Abstract
The objective of this study was to assess estrogen-dependent cellular mechanisms that could contribute to the acid pH of the vaginal lumen. Cultures of normal human cervical-vaginal epithelial (hECE) cells and endocervical cells were grown on filters, and acidification of the extracellular solutions on the luminal (L-pHo) and contraluminal (CL-pHo) sides was measured. The hECE cells and endocervical cells decreased CL-pHo from 7.40 to 7.25 within 20–30 min of incubation in basic salt solution. Endocervical cells also produced a similar decrease in L-pHo. In contrast, hECE cells acidified L-pHo down to pH 7.05 when grown as monoculture and down to pH 6.05 when grown in coculture with human cervical fibroblasts. This enhanced acid secretion into the luminal compartment was estrogen dependent because removal of endogenous steroid hormones attenuated the effect, whereas treatment with 1 7β-estradiol restored it. The 17β-estradiol effect was dose dependent (EC50 0.5 nM) and could be mimicked by diethyl-stilbestrol and in part by estrone and tamoxifen. Preincubation with ICI-182780, but not with progesterone, blocked the estrogen effect. Preincubation of cells with the V-ATPase blocker bafilomycin A1, when administered to the luminal solution, attenuated the baseline and estrogen-dependent acid secretion into the luminal solution. Treatment with EGTA, to abrogate the tight junctional resistance, blocked the decrease in L-pHo and stimulated a decrease in CL-pHo, indicating that the tight junctions are necessary for maintaining luminal acidification. We conclude that vaginal-ectocervical cells acidify the luminal canal by a mechanism of active proton secretion, driven in part by V-H+-ATPase located in the apical plasma membrane and that the baseline active net proton secretion occurs constitutively throughout life and that this acidification is up-regulated by estrogen.
An important function of the vaginal and cervical epithelial cells is to regulate the pH of the lumen of the lower genital tract. During premenopausal years vaginal luminal pH ranges between 4.5 and 6.0 with mild alkalinization to about 6.5 before ovulation. Lack of estrogen, such as after menopause, is associated with alkalinization to about 6.5–7.0, whereas replacement with estrogen can acidify the luminal vaginal pH to about 5.5 (1). Alkalization above 6.5 is associated with increased risk of vaginal infections (2–8), whereas low (acidic) vaginal pH can inhibit the growth of serious pathogens (9). Alkalinization of the vaginal fluid can also cause dyspareunia (10), and abnormally acidic vaginal pH can adversely affect cervical mucus and sperm cell motility and result in infertility (11). Abnormally elevated luminal vaginal pH has been implicated as a potential tumorigenic factor in the cervix (12).
Despite the important implications for women’s health and reproduction, little is known about mechanisms that control and regulate vaginal/cervical pH. The prevailing theory about regulation of vaginal pH was presented more than 60 yr ago, and it postulates that the acidic vaginal luminal pH is produced by cohabituating Doderlein lactobacilli (13). This theory is based on the finding that the bacteria produce hydrogen peroxide and secrete protons into their immediate environment (14, 15). Because lactobacilli can be found in the vagina of most healthy women (1), acidification of the vaginal lumen is thought to result directly from bacterial proton secretion. The Lactobacillus-Doderlein theory is supported by two groups of observations: first, there is a correlation between alkalinization of vaginal luminal pH and decreased density of vaginal Doderlein Lactobacillus (1, 16, 17); second, introduction of Lactobacillus species can acidify vaginal luminal pH (18).
Two studies apparently contradict the Lactobacillus-Doderlein theory. Tsai et al. (19) found that the pH in the vaginal fornices was significantly lower than in the middle portion of the vagina. This finding is at odds with the fact that the presence of Lactobacillus-Doderlein is uniform throughout the vaginal canal (13). De Bernardis et al. (20) studied effects of pH on pathogenicity of Candida albicans and found that the pH of the infection site, rather than that produced by the pathogen, regulates survival of the Candida. This observation implies that environmental factors such as vaginal pH may affect the pathobiology of vaginal microorganisms. Whereas these findings challenge, to a degree, the Lactobacillus-Doderlein theory, no alternative theory was offered for the regulation of vaginal pH.
Based on novel results, we advance a new hypothesis for the regulation of vaginal pH. We propose that the luminal vaginal pH is determined by net proton secretion by vaginal epithelial cells through the coordinated action of ion transport mechanisms located in the apical cell membrane. In this regard vaginal epithelial cells resemble gastric chief cells that regulate net proton secretion into the gastric lumen (21) as well as other types of cells such as renal type A intercalated cells (22) and papillary surface epithelium (23), epididymis and vas deferens epithelial cells (22), macrophages and neutrophils (24), osteoclasts (25, 26), cancer metastatic cells (27), and insect midgut cells (28). In the present study, we began using an in vitro system to test this hypothesis. Our data support the hypothesis and provide evidence for an estrogen-dependent, bafilomycin-A1−-sensitive proton secretory mechanism in the apical plasma membrane of human vaginal/ectocervical epithelial cells. These data suggest involvement of estrogen-dependent V type H+-ATPase (29) in the acidification of the vaginal canal.
Materials and Methods
Determinations of vaginal and cervical pH in vivo
The experiments in vivo were approved by the University Hospitals of Cleveland and Case Western Reserve University Institutional Review Board (protocol 04-01-24) and were conducted after obtaining a signed consent form. A total of 12 women, aged 20–47 yr, were included in the study. Women were selected from among healthy premenopausal patients presenting for their annual (well-being) exam to the gynecology clinics of the authors (G.I.G. and E.M.). Included were women with regular menstrual cycles not using hormonal medications and without clinical evidence of vaginal or cervical infections. Women were divided into three groups according to cycle day based on their last menstrual period: d 6–9 (n = 5), 11–14 (n = 3), and 17–24 (n = 4). Of the 12 women, eight were African American and four were Caucasians. There were no significant differences among the three groups relative to age or gravidity. Before their scheduled routine exam, all women underwent pelvic examination using nonlubricated vaginal speculum. The lateral vaginal wall was gently touched at midvaginal level with a strip of pHydrion paper at its tip (4.5–7.5, Micro Essential Laboratory Inc., Brooklyn NY) attached to uterine forceps. The process was then repeated by touching the cervical os, and the cervical pH was thus determined.
Cell culture techniques
The experiments used secondary/tertiary cultures of human ectocervical-vaginal epithelial (hECE) cells and human endocervical cells. Cultures of hECE cells were generated from minces of the ectocervix/vagina as described (30–32). The discarded tissues were collected by the Cooperative Human Tissue Network at University Hospitals of Cleveland and Case Western Reserve University according to the institutional review board protocol 03–90-TG. Tissues were collected from a total of 11 premenopausal women aged 37–46 yr; seven were African American and four Caucasians. One woman was of Latino origin. hECE cells were grown and maintained in DMEM/Ham’s F12 (3:1) supplemented with nonessential amino acids, adenine (0.2 mM), penicillin (100 U/ml), streptomycin (100 μg/ml), gentamicin (50 ng/ml), L-glutamine (2 mM), insulin (5 μg/ml), hydrocortisone (1 μM), transferrin (5 μg/ml), triiodothyronine (2 nM), epidermal growth factor (0.2 nM), and 8% fetal calf serum, at 37 C in 91% O2-9% CO2 humidified incubator. Culturing techniques and characterization of hECE cells as phenotypically resembling squamous ectocervical/vaginal epithelial cells were described (30–32). Cells chosen for experiments were those obtained from tissues reported as human papillomavirus negative, and the cultured hECE cells were routinely tested for mycoplasma.
