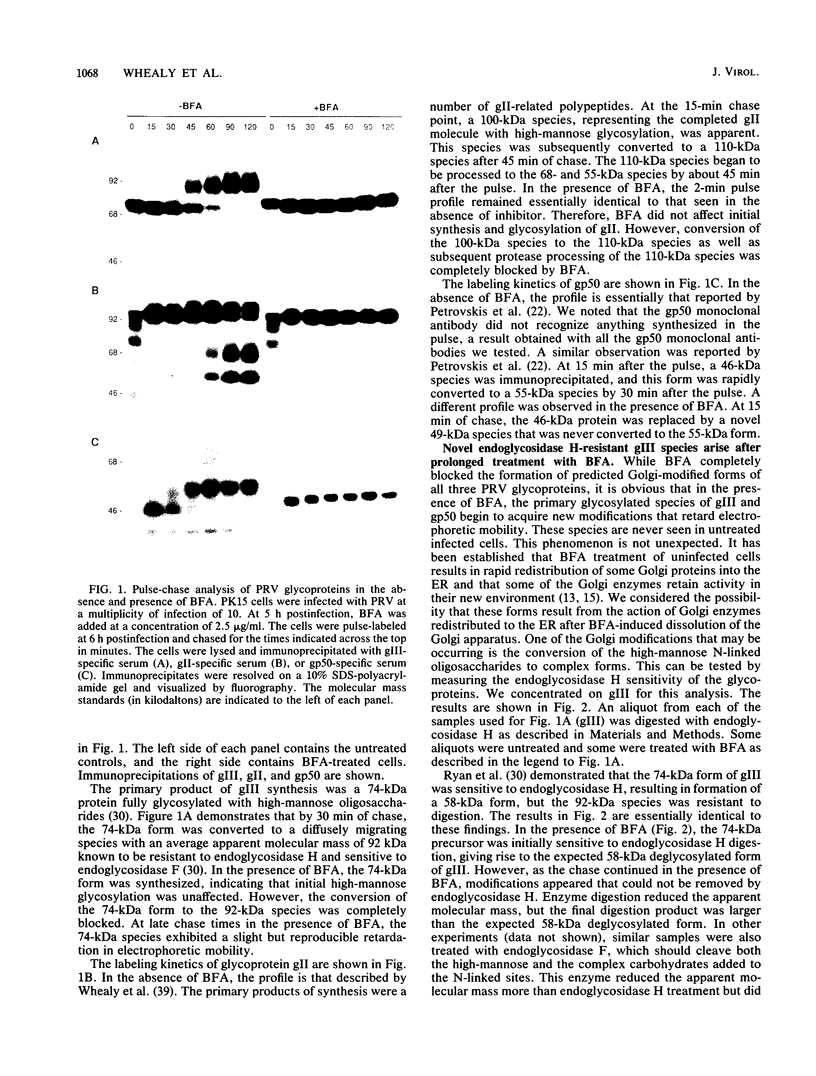
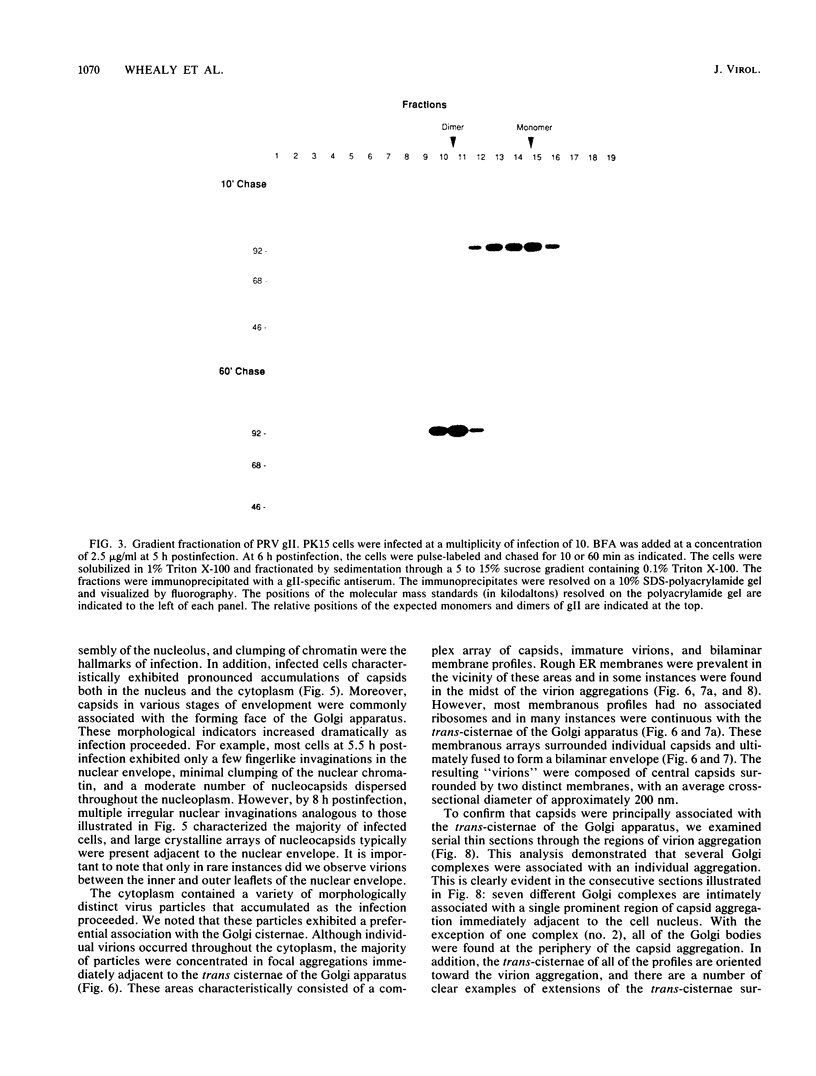
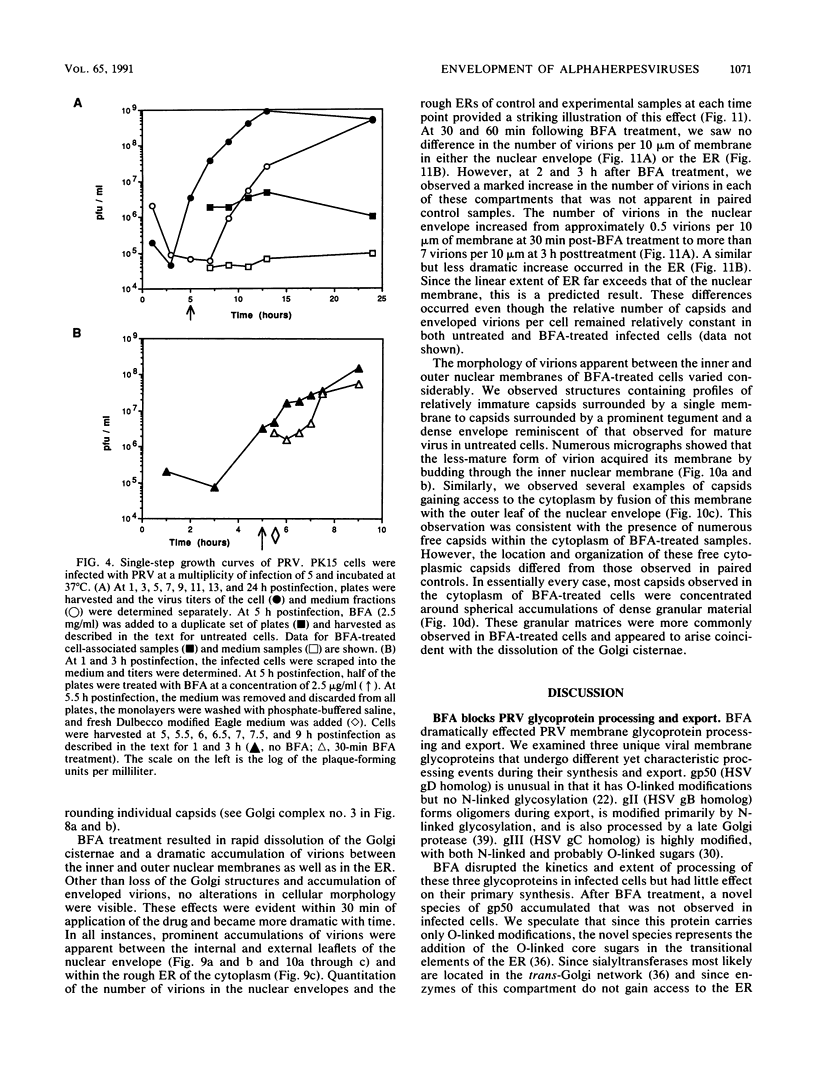
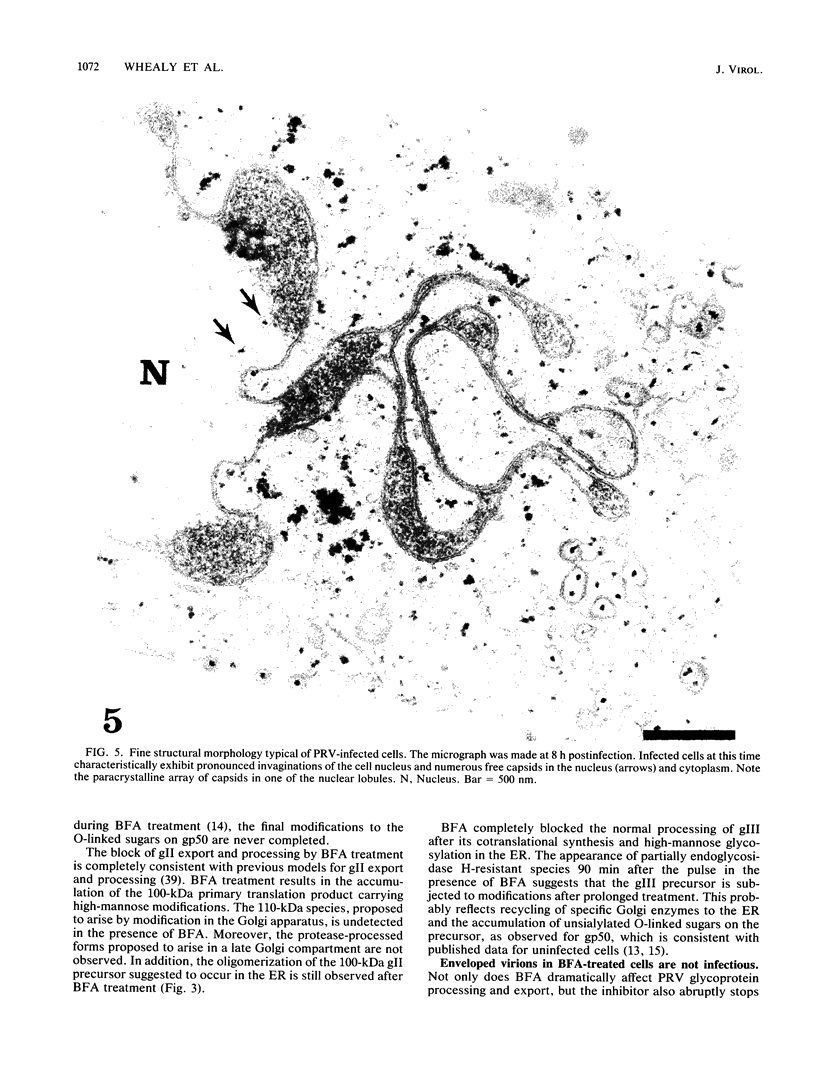
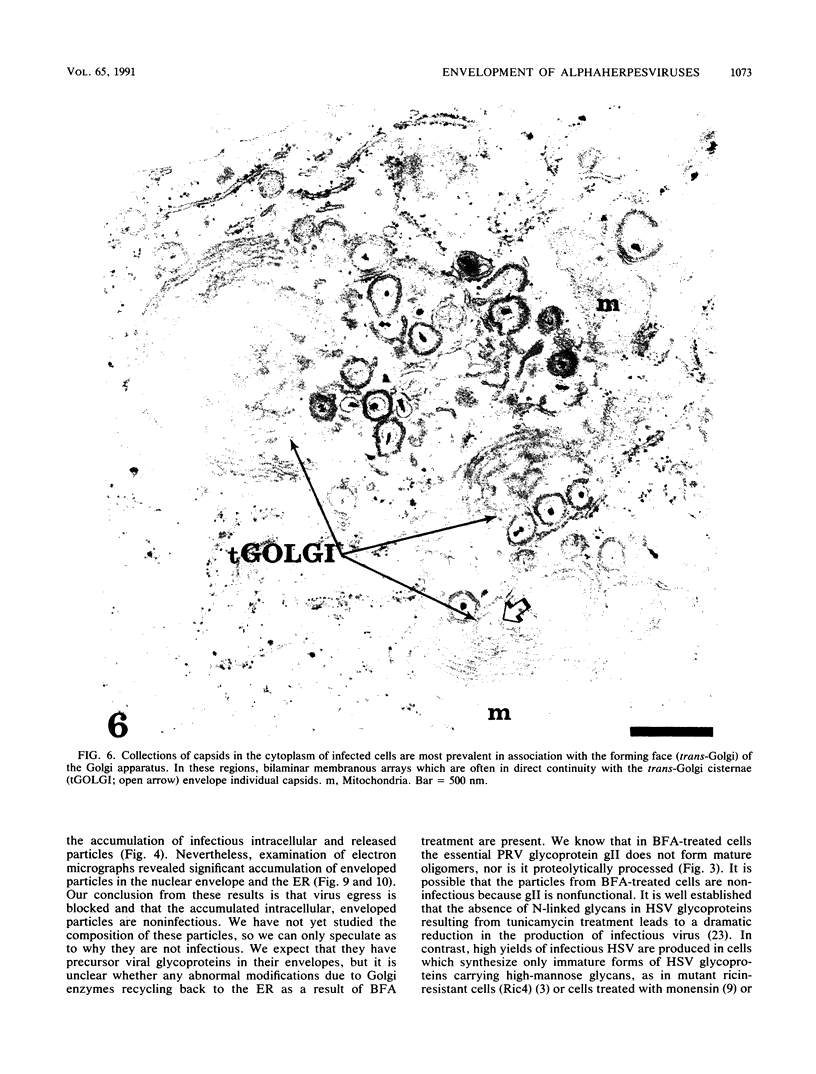
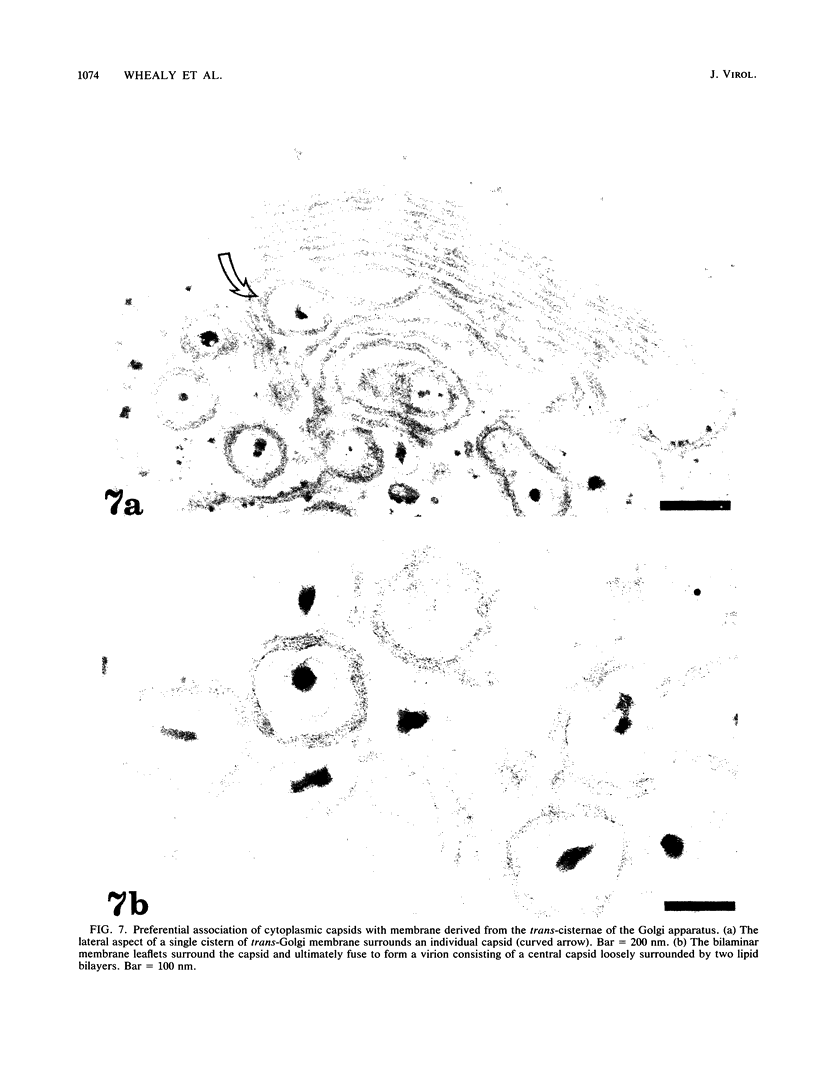
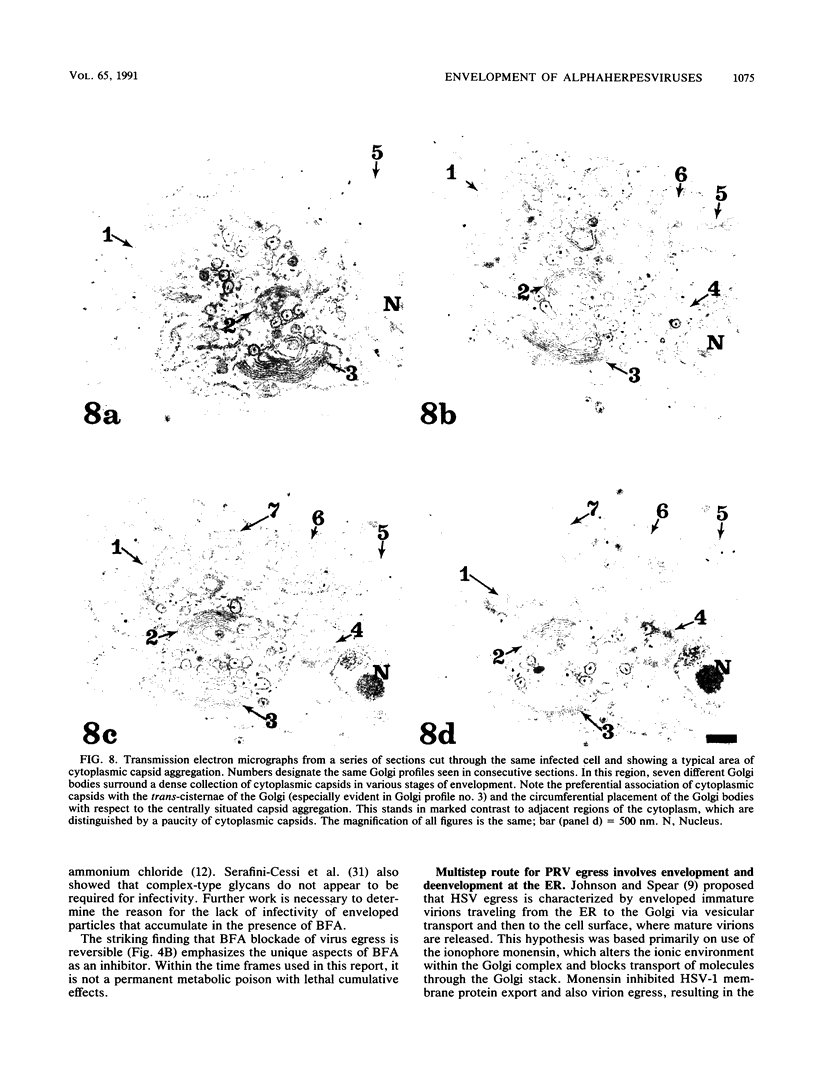
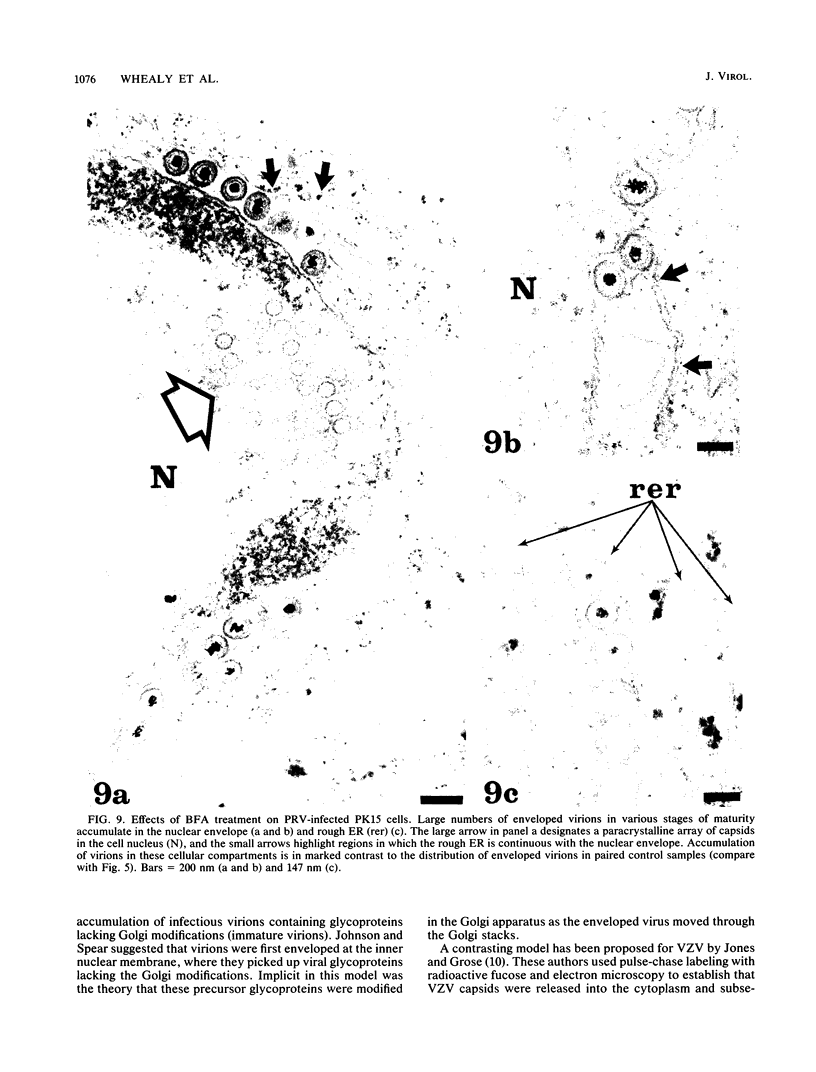
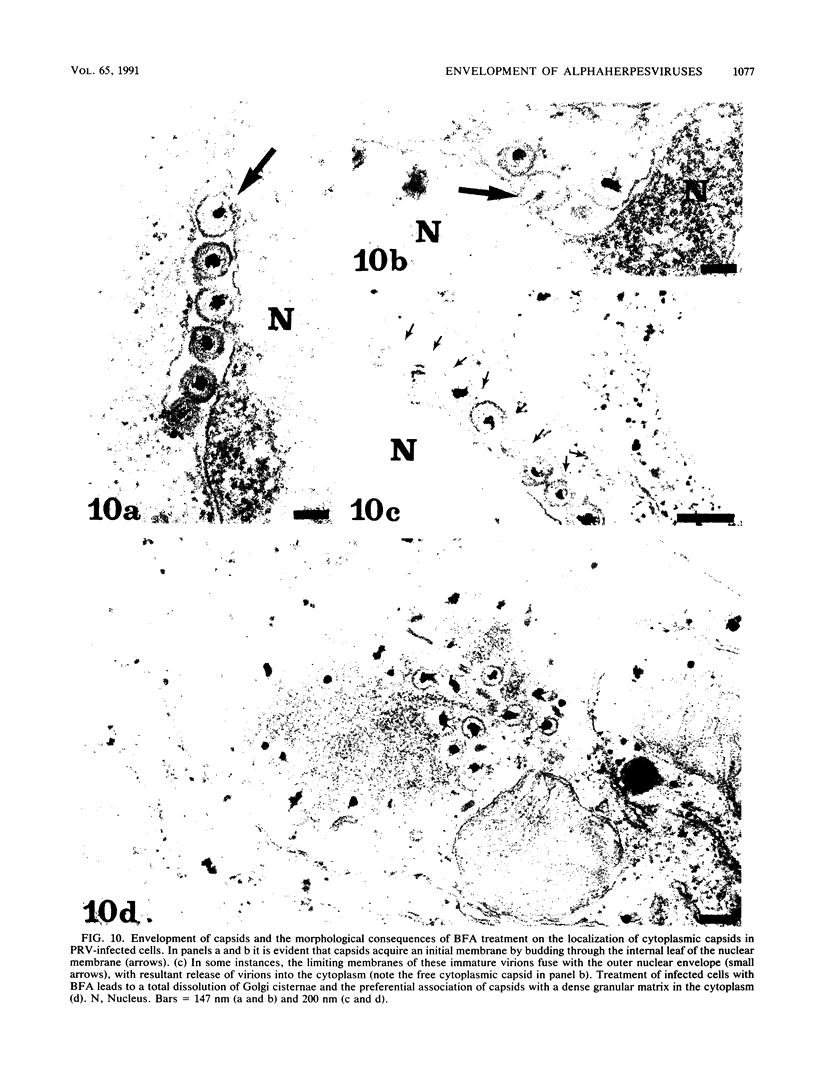
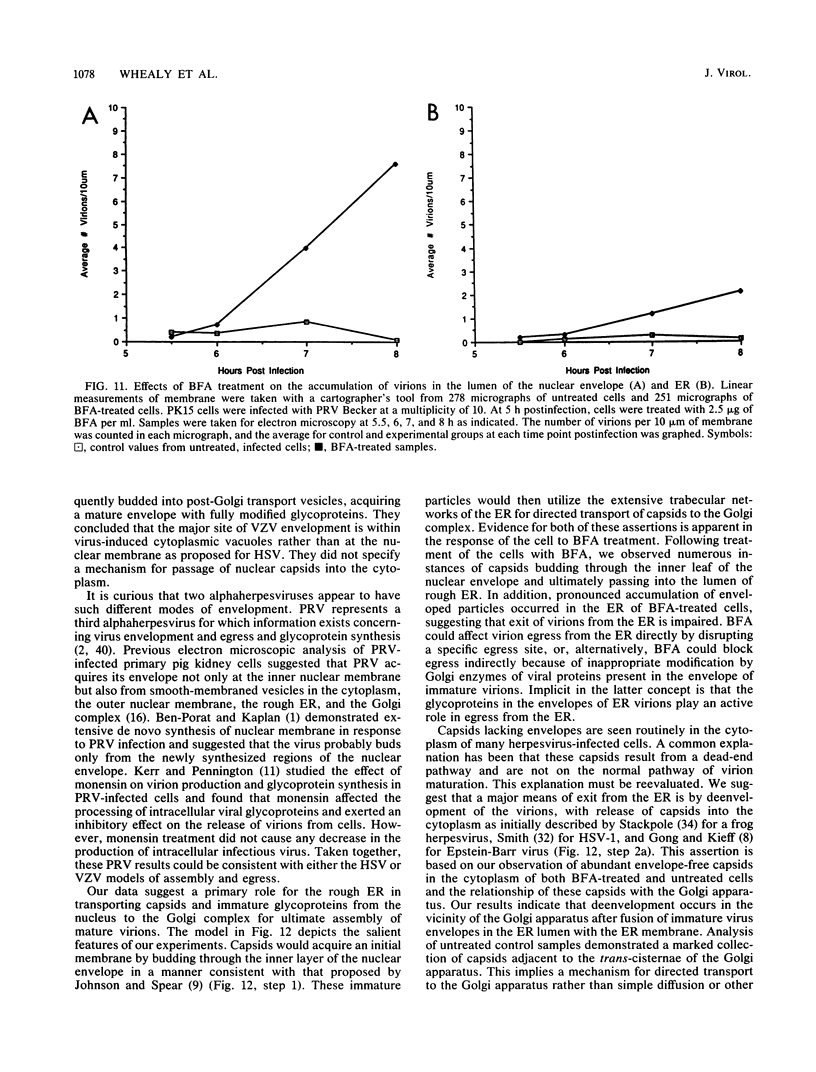
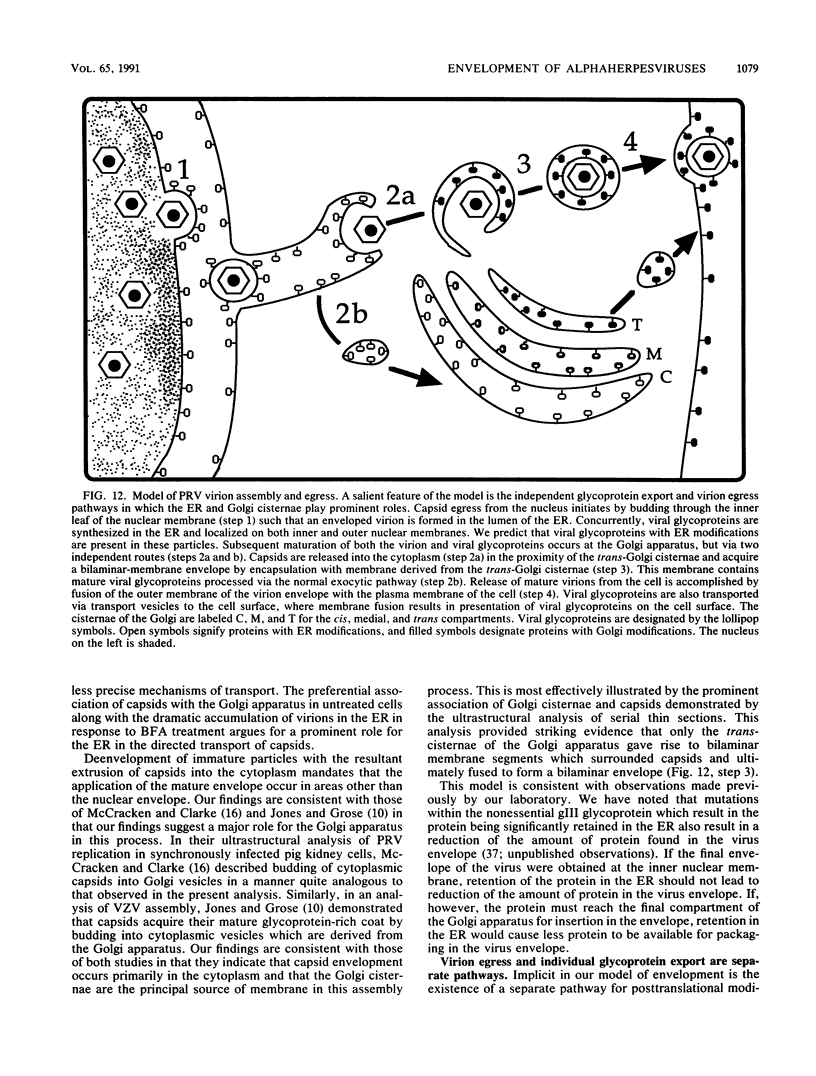
Abstract
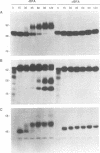
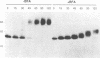
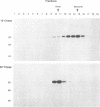
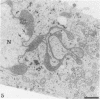
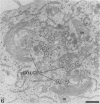
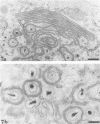
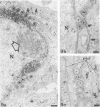
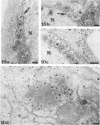
In this work we used brefeldin A (BFA), a specific inhibitor of export to the Golgi apparatus, to study pseudorabies virus viral glycoprotein processing and virus egress. BFA had little effect on initial synthesis and cotranslational modification of viral glycoproteins in the endoplasmic reticulum (ER), but it disrupted subsequent glycoprotein maturation and export. Additionally, single-step growth experiments demonstrated that after the addition of BFA, accumulation of infectious virus stopped abruptly. BFA interruption of virus egress was reversible. Electron microscopic analysis of infected cells demonstrated BFA-induced disappearance of the Golgi apparatus