothesis of aging.
The cage treatment in the first experiment reduced forager mortality rate. However, this effect was smaller than the effect of just reducing foraging quantity in the second experiment. The former effect may also be entirely related to the reduction of foraging quantity, not the protected flight cage environment. Thus, our prediction that protection from external mortality factors leads to a significant increase in lifespan, particularly as forager, could not be confirmed. Flight cages themselves may have a detrimental effect on honey bee worker life expectancy (Herbert and Shimanuki, 1978). However, we are confident that our results are not confounded by these cage effects because no disorientation or accumulation of large number of bees outside the hive were observed after the initial training phase (which was excluded from the analyses). Thus, the protection from external mortality factors does not explain the high mortality rate of foragers. Instead, we found a slight but significant effect of foraging activity, consistent with a minor role of wear-and-tear (Page and Peng, 2001) or internal resource depletion (Neukirch, 1982).
Overall, the experimentally decreased forager mortality rate did not translate in pronounced lifespan differences for two reasons: First, the experimental manipulations increased forager flightspan only slightly. Second, only a part of the workers were actually observed foraging. Thus, our third prediction that quantitatively reduced foraging activity lead to a significant lifespan extension could only partly be confirmed.
We are confident that our experimental manipulations were successful. Flight activity was dramatically different between experimental treatments because honey bees learn quickly the availability of food resources in space and time and adapt their foraging activity (Seeley, 1995). Significant numbers of honey bees trying to escape the cages were only observed during the first few days of each experiment (which were therefore excluded from the analyses). Thus, foraging effort and many external mortality hazards were effectively reduced without increasing honey bee worker lifespan as much as would be expected based on the differences between summer and winter bees (Page and Peng, 2001; Omholt and Amdam, 2004) and temporal mortality dynamics of workers under natural conditions (Sakagami and Fukuda, 1968; Visscher and Dukas, 1997).
The high number of bees that died before being observed as foragers and the analysis of the experience-specific mortality of foragers (Fig. 4) point to a high mortality rate at the transition from hive bee to of foraging, irrespective of age and foraging environment. The mortality peak at the onset of foraging occurred in all experimental groups demonstrating that a protected cage environment does not prevent the death of early, inexperienced foragers. Wear-and- tear or forager exhaustion (Neukirch, 1982) cannot account for the early mortality of foragers. Regulatory events at the onset of foraging, such as the decrease in vitellogenin (Amdam and Omholt, 2002) are more consistent with the observed patterns of foraging mortality. Down-regulation of vitellogenin, which limits the foragers’ resistance to pathogens (Amdam et al., 2005, 2007) and oxidative stress (Seehuus et al., 2006) could explain in the observed age-independent mortality peak at the onset of foraging.
After an initial decline, the mortality rate of foragers increased in all cohorts relative to the number of foraging days. This demographic senescence is paralleled by the finding of functional senescence in honey bee foragers (Tofilski, 2000) and could be due to mechanical wear-and- tear (Higginson and Gilbert, 2004). Our data argue against a fixed amount of glycogen reserves as a deterministic lifespan delimiter (Neukirch, 1982) because the mortality increase is gradual and only loosely linked to experimental condition (Fig. 4).
4.3. Foraging specialization
Pollen specialization decreased the overall mortality hazard in the first and second experiment. Contrary to previous observations (Pankiw, 2003) pollen specialists initiated foraging significantly earlier then bees less specialized on pollen in the first experiment. This effect may be due to quantity and quality of available pollen or due to colony-internal conditions, specifically the brood to fresh pollen ratio, which is known to affect pollen foraging (Winston, 1987; Pankiw and Rubink, 2002). Pollen specialization in the first and second experiment also affected the foraging period: Bees that collected a higher proportion of pollen had longer flightspans. This effect was consistent but stronger under caged than under free-flying conditions. Because pollen and nectar feeders were easily accessible at equal distance for the caged bees, this difference in flightspan is more likely due to intrinsic differences between nectar and pollen specialists than to external mortality risks associated with the different resources. Alternatively, individual foraging intensity between pollen and nectar specialists might have differed.