Primary cultures of human endocervical cells were obtained through Clonetics (Walkersville, MD). Cells chosen for experiments were those obtained from tissues of two women reported as human papillomavirus negative. All cultured endocervical cells were tested for mycoplasma. Primary endocervical cultures were grown and subcultured into second passage using Clonetics proprietary endocervical medium (supplied with the cells) and thereafter maintained in hECE culture medium. Cells were characterized as epithelial cells based on typical morphology of a monolayered epithelium; the expression of involucrin; lack of expression of vimentin; and expression of tight junctions (not shown). Confluent endocervical cultures on filters (see below) were tested in Ussing diffusion chambers (33) and were found to generate transepithelial resistance levels of about 35 Ω/cm2, similar to hECE cultures (31), indicating relatively low-resistance cultures. The cells were defined as phenotypically endocervical (in contrast to ectocervical/vaginal) based on expression of apical villi, the abundant expression of cytokeratins 18/19, and minimal expression of cytokeratins 4/5 and 13 [experiments were done as described (30, 31), results not shown]. Both hECE cells (34) and the human endocervical cells (not shown) expressed functional estrogen receptors.
Cocultures of hECE cells and human cervical fibroblasts (HCF) were generated by plating irradiated HCF cells on one side of the filter (Fig. 1A, gray bars) and hECE on the opposing side (Fig. 1B, bars with boxes). Primary cultures of HCF cells were generated from discarded ectocervix/vaginal tissues after the surface epithelium was dissected to generate the hECE cultures. Tissues were immersed in Hanks’ balanced salt solution (HBSS) plus 2.5% collagenase for 30 min at 37 C. The subepithelial surface was gently scraped with scalpel; the resulting suspension of cells was incubated for 15 min at 37 C in HBSS plus 1.5% trypsin and 5 mM EDTA followed by two washes and incubation for 5 min at 37 C in medium composed of DMEM and Ham’s F-12 (3:1) supplemented with 5% calf serum, L-glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 μg/ml), and gentamicin (50 ng/ml). Cells were plated in the same medium on plates, and the resulting secondary-tertiary cultures were composed of fibroblasts as determined by morphology, expression of vimentin, and lack of expression of involucrin and epithelial-type cytokeratins. Before plating on filters, HCF cells were irradiated (32) to block further proliferation.
Fig. 1.
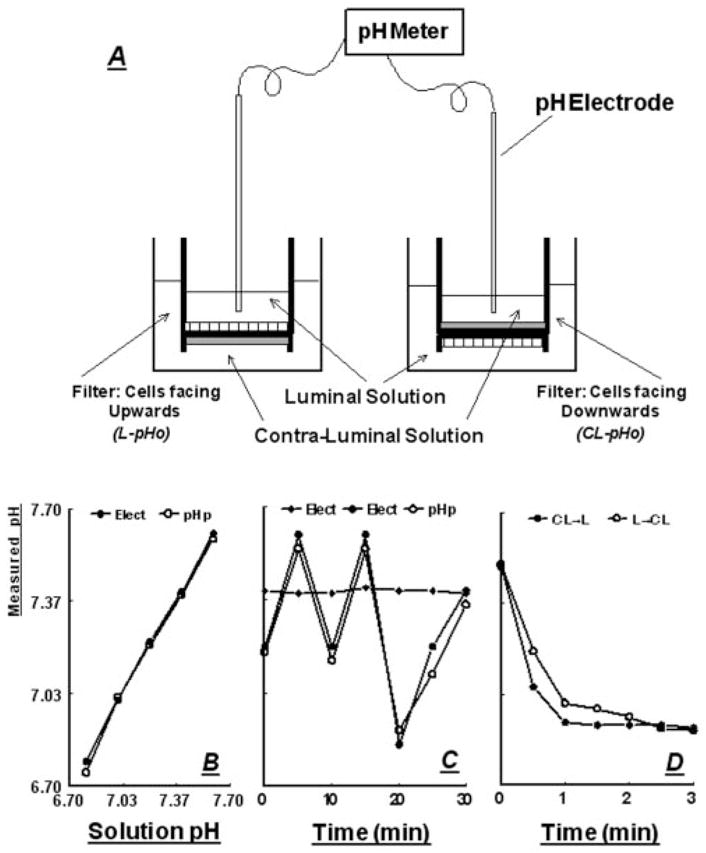
A, Schema of the experimental system for determinations of L-pHo (left) and CL-pHo (right) across cultured epithelial cells. Epithelial cells are shown as hatched bars and fibroblasts as gray bars. B, Correlation of pH measurements using the pH electrodes (Elect) and pH paper (pH p) in standard pH solutions. C, Filled diamonds, stability of pH recordings using blank filter (without cells) in a setup as in A; circles, pH measurements using the pH electrodes (Elect, filled circles) and pH paper (pH p, open circles) in a blank filter after pH was changed by adding aliquots from 0.1 N NaCl or 0.1 N HCl. D, Filled circles, pH measurements in a blank filter using the pH electrode (placed in the luminal [L] compartment) after acidification of the contraluminal solution (filled circles, CL → L); open circles, pH measurements in a blank filter using the pH electrode (placed in the contraluminal [CL] compartment) after acidification of the luminal solution (filled circles, L → CL). Acidification in the cis compartment was induced by adding aliquots of 0.1 N HCl.
Determinations of extracellular pH (pHo)
hECE cells or human endocervical cells were plated on Anocell filters (Anocell-10, Oxon, UK, obtained through Sigma Chemicals, St. Louis, MO), which are ceramic-base filters, pore size of 0.02 μm width, 50 μm depth, and surface area of 0.6 cm2. Filters were coated with 4 μg/cm2 collagen type IV and incubated at 37 C overnight. The remaining collagen solution was aspirated and the filter was dried at 37 C. Before plating, filters were rinsed three times with HBSS. Cells were plated either on the upper surface of the filter (for determinations of luminal pHo) or the bottom surface of the filter (for determinations of the contraluminal pHo) at 3 × 105 cells/cm2. By plating at this relatively high density, the cultures became confluent within 12 h after plating (31). To generate cocultures of hECE and HCF cells, irradiated HCF cells were plated on one side of the filter at 5 × 104 cells/cm2 for 12 h, followed by shifting the cells to hECE medium and plating hECE cells on the opposite surface of the filter (Fig. 1A). Plates containing the filters were placed in a tissue culture incubator (37 C), and pHo changes in the luminal and contraluminal solutions were determined using AMANI-1000 microcombination pH electrodes (Harvard Apparatus, Holliston, MA, obtained through Genomics Solutions, Ann Harbor, MI). The AMANI-1000 pH sensor (stored in pH 7.0) is based on metal-metal oxide pH measurements, and the pH sensitive layer is immobilized on plastic. The reference electrode employs an Ag/AgCl in 3.4 M KCl, and the reference electrode junction is leak free, ensuring no leaks of KCl out of the reference electrode. The design of the pH electrodes enabled their use for the purpose of the present experiments: tip diameter of 1 mm, depth of immersion 1 mm, and response time of less than 5 sec.
Electrodes attached to a stand were held stable with the tip immersed in the solution within the upper compartment of the filter to a depth of about 2 mm (described in Fig. 1A), avoiding direct contact between the electrode tip and the cultured cells. The electrodes were connected directly to an Accumet pH meter 910 (Fisher Scientific, Suwanee, GA). After stabilization, the luminal and contraluminal solutions were gently aspirated using glass pipette and replaced with fresh warmed (37 C) basic salt solution containing (in millimoles) NaCl (140), KC1 (5), MgC12 (1), CaCl2 (1), glucose (10), and 0.1% BSA (pH 7.4). The volumes in the luminal and contraluminal compartments were 150 and 500 μl, respectively. Determinations of changes in pHo were made at time 0, and at 5-min intervals thereafter for up to 30 min. The experimental system is shown schematically in Fig. 1A.