accompanied by a dramatic accumulation of enveloped virions between the inner and outer nuclear membranes and also in the ER. Large numbers of envelope-free capsids were also present in the cytoplasm of all samples. In control samples, these capsids were preferentially associated with the forming face of Golgi bodies and acquired a membrane envelope derived from the trans-cisternae. Our results are consistent with a multistep pathway for envelopment of pseudorabies virus that involves initial acquisition of a membrane by budding of capsids through the inner leaf of the nuclear envelope followed by deenvelopment and release of these capsids from the ER into the cytoplasm in proximity to the trans-Golgi. The released capsids then acquire a bilaminar double envelope containing mature viral glycoproteins at the trans-Golgi. The resulting double-membraned virus is transported to the plasma membrane, where membrane fusion releases a mature, enveloped virus particle from the cell.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Porat T., Kaplan A. S. Studies on the biogenesis of herpesvirus envelope. Nature. 1972 Jan 21;235(5334):165–166. doi: 10.1038/235165a0. [DOI] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Poletti L., Dall'Olio F., Serafini-Cessi F. Infectivity and glycoprotein processing of herpes simplex virus type 1 grown in a ricin-resistant cell line deficient in N-acetylglucosaminyl transferase I. J Virol. 1982 Sep;43(3):1061–1071. doi: 10.1128/jvi.43.3.1061-1071.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Darlington R. W., Moss L. H., 3rd Herpesvirus envelopment. J Virol. 1968 Jan;2(1):48–55. doi: 10.1128/jvi.2.1.48-55.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Edson C. M., Ellis R. W., Forghani B., Gilden D., Grose C., Keller P. M., Vafai A., Wroblewska Z., Yamanishi K. New common nomenclature for glycoprotein genes of varicella-zoster virus and their glycosylated products. J Virol. 1986 Mar;57(3):1195–1197. doi: 10.1128/jvi.57.3.1195-1197.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M., Kieff E. Intracellular trafficking of two major Epstein-Barr virus glycoproteins, gp350/220 and gp110. J Virol. 1990 Apr;64(4):1507–1516. doi: 10.1128/jvi.64.4.1507-1516.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihashi Y., Matsumoto S., Dales S. Biogenesis of poxviruses: role of A-type inclusions and host cell membranes in virus dissemination. Virology. 1971 Dec;46(3):507–532. doi: 10.1016/0042-6822(71)90056-0. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J Virol. 1982 Sep;43(3):1102–1112. doi: 10.1128/jvi.43.3.1102-1112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones F., Grose C. Role of cytoplasmic vacuoles in varicella-zoster virus glycoprotein trafficking and virion envelopment. J Virol. 1988 Aug;62(8):2701–2711. doi: 10.1128/jvi.62.8.2701-2711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr C. L., Pennington T. H. The effect of monensin on virion production and protein secretion in pseudorabies virus-infected cells. J Gen Virol. 1984 Jun;65(Pt 6):1033–1041. doi: 10.1099/0022-1317-65-6-1033. [DOI] [PubMed] [Google Scholar]