The strain effects on the different life-history components tested in the third experiment proved to be consistent for the two hive replicates and with our predictions. The low pollen-hoarding strain bees transitioned at significantly older age from in-hive tasks to foraging than high pollen-hoarding strain bees and collected less pollen (Guzmán-Novoa et al., 1994; Page et al., 1995; Pankiw and Page, 2001; Rueppell et al., 2004b). The dependency of flightspan on AFF was not different in the two strains and low strain bees lived longer because of their higher AFF. The correlation of the AFF, strain and foraging specialization may have masked any independent effect of pollen collection behavior on life expectancy. The behavioral and life-history differences between the high and low pollen-hoarding strains are orchestrated by the hemolymph titer of vitellogenin (Amdam et al., 2004, 2007) and the mortality differences between the strains were consistent with the predictions that we made based on vitellogenin dynamics in the two strains. The mortality rate of high strain workers exceeded that of low strain workers for intermediate ages, coinciding with the beginning of foraging life, when vitellogenin titers in high strain individuals are lower than in low strain individuals (Amdam et al., 2007).
5. Conclusion
Overall, our experiments confirmed the type-2 survivorship patterns of honey bee workers (Sakagami and Fukuda, 1968). The age-dependent mortality followed in most cases a logistic model (Pletcher, 1999). The main reason for these logistic mortality dynamics in honey bee workers is the age-dependent pattern of the onset of flight activity, which also follows a logistic pattern (Rueppell et al., 2004b). A logistic mortality pattern has been reported from a variety of organisms with sufficiently large cohorts studied (Vaupel et al., 1998) but the transition between two hazard states (pre-foraging bee and forager) is a novel explanation for a logistic mortality pattern that depends on the shape of the transition function and the mortality peak associated with the transition. Similar mortality dynamics were reported previously for honey bee males (Rueppell et al., 2005) that also transition from the protected hive environment to flight activity at older ages (Winston, 1987).
Our experiments confirmed a higher mortality rate of foragers than non-foraging bees. However, the experimental reduction of foraging activity and elimination of a number of external mortality factors increased the life expectancy of workers only by 4–5 days. This is not comparable to the nearly tenfold lifespan differences between winter and summer bees (Omholt and Amdam, 2004) or the typical mortality differences between young nurse bees and old foragers (Sakagami and Fukuda, 1968). Our results suggest that the mortality patterns in worker honey bees are mainly influenced by the transition from in-hive workers to foragers. Activity levels had a small significant effect, suggesting that wear-and-tear or exhaustion cannot be completely ignored but are not the main determinant of honey bee worker life expectancy. Our demographic data corroborates mechanistic hypotheses that explain the extended lifespan of non-foraging bees, winter bees, and queens, with the internal regulation of defense and repair mechanisms (Amdam et al., 2005; Seehuus et al., 2006; Corona et al., 2007). The high plasticity of aging in individual honey bees may thus be best interpreted as an optimization of resources at the colony level due to kin selection.
Acknowledgments
We thank LoriAnn Rodriguez for great practical help with the third experiment. Two anonymous reviewers made helpful suggestions to improve the manuscript. This research was supported by the National Institute on Aging (PO1 AG22500).