A number of experiments with blank filters (without cells) were done to test the validity of the system, revealing the accuracy (Fig. 1, B and C) and stability (Fig. 1C) of the pH electrodes. Also shown in Fig. 1D is that acidification of the solution in a cis compartment leads to acidification across the filter in the trans compartment within seconds, indicating that the semipermeable material of the filter does not impede movement of protons or proton equivalents.
In some experiments pHo determinations were validated using pH paper (Hydrion pH test paper, MSD-2943, Analytical Scientific, Helotes, TX). Ministrips of the pH papers held by microsurgical pickups were dipped into the luminal solution to a depth of less than 1 mm, and changes in pHo were determined by the change of color of the pH paper strip. The results were similar to those using pH electrodes. The data presented in this paper represent pH determinations using the pH electrodes. Similar trends of changes in pHo were obtained if cultures were immersed in HEPES-buffered solution [containing (in millimoles) NaCl (140), KCl (5), MgC12 (1), CaCl2 (l), glucose (10), and HEPES/Tris (10) (pH 7.4)] (not shown).
Deprivation of estrogens
To remove estrogens, cells were grown in steroid-free medium as described (35). This procedure involved shifting cells on filters for 3 d before pHo assays to a medium composed of phenol-red-deficient (DMEM)/Ham’s F12 (Sigma) or containing 8% heat-inactivated charcoal-treated fetal bovine serum (35). For experiments, cells were shifted to steroid-free medium for 3 d; alternatively, cells were shifted to the steroid-free medium for 24 h and treated with 17β-estradiol for an additional 2 d.
Cell-vitality staining was done using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assay (36). MTT serves as a substrate for mitochondrial dehydrogenases, and only viable functional mitochondria are capable of cleaving MTT to generate the colored product formazan (37). MTT solution was prepared by dissolving MTT at a concentration of 5 mg/ml in PBS composed of (in millimoles) NaCl (137), KCl (2.68), Na2HPO4 (10), and KH2PO4 (1.76, pH 7.4) and filtering the solution through a filter from Millipore (Burlington, MA). After treatments/assays, cells on filters were washed with fresh and warm (37 C) PBS and incubated for 60 min at 37 C in MTT solution. At the completion of incubation, cells were washed with PBS and solubilized in isopropanol containing 0.1 M HCl plus 1% Triton X-100. Lysates were mixed by pipetting to dissolve the reduced MTT crystals (during which time the colorless solution turns purple) and were spun at 10,000 × g for 5 min. The solubilized formazan was measured by determining absorption at 570 nm. Background absorbance at 690 nm was subtracted for each sample, and values were normalized to OD570 of control unperturbed cells.
Statistical analysis of the data
Data are presented as means (± SD) and significance of differences among means was estimated by Student’s t test or with ANOVA. Trends were calculated using GB-STAT version 5.3 (Dynamic Microsystems Inc., Silver Spring, MD) and analyzed with ANOVA.
Chemicals and supplies, unless specified otherwise, were obtained from Sigma. Stock chemicals and drug solutions were titrated to pH 7.4 or 7.35 before cell treatments and were administered from ×1000 stocks.
Results
pHo changes in vivo
A total of 12 premenopausal women aged 20–47 yr were included in the study. Measurements of cervical pHo revealed a mild acidic pHo of 5.7 ± 0.6 in d 6–9 of the menstrual cycle, which increased to 7.2 ± 0.3 during d 11–14 (P < 0.01) and remained elevated at 6.1 ± 0.8 during d 17–24 of the menstrual cycle (Fig. 2). In contrast, vaginal pH remained low at about 5.1 throughout d 6–24 of the menstrual cycle (Fig. 2). The most likely explanation for the changes in cervical pHo are ovulation-dependent changes in cervical mucus secretion, which buffer the acidic pHo at the level of the cervical os. It is therefore concluded that vaginal pHo remains acidic throughout the menstrual cycle.
Fig. 2.
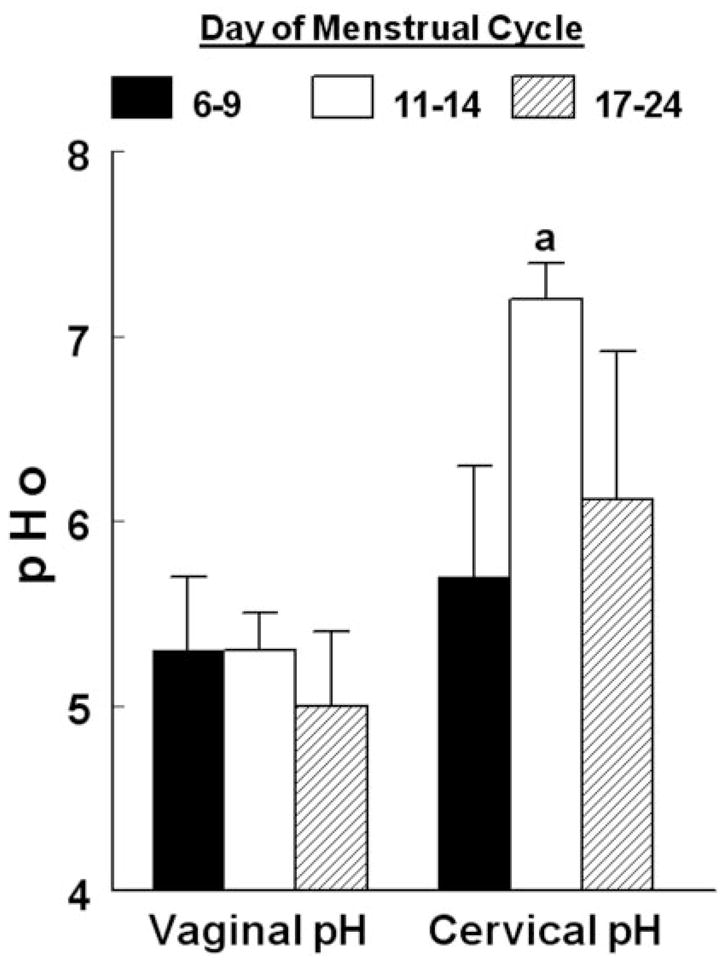
Changes in vaginal and cervical pHo according to the phase of the menstrual cycle. a, P < 0.01, compared with cervical pHo in d 6–9.
pHo changes in vitro
The levels of vaginal pHo found in Fig. 2 are significantly lower than plasma pH (7.2–7.4). One of the explanations is that vaginal-ectocervical epithelial cells secrete acid actively and selectively into the lumen. To test this hypothesis directly, cultures of human epithelial vaginal-ectocervical and endocervical cells were generated on filters as models for these epithelia, using previously described methodology (38–41). For some cultures cells were grown on the upper surface of the filter (facing upward when the filter is placed horizontally, Fig. 1, left panel). This established the filter’s upper compartment as housing the luminal solution, and the compartment in which the filter was bathed as housing the contraluminal solution. In other experiments, cells were plated on the bottom part of the filter that was then flipped back to their original position for assays (Fig. 1A, right panel). This procedure established the filter’s upper compartment as the contraluminal solution, and the compartment in which the filter was bathed as the luminal solution (Fig. 1A, right panel). Preliminary experiments validated that cultures remain confluent when plated on the bottom part of the filter (not shown).