- Kousoulas K. G., Bzik D. J., DeLuca N., Person S. The effect of ammonium chloride and tunicamycin on the glycoprotein content and infectivity of herpes simplex virus type 1. Virology. 1983 Mar;125(2):468–474. doi: 10.1016/0042-6822(83)90217-9. [DOI] [PubMed] [Google Scholar]
- Langford L. A., Coggeshall R. E. The use of potassium ferricyanide in neural fixation. Anat Rec. 1980 Jul;197(3):297–303. doi: 10.1002/ar.1091970304. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Donaldson J. G., Schweizer A., Berger E. G., Hauri H. P., Yuan L. C., Klausner R. D. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990 Mar 9;60(5):821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L. C., Bonifacino J. S., Klausner R. D. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989 Mar 10;56(5):801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN C., ELLISON S. A., ROSE H. M., MOORE D. H. Structure and development of viruses as observed in the electron microscope. I. Herpes simplex virus. J Exp Med. 1954 Aug 1;100(2):195–202. doi: 10.1084/jem.100.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken R. M., Clarke J. K. A thin-section study of the morphogenesis of Aujeszky's disease virus in synchronously infected cell cultures. Arch Gesamte Virusforsch. 1971;34(3):189–201. doi: 10.1007/BF01242992. [DOI] [PubMed] [Google Scholar]