References
- Amdam GV, Aase ALTO, Seehuus SC, Fondrk MK, Norberg K, Hartfelder K. Social reversal of immunosenescence in honey bee workers. Exp Gerontol. 2005;40:939–947. doi: 10.1016/j.exger.2005.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Csondes A, Fondrk MK, Page RE. Complex social behaviour derived from maternal reproductive traits. Nature. 2006;439:76–78. doi: 10.1038/nature04340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Nilsen K-A, Norberg K, Fondrk MK, Hartfelder K. Variation in endocrine signaling underlies variation in social life-history. Am Nat. 2007;170:37–46. doi: 10.1086/518183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Norberg K, Fondrk MK, Page RE. Reproductive ground plan may mediate colony-level selection effects on individual foraging behavior in honey bees. Proc Nat Acad Sci USA. 2004;101:11350–11355. doi: 10.1073/pnas.0403073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Omholt SW. The regulatory anatomy of honeybee lifespan. J Theor Biol. 2002;216:209–228. doi: 10.1006/jtbi.2002.2545. [DOI] [PubMed] [Google Scholar]
- Beshers SN, Fewell JH. Models of division of labor in social insects. Ann Rev Entomol. 2001;46:413–440. doi: 10.1146/annurev.ento.46.1.413. [DOI] [PubMed] [Google Scholar]
- Carey JR. Insect biodemography. Annu Rev Entomol. 2001;46:79–110. doi: 10.1146/annurev.ento.46.1.79. [DOI] [PubMed] [Google Scholar]
- Chapuisat M, Keller L. Division of labour influences the rate of ageing in weaver ant workers. Proc R Soc Lond B. 2002;269:909–913. doi: 10.1098/rspb.2002.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona M, Hughes KA, Weaver DB, Robinson GE. Gene expression patterns associated with queen honey bee longevity. Mech Ageing Dev. 2005;126:1230–1238. doi: 10.1016/j.mad.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Corona M, Velarde RA, Remolina S, Moran-Lauter A, Wang Y, Hughes KA, Robinson GE. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc Nat Acad Sci USA. 2007;104:7128–7133. doi: 10.1073/pnas.0701909104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornhaus A, Chittka L. Why do honey bees dance? Behav Ecol Sociobiol. 2004;55:395–401. [Google Scholar]
- Finch CE. Longevity, Senescence, and the Genome. University of Chicago Press; Chicago, Il: 1990. [Google Scholar]
- Guzmán-Novoa E, Page RE, Gary NE. Behavioral and life-history components of division of labor in honey bees (Apis mellifera L.) Behav Ecol Sociobiol. 1994;34:409–417. [Google Scholar]
- Herbert EW, Shimanuki H. Effect of the size of outdoor flight cages on brood rearing and food consumption by honeybees. J Apicult Res. 1978;17:114–117. [Google Scholar]
- Higginson AD, Gilbert F. Paying for nectar with wingbeats: a new model of honeybee foraging. Proc R Soc Lond B. 2004;271:2595–2603. doi: 10.1098/rspb.2004.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZY, Robinson GE. Regulation of honey bee division of labor by colony age demography. Behav Ecol Sociobiol. 1996;39:147–158. [Google Scholar]
- Keller L, Genoud M. Extraordinary lifespans in ants: a test of evolutionary theories of ageing. Nature. 1997;389:958–960. [Google Scholar]
- Kirkwood TBL, Austad SN. Why do we age? Nature. 2000;408:233–238. doi: 10.1038/35041682. [DOI] [PubMed] [Google Scholar]
- Lundie AE. The flight activity of the honeybee. Department Bulletin (United States Department of Agriculture) 1925;1328:1–37. [Google Scholar]
- Nelson CM, Ihle KE, Fondrk MK, Page RE, Amdam GV. The gene vitellogenin has multiple coordinating effects on social organization. PLoS Biol. 2007;5:e62. doi: 10.1371/journal.pbio.005006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neukirch A. Dependence of the life span of the honeybee (Apis mellifica) upon flight performance and energy consumption. J Comp Physiol. 1982;146:35–40. [Google Scholar]