When hECE cells were exposed to basic salt solution, the luminal pHo decreased from 7.40 ± 0.03 to 7.01 ± 0.04 within 25 min and remained at that level for the duration of the experiment (30 min) [Fig. 3A, five independent repeats (cells from three different women), P < 0.01]. The mean levels of contraluminal pHo dropped from 7.41 ± 0.04 to 7.28 ± 0.04 [Fig. 3A, five independent repeats (cells from three different women), P < 0.01]. In endocervical cells mean levels of luminal pHo and contraluminal pHo dropped from 7.41 ± 0.04 to only 7.27 ± 0.04 [Fig. 3B, four independent repeats (cells from two different women), P < 0.01]. The mild acidification of the contraluminal pHo in hECE cells and the luminal and contraluminal pHo in endocervical cells can be explained by acid generation and extrusion from a more acidic intracellular pH compartment (pH about 7.25). In contrast, the significant acidification of the luminal pHo in hECE cells suggests active proton (acid) extrusion across the apical plasma membrane.
Fig. 3.
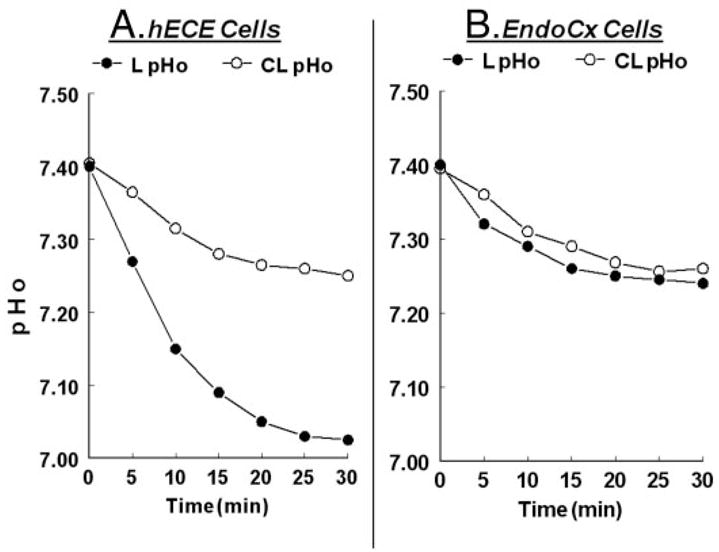
Changes in extracellular pH in the L-pHo and CL pHo solutions of hECE (A) and endocervical cells (B). The experiments were repeated three to four times with similar trends. Data are summarized in the text.
Estrogens regulate acidification of luminal pHo
In vivo, estrogens modulate vaginal pH, and the next set of experiments tested the hypothesis that estrogens regulate luminal acidification. hECE and endocervical cells were grown in steroid-free medium to deprive the cells of estrogens and then treated with 17β-estradiol at the physiological concentration of 10 nM. This low concentration is near maximal for increasing transepithelial, paracellular permeability across cultured human ectocervical-vaginal and endocervical cells (35). Incubation of endocervical cells in steroid-free medium and treatment with estradiol had no effect on contraluminal pHo or on luminal pHo, which remained in both cases around 7.25 (Fig. 4). Incubation of hECE cells in steroid-free medium and treatment with estradiol had no effect on contraluminal pHo, which also remained around 7.25 (Figs. 3 and 4). In contrast, incubation of hECE cells in steroid-free medium attenuated the acidification of the luminal compartment (pHo of 7.11 ± 0.02) (Fig. 4) vs. 7.01 ± 0.04 in cells grown in regular culture medium (Fig. 3A, P < 0.01). Treatment with estradiol resulted in significant acidification of the luminal compartment to pHo of 7.03 ± 0.02 (Fig. 4, P < 0.01).
Fig. 4.
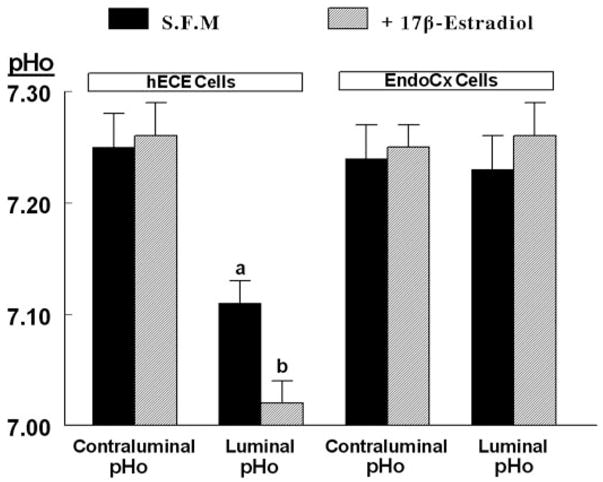
Effects of estrogen on changes in pHo. hECE cells were plated on filters and shifted to steroid-free medium for 1 d and then maintained in the same medium for an additional 2 d in the absence (S.F.M) or presence of 10 nM 17β-estradiol (added to both the luminal and contraluminal solutions) (+17β-estradiol). Determinations of pHo were described in the text. Shown are means (± SD) of three to five repeats per point of pHo determinations 30 min after mounting filters for assays. a, P < 0.01, compared with contraluminal pHo, hECE cells (S.F.M.); b, P < 0.01, compared with luminal pHo, hECE cells (S.F.M.).
The effect of estrogen was specific, and in hECE cells grown in steroid-free medium, only the potent estrogen diethylstilbestrol could mimic 17β-estradiol decrease in luminal pHo. The weak estrogen estrone had only a mild effect, whereas testosterone had no effect on luminal pHo (Fig. 5A). The effect of 17β-estradiol was dose dependent: in hECE cells grown in steroid-free medium, treatment with 0.1 nM 17β-estradiol already decreased luminal pHo from 7.26 ± 0.03 to 7.13 ± 0.03, and the effect reached near saturation at 1 nM 17β-estradiol, suggesting an EC50 of 17β-estradiol of about 0.5 nM (Fig. 5B). This level is similar to estrogen effect on paracellular permeability (35) and is compatible with activation of the classical nuclear estrogen receptor(s) mechanism. Treatment with the higher concentration of 100 nM 17β-estradiol had no additional effect on luminal pHo (Fig. 5B).
Fig. 5.
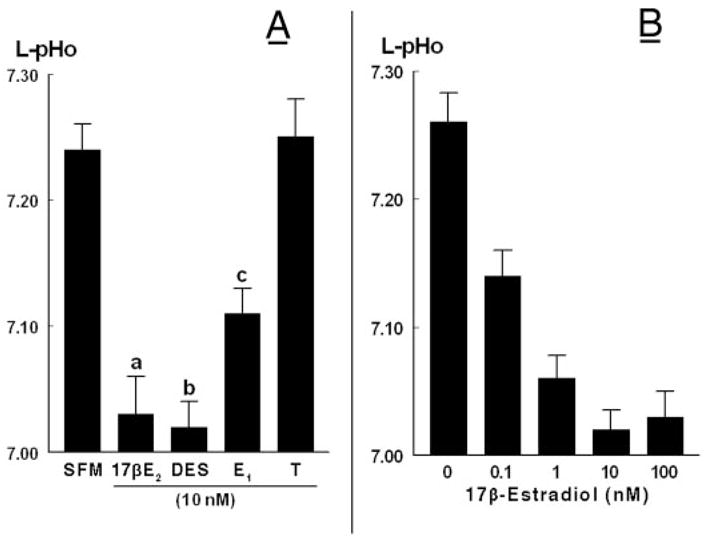
A, Specificity of estrogen-induced decrease of L-pHo. Experiments were done as in Fig. 4. hECE cells were grown in steroid-free medium (SFM) and treated with one of the following hormones (all added at 10 nM to both the luminal and contraluminal solutions): 17β-estradiol (17βE2), diethylstilbestrol (DES), estrone (E1), or testosterone. Shown are means (± SD) of L-pHo determinations in three to five repeats. a, b, c, P < 0.01, compared with SFM. B, Dose-response effect of 17β-estradiol. Shown are means (± SD) of three repeats per point. The decrease in mean (L-pHo) vs. (17β-estradiol) was significant (P < 0.01).