- Mettenleiter T. C. Glycoprotein gIII deletion mutants of pseudorabies virus are impaired in virus entry. Virology. 1989 Aug;171(2):623–625. doi: 10.1016/0042-6822(89)90635-1. [DOI] [PubMed] [Google Scholar]
- Morgan C. Vaccinia virus reexamined: development and release. Virology. 1976 Aug;73(1):43–58. doi: 10.1016/0042-6822(76)90059-3. [DOI] [PubMed] [Google Scholar]
- Nii S. Electron microscopic observations on FL cells infected with herpes simplex virus. I. Viral forms. Biken J. 1971 Jun;14(2):177–190. [PubMed] [Google Scholar]
- Nii S. Electron microscopic observations on FL cells infected with herpes simplex virus. II. Envelopment. Biken J. 1971 Sep;14(3):325–347. [PubMed] [Google Scholar]
- O'Callaghan D. J., Randall C. C. Molecular anatomy of herpesviruses: recent studies. Prog Med Virol. 1976;22:152–210. [PubMed] [Google Scholar]
- Payne L. G., Kristenson K. Mechanism of vaccinia virus release and its specific inhibition by N1-isonicotinoyl-N2-3-methyl-4-chlorobenzoylhydrazine. J Virol. 1979 Nov;32(2):614–622. doi: 10.1128/jvi.32.2.614-622.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovskis E. A., Timmins J. G., Armentrout M. A., Marchioli C. C., Yancey R. J., Jr, Post L. E. DNA sequence of the gene for pseudorabies virus gp50, a glycoprotein without N-linked glycosylation. J Virol. 1986 Aug;59(2):216–223. doi: 10.1128/jvi.59.2.216-223.