- Omholt SW, Amdam GV. Epigenic regulation of aging in honeybee workers. Sci Aging Knowledge Environ. 2004;26:pe28. doi: 10.1126/sageke.2004.26.pe28. [DOI] [PubMed] [Google Scholar]
- Page RE, Fondrk MK. The effects of colony level selection on the social organization of honey bee (Apis mellifera L.) colonies – colony level components of pollen hoarding. Behav Ecol Sociobiol. 1995;36:135–144. [Google Scholar]
- Page RE, Peng Y-SC. Aging and development in social insects with emphasis on the honey bee, Apis mellifera L. Exp. Gerontol. 2001;36:695–711. doi: 10.1016/s0531-5565(00)00236-9. [DOI] [PubMed] [Google Scholar]
- Page RE, Waddington KD, Hunt GJ, Fondrk MK. Genetic determinants of honey bee foraging behaviour. Anim Behav. 1995;50:1617–1625. [Google Scholar]
- Pankiw T. Directional change in a suite of foraging behaviors in tropical and temperate evolved honey bees (Apis mellifera L.) Behav Ecol Sociobiol. 2003;54:458–464. [Google Scholar]
- Pankiw T, Page RE. Genotype and colony environment affect honeybee (Apis mellifera L.) development and foraging behavior. Behav Ecol Sociobiol. 2001;51:87–94. [Google Scholar]
- Pankiw T, Rubink WL. Pollen foraging response to brood pheromone by Africanized and European honey bees (Apis mellifera L.) Ann Entomol Soc Am. 2002;95:761–767. [Google Scholar]
- Pletcher SD. Model fitting and hypothesis testing for age-specific mortality data. J Evol Biol. 1999;12:430–439. [Google Scholar]
- Robinson G. Genomics and integrative analyses of division of labor in honeybee colonies. Am Nat. 2002;160:S160–S172. doi: 10.1086/342901. [DOI] [PubMed] [Google Scholar]
- Rose M. Evolutionary Biology of Aging. Oxford University Press; New York, NY: 1991. [Google Scholar]
- Rueppell O, Amdam GV, Page RE, Carey JR. From genes to society: Social insects as models for research on aging. Sci Aging Knowledge Environ. 2004a;5:pe5. doi: 10.1126/sageke.2004.5.pe5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueppell O, Pankiw T, Nielson DI, Fondrk MK, Beye M, Page RE. The genetic architecture of the behavioral ontogeny of foraging in honey bee workers. Genetics. 2004b;167:1767–1779. doi: 10.1534/genetics.103.021949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueppell O, Fondrk MK, Page RE. Biodemographic analysis of male honey bee mortality. Aging Cell. 2005;4:13–19. doi: 10.1111/j.1474-9728.2004.00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueppell O, Christine S, Mulcrone C, Groves L. Aging without functional senescence in honey bee workers. Curr Biol. 2007;17:R274– R275. doi: 10.1016/j.cub.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami SF, Fukuda H. Life tables for worker honeybees. Res Popul Ecol. 1968;10:127–139. [Google Scholar]
- Schulz DJ, Huang ZY, Robinson GE. Effects of colony food shortage on behavioral development in honey bees. Behav Ecol Sociobiol. 1998;42:295–303. [Google Scholar]
- Seehuus SC, Norberg K, Gimsa U, Krekling T, Amdam GV. Reproductive protein protects sterile honey bee workers from oxidative stress. Proc Nat Acad Sci USA. 2006;103:962–967. doi: 10.1073/pnas.0502681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley TD. The Wisdom of the Hive. Harvard University Press; Cambridge, Ma: 1995. [Google Scholar]
- Tofilski A. Senescence and learning in honeybee (Apis mellifera) workers. Acta Neurobiol Exp. 2000;60:35–39. doi: 10.55782/ane-2000-1323. [DOI] [PubMed] [Google Scholar]
- Vaupel JW, Carey JR, Christensen K, Johnson TE, Yashin AI, Holm NV, Ichine IA, Kannisto V, Khazaeli AA, Liedo P, Longo VD, Zeng Y, Manton KG, Curtsinger JW. Biodemographic trajectories of longevity. Science. 1998;280:855–859. doi: 10.1126/science.280.5365.855. [DOI] [PubMed] [Google Scholar]
- Visscher PK, Dukas R. Survivorship of foraging honey bees. Ins Soc. 1997;44:1–5. [Google Scholar]
- Winston ML. The Biology of the Honey Bee. Harvard University Press; Cambridge, Massachusetts: 1987. [Google Scholar]