Specific estrogen receptor modulator (SERM) effects on estrogen modulation of luminal pHo
Treatment with ICI-182780 (10 μM) or progesterone (1 μM) alone had no effect on luminal pHo (Fig. 6). In contrast, tamoxifen alone decreased luminal pHo from 7.24 ± 0.02 to 7.15 ± 0.02 (Fig. 6, P < 0.01). Cotreatment with tamoxifen or with progesterone had no significant effect on the 17β-estradiol (10 nM)-induced decrease in luminal pHo (Fig. 6). In contrast, cotreatment with ICI-182780 blocked estrogen-induced decrease in luminal pHo (Fig. 6). Neither tamoxifen ICI-182780 nor progesterone had any appreciable effect on contraluminal pHo (not shown).
Fig. 6.
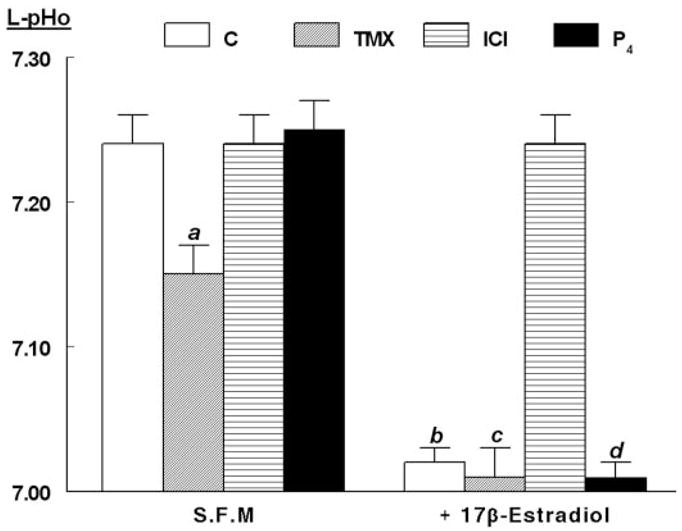
Modulation of estrogen decrease in L-pHo by specific estrogen-receptor modulators (SERMs). Experiments were done as in Fig. 4. hECE cells were grown in steroid-free medium (S.F.M.) in the absence or presence of 10 nM 17β-estradiol as well as with one of the following SERMs (all added 24 h before assays to both the luminal and contraluminal solutions): 10 μM tamoxifen (TMX), 10 μM ICI-182780 (ICI), or 1 μM progesterone (P4). Shown are means (± SD) of L-pHo determinations in three to four repeats per point. a, P < 0.01, compared with control (C), S.F.M. group; b, c, and d, P < 0.01, compared with S.F.M. group.
Mechanism of the luminal proton secretion
The results in Figs. 3–6 suggest that hECE cells decrease luminal pHo by active net proton secretion. In other types of cells, several types of H+-ATPases, which use cellular energy by hydrolyzing ATP to effect extrusion of hydrogen ion, have been described. These pumps are either electrogenic and require interaction with a parallel ion conductance (e.g. K+ channel) to maintain electroneutrality or involve the countertransport of another cation (e.g. the gastric H+/K+-ATPase). Three classes of H+-ATPases have been described: the E1-E2-ATPases [including the Na+/K+-ATPase (inhibited by ouabain), gastric H+/K+-ATPase (inhibited by omeprazole), and the Ca2+-ATPase]; the mitochondrial, chloroplastic, and bacterial membranal F1-F0-ATPases; and the vacuolar-type H+-ATPases (V-H+-ATPase), which are responsible for acidification of extracellular milieus, including the endosomal and lysosomal compartments and the cell exterior (reviewed in Ref. 42). The latter are most frequently associated with transepithelial acid secretion (21–28). Therefore, we hypothesized that hECE cells decrease luminal pHo by a mechanism that involves proton secretion using a V-H+-ATPase.
This hypothesis was tested by treating hECE cells with 1 μM bafilomycin A1, a specific inhibitor of V-H+-ATPase (43). As can be seen in Fig. 7, pretreatment for 30 min blocked the estrogen-induced acidification of the luminal pHo. Moreover, luminal pHo increased to pHo levels measured in the contraluminal compartment (compare Fig. 7 with Figs. 3A and 4). A possible explanation for the results shown in Fig. 7 is that hECE cells express an estrogen-dependent V-H+-ATPase that operates constitutively to acidify the luminal pHo. The controls for this experiment were ouabain and omeprazole. Ouabain (1 μM) was used for 30 min before the assay, and it was added to the contraluminal solution to block the Na+/K+-ATPase that, similar to other cells, is located in hECE cells in the basolateral membrane (not shown). In cells grown in steroid-free medium, pretreatment with ouabain resulted in mild alkalinization of luminal pHo to levels similar to the contraluminal pHo (compare Fig. 7 with Figs. 3A and 4). Pretreatment with ouabain also attenuated mildly estradiol effect, but luminal pHo still remained significantly more acidic, compared with cells not treated with estradiol (Fig. 7). A possible explanation for the mild effects of ouabain is a toxic cellular effect (see below). Pre-treatment for 30 min with 100 μM omeprazole had no significant effect (Fig. 7).
Fig. 7.
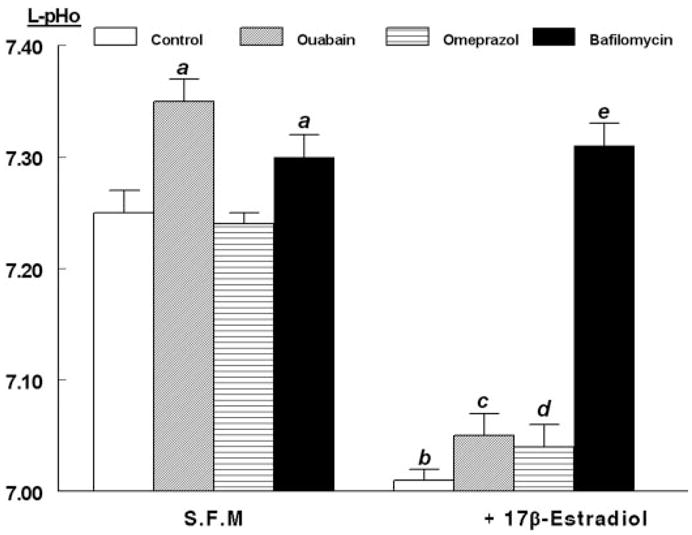
Modulation of estrogen decrease in L-pHo by ATPase inhibitors. Experiments were done as in Fig. 4. hECE cells were grown in steroid-free medium (S.F.M.) in the absence or presence of 10 nM 17β-estradiol as well as with one of the following drugs (all added 30 min before assays): ouabain (1 μM, added to the contraluminal solution), omeprazole (100 μM, added to both the luminal and contraluminal solutions), and bafilomycin A1 (1 μM, added to the luminal solution). Shown are means (± SD) of L-pHo determinations in three repeats per point 30 min after mounting filters for assays. a, P < 0.01, compared with control (C), S.F.M. group; b, c, and d, P < 0.01, compared with respective treatments in S.F.M. group; c, P < 0.02, compared with C, 17β-estradiol group, but P > 0.1, compared with omeprazole, 17β-estradiol group; e, P < 0.01, compared with b–d, and P < 0.01, compared with C, S.F.M. group.