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizer L. I., Cohen G. H., Eisenberg R. J. Effect of tunicamycin on herpes simplex virus glycoproteins and infectious virus production. J Virol. 1980 Apr;34(1):142–153. doi: 10.1128/jvi.34.1.142-153.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliquin L., Levine G., Shore G. C. Involvement of Golgi apparatus and a restructured nuclear envelope during biogenesis and transport of herpes simplex virus glycoproteins. J Histochem Cytochem. 1985 Sep;33(9):875–883. doi: 10.1177/33.9.2991363. [DOI] [PubMed] [Google Scholar]
- Robbins A. K., Dorney D. J., Wathen M. W., Whealy M. E., Gold C., Watson R. J., Holland L. E., Weed S. D., Levine M., Glorioso J. C. The pseudorabies virus gII gene is closely related to the gB glycoprotein gene of herpes simplex virus. J Virol. 1987 Sep;61(9):2691–2701. doi: 10.1128/jvi.61.9.2691-2701.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. K., Watson R. J., Whealy M. E., Hays W. W., Enquist L. W. Characterization of a pseudorabies virus glycoprotein gene with homology to herpes simplex virus type 1 and type 2 glycoprotein C. J Virol. 1986 May;58(2):339–347. doi: 10.1128/jvi.58.2.339-347.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. K., Whealy M. E., Watson R. J., Enquist L. W. Pseudorabies virus gene encoding glycoprotein gIII is not essential for growth in tissue culture. J Virol. 1986 Sep;59(3):635–645. doi: 10.1128/jvi.59.3.635-645.