Sidedness of the proton secretion mechanism
In polarized epithelial cells, ion transport mechanisms are usually sorted to either apical or basolateral domains of the plasma membrane. This separation is critical for net transepithelial vectorial ion transport. To test more directly the hypothesis that luminal acidification in hECE cells is mediated by apically located proton transport mechanism, bafilomycin A1 was administered selectively to either the contraluminal or to the luminal solutions. Pretreatment for 30 min with 1 μM of bafilomycin added to the contraluminal solution had no effect on the pHo of the contraluminal or luminal solutions (Fig. 8, bafilomycin CL). When added to the luminal solution, bafilomycin A1 had little effect on the pHo of the contraluminal solution (Fig. 8, bafilomycin L); however, it significantly increased luminal pHo to levels measured in the contraluminal compartment (Fig. 8). These results indicate that bafilomycin A1 targets primarily apically located proton extrusion mechanisms, suggesting that the estrogen-regulated V-H+-ATPase is expressed mainly in the apical membrane.
Fig. 8.
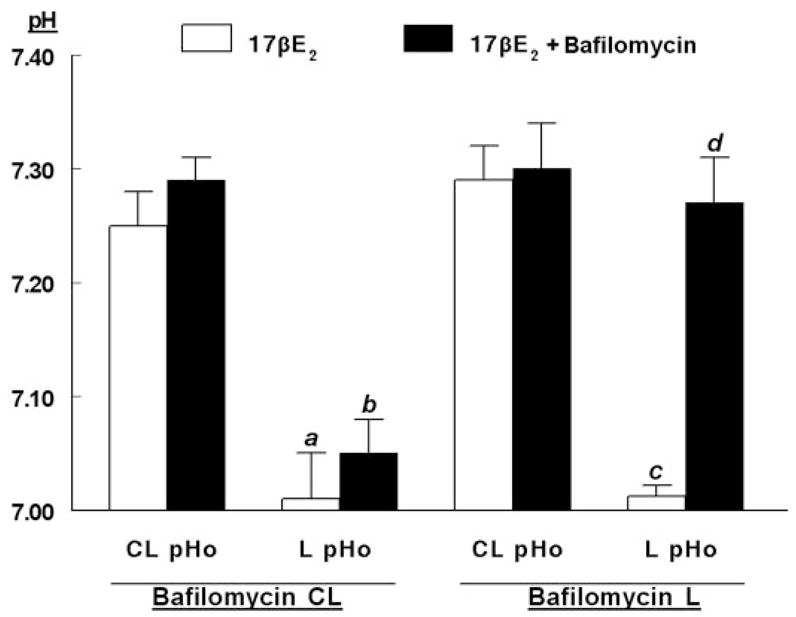
Sidedness of the acidification. Experiments were done as in Fig. 4. hECE cells were grown in steroid-free medium (S.F.M.) plus 10 nM 17β-estradiol. Thirty minutes before assays bafilomycin A1 (1 μM) was added to either the CL or L solution. Determinations of pHo in the CL-pHo and L-pHo were described in Materials and Methods. Shown are means (± SD) of three to five repeats per point 30 min after mounting filters for assays. a, b, c, P < 0.01, compared with CL-pHo; d, P < 0.01, compared with c.
Role of extracellular calcium and the tight junctions
To determine the role of the intercellular tight junctions in the control of luminal acidification, hECE cells cultured on filters were treated with 1.2 mM EGTA to chelate extracellular calcium to less than 0.1 mM. It was previously shown that Ca2+ is required at extracellular domains of the tight junctions for effective occlusion of the intercellular space and that lowering extracellular calcium decreases tight junctional resistance within minutes (44). Calcium was replaced with Cd2+ that was added to the bathing solutions at the equimolar concentration of 1.2 mM. Cd2+ cannot substitute for Ca2+ for tight junctional occlusion, and it has minimal effects on permeability across hECE cultures (45). Lowering Ca2+ blocked the luminal acidification (Fig. 9A) and resulted in acidification of the contraluminal solution (Fig. 9B), and the luminal and contraluminal pHo both reached a pH of about 7.2. These results indicate that in hECE cells the tight junctions are necessary for maintaining luminal acidification.
Fig. 9.
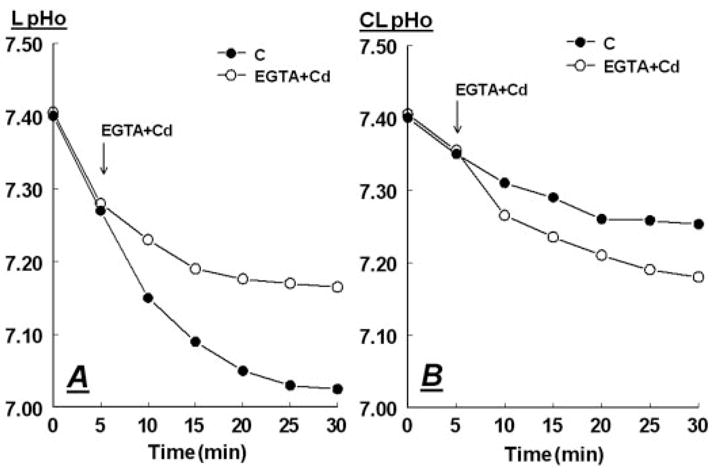
Abrogation of the tight junctions modulates luminal acidification. Filters with hECE cells were mounted with pH electrodes and shifted to basic salt solution. After 5 min 1.2 mM EGTA plus 1.2 mM CdCl2 (Cd) were added to the luminal and contraluminal solutions from concentrated ×1000 stocks (pH 7.35). Determinations of L-pHo (A) and CL-pHo (B) were described in Materials and Methods. The experiment was repeated twice with similar trends.
Coculturing with fibroblasts augments proton secretion
The degree of luminal acidification in monocultures of hECE cells was significant (ΔpHo of about −0.35), but smaller in magnitude compared with the situation in vivo (ΔpHo of −1.2 to −2.7). In vivo, epithelial cells rest on a basement membrane that separates them from stromal fibroblasts. To better mimic the in vivo conditions, cocultures of hECE cells and HCFs were generated by plating hECE cells and HCF cells on opposite surfaces of the filter (Fig. 1A). Plating irradiated HCF at the relatively low density of 5 × 104 cells/cm2 resulted in nonconfluent cultures of HCF, which did not affect per se the electrical resistance across the filter insert (not shown). In addition, in filters plated with only HCF levels of contraluminal (CL)-pHo or luminal (L) L-pHo remained at about 7.4 (not shown). Coplating of HCF improved the attachment of hECE cells to the trans surface of the filter and resulted in higher transepithelial electrical resistance (about 55 Ω/cm2, compared with about 35 Ω/cm2 in monocultures of hECE cells). Cocultured hECE cells lowered CL-pHo to pH 7.25 by 30 min, similar to monocultures of hECE cells (Fig. 10). In contrast, cocultured hECE cells lowered L-pHo to 6.05 ± 0.02 (Fig. 10, three independent experiments, P < 0.01). Thus, the cocultured hECE cells acidified the luminal compartment by more than 1 pH unit, compared with monocultured hECE cells.
Fig. 10.