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B., Carmichael L. E., Deinhardt F., de-The G., Nahmias A. J., Plowright W., Rapp F., Sheldrick P., Takahashi M., Wolf K. Herpesviridae. Definition, provisional nomenclature, and taxonomy. The Herpesvirus Study Group, the International Committee on Taxonomy of Viruses. Intervirology. 1981;16(4):201–217. doi: 10.1159/000149269. [DOI] [PubMed] [Google Scholar]
- Ryan J. P., Whealy M. E., Robbins A. K., Enquist L. W. Analysis of pseudorabies virus glycoprotein gIII localization and modification by using novel infectious viral mutants carrying unique EcoRI sites. J Virol. 1987 Oct;61(10):2962–2972. doi: 10.1128/jvi.61.10.2962-2972.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini-Cessi F., Dall'Olio F., Scannavini M., Campadelli-Fiume G. Processing of herpes simplex virus-1 glycans in cells defective in glycosyl transferases of the Golgi system: relationship to cell fusion and virion egress. Virology. 1983 Nov;131(1):59–70. doi: 10.1016/0042-6822(83)90533-0. [DOI] [PubMed] [Google Scholar]
- Smith J. D. An additional role for the outer nuclear membrane in the morphogenesis of herpes simplex virus. Intervirology. 1980;13(5):312–316. doi: 10.1159/000149140. [DOI] [PubMed] [Google Scholar]