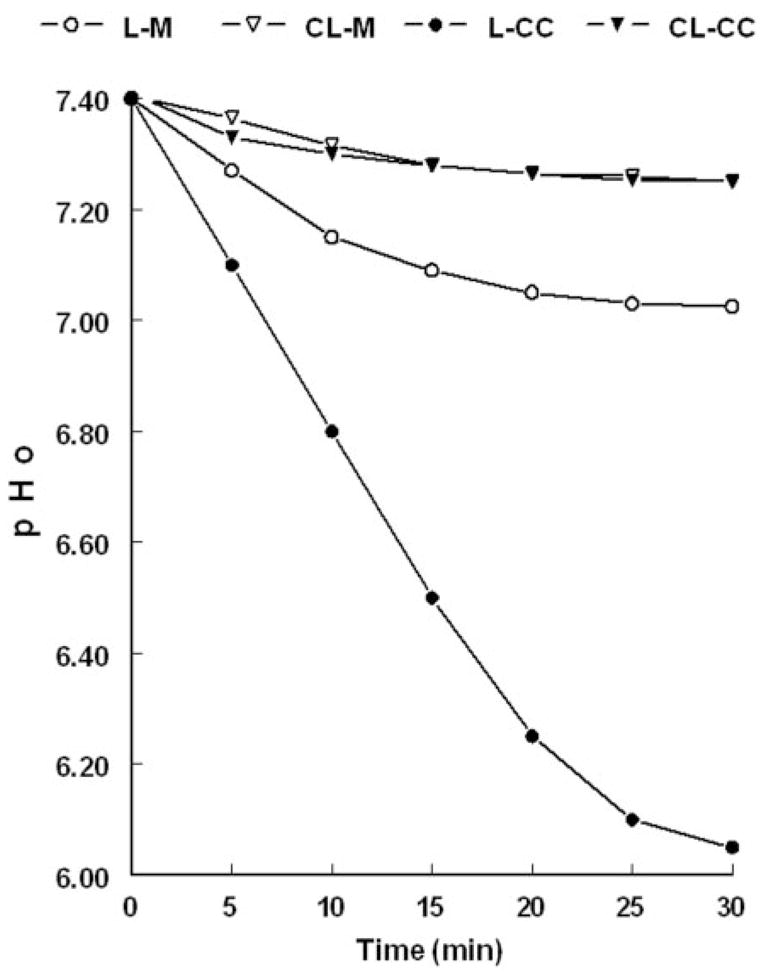
Determinations of L-pHo and CL-pHo in monocultures of hECE cells (M) and cocultures (CC) of hECE and HCF cells. The experiment was repeated three times with similar trends.
Effects on cell toxicity
The experimental procedures associated with pH measurements had no effect on cell viability, as determined with the MTT cleavage assay. With the exception of ouabain, all other treatments and procedures, including incubations in steroid-free medium, did not produce significant decreases in MTT cleavage, compared with control cells (not shown). As expected, treatments with ouabain decreased MTT incorporation and staining to about 65% of control. In this context, the mild inhibitory effect of ouabain on acidification (Fig. 7) was probably the result of its toxic effect on the cells.
Discussion
The present results suggest that vaginal-ectocervical cells acidify the luminal canal by a mechanism of active proton secretion, possibly via a V-H+-ATPase, which is located in the apical membrane. The in vivo experiments showed that the pHo of the apical vaginal surface remains acidic throughout the menstrual cycle and is unrelated to changes in L-pHo induced by the cervical mucus. The in vitro experiments showed that cultured hECE cells express a bafilomycin-A1−-sensitive proton secretory mechanism located in apical domain of the plasma membrane. The sensitivity to bafilomycin-A1 and lack of appreciable effects by omeprazole and ouabain suggest involvement of a V-H+-ATPase mechanism. Bafilomycin-A1 inhibits V-H+-ATPases by binding directly to the transmembrane V0 subunit c (46–48), and the finding that bafilomycin-A1 effect was most pronounced when the drug was administered to the luminal solution (Fig. 8) suggests that the putative V-H+-ATPase in vaginal-ectocervical cells is expressed predominantly in the apical plasma membrane. Because intracellular pH did not change significantly and remained about 7.25 (not shown), the constitutive acidification of the luminal solution is possibly the result of V-H+-ATPase-dependent proton secretion along with anion efflux. The latter could be secreted inorganic anions but not CO2 or HCO3+ because experiments were carried out at a fixed partial pressure CO2.
Although the V-H+-ATPase is probably the main vaginal proton extrusion mechanism, other proton transporters could also be involved in the regulation of pHo. For instance, the acidification of the contraluminal solution could be due to extrusion of proton or proton equivalents to equilibrate the intracellular pH (7.25 in hECE cells; Gorodeski, G. I., unpublished data) with the pH of the bathing solution. hECE express the Na+/H+ exchanger(s) (not shown), which electroneutrally decrease pHo (49, 50). Studies in human breast cancer tissues (51) and the rat epididymis (52) have shown estrogen regulation of the Na+/H+ exchanger similar to the effects in hECE cells. In hECE cells the Na+/H+ exchanger(s) are located predominantly in the basolateral membrane (not shown) and are therefore likely to play a role in the acidification of the contraluminal solution. Because neither estrogen deprivation nor treatment with 17β-estradiol affected contraluminal acidification, it is unlikely that in hECE cells the activity of the Na+/H+ exchanger(s) is modulated by estrogen. Whether Na+-dependent HCO3−transporters (53) and carbonic anhydrases (54, 55) contribute to the luminal acidification in hECE cells remains to be determined.
Estrogen deprivation did not abolish acidification of the luminal solution. This finding and the observations that in hypoestrogenic postmenopausal women vaginal pH ranges 6.5–7.0 (1) and is still lower than plasma pH (7.2–7.4) suggest that the apically located proton extrusion mechanism acidifies constitutively the luminal fluid, regardless of estrogen status. Treatment with estrogens up-regulated proton secretion into the luminal fluid; a low concentration of 1 nM 17β-estradiol sufficed to exert near maximum effect, suggesting that during premenopausal years the proton extrusion mechanism operates at its near maximum capacity and that cycle-related increases in plasma estradiol do not exert an additional effect on the activity of the proton extrusion mechanism. However, plasma 17β-estradiol less than 0.1 nM, such as after menopause, could result in decreased luminal acidification.
The mechanism by which estrogen up-regulates proton extrusion in hECE cells is not entirely understood. The agonist profile (17β-estradiol = diethylstilbestrol > estrone ≫ testosterone), the 17β-estradiol concentration-response profile (EC50 ~ 0.5 nM), and the inhibitory effect by ICI-182780 suggest involvement of the classical estrogen receptor(s) mechanism. The present results differ from inhibitory effects of estrogens in male rats on gastric H+/K+-ATPase (56) and hepatic acidification of endocytic vesicles (57). In addition, in rat epididymis and vas deferens diethylstilbestrol blocks androgen-dependent expression and activity of the V-H+-ATPase (58), which is involved in luminal acidification (22). Based on these data, it appears that the effects of estrogens on V-H+-ATPases could be gender/tissue related: facilitatory in female vagina and inhibitory in male certain tissues.
In hECE cells, tamoxifen augmented luminal acidification to a lesser degree than 17β-estradiol, suggesting partial estrogen agonistic effect. However, unlike ICI-182780, tamoxifen did not block the estrogen increase in acidification. Tamoxifen effect on L-pHo differed from its effect on permeability, in which tamoxifen attenuates estrogen increase in permeability (35) by blocking estrogen-dependent increase in estrogen receptor-α (34). These data raise the possibility of nongenomic effect (59). In fact, Chen et al. (60) suggested that most nongenomic effects of tamoxifen (61–64) are the result of tamoxifen inhibition of acidification of cytoplasmic organelles. However, whether the effect of tamoxifen in hECE cells involves up-regulation of V-H+-ATPases activity remains to be determined.