- Stackpole C. W. Herpes-type virus of the frog renal adenocarcinoma. I. Virus development in tumor transplants maintained at low temperature. J Virol. 1969 Jul;4(1):75–93. doi: 10.1128/jvi.4.1.75-93.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura G., Ando K., Suzuki S., Takatsuki A., Arima K. Antiviral activity of brefeldin A and verrucarin A. J Antibiot (Tokyo) 1968 Feb;21(2):160–161. doi: 10.7164/antibiotics.21.160. [DOI] [PubMed] [Google Scholar]
- Tooze S. A., Tooze J., Warren G. Site of addition of N-acetyl-galactosamine to the E1 glycoprotein of mouse hepatitis virus-A59. J Cell Biol. 1988 May;106(5):1475–1487. doi: 10.1083/jcb.106.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whealy M. E., Baumeister K., Robbins A. K., Enquist L. W. A herpesvirus vector for expression of glycosylated membrane antigens: fusion proteins of pseudorabies virus gIII and human immunodeficiency virus type 1 envelope glycoproteins. J Virol. 1988 Nov;62(11):4185–4194. doi: 10.1128/jvi.62.11.4185-4194.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whealy M. E., Robbins A. K., Enquist L. W. Pseudorabies virus glycoprotein gIII is required for efficient virus growth in tissue culture. J Virol. 1988 Jul;62(7):2512–2515. doi: 10.1128/jvi.62.7.2512-2515.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whealy M. E., Robbins A. K., Enquist L. W. The export pathway of the pseudorabies virus gB homolog gII involves oligomer formation in the endoplasmic reticulum and protease processing in the Golgi apparatus. J Virol. 1990 May;64(5):1946–1955. doi: 10.1128/jvi.64.5.1946-1955.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]