One of our objectives was also to improve the culture techniques and obtain a system of hECE cells on filters that would generate acidification of the luminal solution to a degree that resembles effects in vivo. This was accomplished in part by coculturing hECE and HCF cells on the opposing surfaces of the filter. This more physiological system produced significantly greater acidification of the luminal solution, to pHo of about 6.0. Furthermore, in preliminary experiments (not shown), the acidification of the luminal solution was also sensitive to bafilomycin-A1, suggesting that in both the hECE and hECE/HCF experimental systems, the apically located V-H+-ATPase is the main cellular mechanism of active net proton extrusion.
The mechanism by which coculturing of hECE cells with HCF enhances active net proton secretion via the apical plasma membrane is unclear. One possibility could be modulation of hECE tight-junctional resistance, which would affect luminal acidification by impeding paracellular proton transport from the luminal to the contraluminal solution and the prevention of proton loss in the luminal solution. Similarly, greater restriction of lateral movement of plasma membrane proteins by the tight junctions could allow more efficient sorting of proton extrusion mechanisms into the apical membrane. An additional mechanism could be HCF-dependent enhanced differentiation of hECE cells with secondary up-regulation of proton extrusion mechanisms in the apical membrane.
In summary, the present data provide a novel mechanistic explanation for the vaginal luminal acidic pH. Based on these results, our hypothesis is that vaginal-ectocervical cells acidify the luminal canal by active proton secretion, possibly via V-H+-ATPase that is located in the apical membrane. We also propose that active net proton secretion occurs constitutively throughout a woman’s life, but the degree of acidification is estrogen dependent. The mechanism of the estrogen effect is at present unclear, although our data suggest involvement of the classical estrogen receptor(s) mechanism.
Supplementary Material
Acknowledgments
The technical support of Kimberley Frieden, Brian De-Santis, and Dipika Pal is acknowledged. Tissue samples were provided by the Cooperative Human Tissue Network, which is funded by the National Cancer Institute.
This work was supported by National Institutes of Health Grants HD29924 and AG15955 (to G.I.G.).
Abbreviations
- CL-pHo
Contraluminal pHo
- HBSS
Hanks’ balanced salt solution
- HCF
human cervical fibroblast
- hECE
human cervical-vaginal epithelial
- L-pHo
luminal pHo
- MTT
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide
- pHo
extracellular pH
- V-H+-ATPase
vacuolar-type H+-ATPase
References
- 1.Moller BR, Kaspersen P. The acidity of the vagina. In: Horowitz B, Mare PA, editors. Vaginitis and vaginosis. New York: Wiley-Liss; 1991. pp. 63–67. [Google Scholar]
- 2.Pedraza Aviles GA, Zaragoza OMC, Coria IA. Bacterial vaginosis a “broad overview. Rev Latinoam Microbiol. 1999;41:25–34. [PubMed] [Google Scholar]
- 3.Kostara I, Carageorgiou H, Varonos D, Tzannetis S. Growth and survival of Trichomonas vaginalis. J Med Microbiol. 1998;47:555–560. doi: 10.1099/00222615-47-6-555. [DOI] [PubMed] [Google Scholar]
- 4.Donders GG, Bosmans E, Dekeersmaecker A, Vereecken A, Van Bulck B, Spitz B. Pathogenesis of abnormal vaginal bacterial flora. Am J Obstet Gynecol. 2000;182:872–878. doi: 10.1016/s0002-9378(00)70338-3. [DOI] [PubMed] [Google Scholar]
- 5.Pandit L, Ouslander JG. Postmenopausal vaginal atrophy and atrophic vaginitis. Am J Med Sci. 1997;314:228–231. doi: 10.1097/00000441-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Sagawa T, Negishi H, Kishida T, Yamada H, Fujimoto S. Vaginal and cervical pH in bacterial vaginosis and cervicitis during pregnancy. Hokkaido Igaku Zasshi. 1995;70:839–846. [PubMed] [Google Scholar]
- 7.Tevi-Benissan C, Belec L, Levy M, Schneider-Fauveau V, Si Mohamed A, Hallouin MC, Matta M, Gresenguet G. In vivo semen-associated pH neutralization of cervicovaginal secretions. Clin Diagn Lab Immunol. 1997;4:367–374. doi: 10.1128/cdli.4.3.367-374.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329:753–756. doi: 10.1056/NEJM199309093291102. [DOI] [PubMed] [Google Scholar]
- 9.Zheng HY, Alcorn TM, Cohen MS. Effects of H2O2-producing lactobacilli on Neisseria gonorrhoeae growth and catalase activity. J Infect Dis. 1994;170:1209–1215. doi: 10.1093/infdis/170.5.1209. [DOI] [PubMed] [Google Scholar]
- 10.Berman JR, Berman LA, Werbin TJ, Flaherty EE, Leahy NM, Goldstein I. Clinical evaluation of female sexual function: effects of age and estrogen status on subjective and physiologic sexual responses. Int J Impot Res. 1999;11:S31–S38. doi: 10.1038/sj.ijir.3900468. [DOI] [PubMed] [Google Scholar]
- 11.Eggert-Kruse W, Kohler A, Rohr G, Runnebaum B. The pH as an important determinant of sperm-mucus interaction. Fertil Steril. 1993;59:617–628. [PubMed] [Google Scholar]
- 12.Frega A, Stentella P, Spera G, Pace S, Cipriano L, Di Ruzza D, Villani C, Pachi A. Cervical intraepithelial neoplasia and bacterial vaginosis: correlation or risk factor? Eur J Gynaecol Oncol. 1997;18:76–77. [PubMed] [Google Scholar]
- 13.Weinstien L, Howard JH. The effect of estrogenic hormone on the H-ion concentration and the bacterial content of the human vagina with special reference to the Doderlein bacillus. Am J Obstet Gynecol. 1939;37:698–706. [Google Scholar]
- 14.Fontaine EA, Taylor-Robinson D. Comparison of quantitative and qualitative methods of detecting hydrogen peroxide produced by human vaginal strains of lactobacilli. J Appl Bacteriol. 1990;69:326–331. doi: 10.1111/j.1365-2672.1990.tb01523.x. [DOI] [PubMed] [Google Scholar]
- 15.Klebanoff SJ, Hillier SL, Eschenbach DA, Waltersdorph AM. Control of the microbial flora of the vagina by H2O2-generating lactobacilli. J Infect Dis. 1991;164:94–100. doi: 10.1093/infdis/164.1.94. [DOI] [PubMed] [Google Scholar]
- 16.Boskey ER, Telsch KM, Whaley KJ, Moench TR, Cone RA. Acid production by vaginal flora in vitro is consistent with the rate and extent of vaginal acidification. Infect Immun. 1999;67:5170–5175. doi: 10.1128/iai.67.10.5170-5175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keane FE, Ison CA, Taylor-Robinson D. A longitudinal study of the vaginal flora over a menstrual cycle. Int J STD AIDS. 1997;8:489–494. doi: 10.1258/0956462971920631. [DOI] [PubMed] [Google Scholar]
- 18.Melis GB, Ibba MT, Steri B, Kotsonis P, Matta V, Paoletti AM. Role of pH as a regulator of vaginal physiological environment. Minerva Ginecol. 2000;52:111–121. [PubMed] [Google Scholar]
- 19.Tsai CC, Semmens JP, Semmens EC, Lam CF, Lee FS. Vaginal physiology in postmenopausal women: pH value, transvaginal electropotential difference, and estimated blood flow. South Med J. 1987;80:987–990. doi: 10.1097/00007611-198708000-00013. [DOI] [PubMed] [Google Scholar]
- 20.De Bernardis F, Mühlschlegel FA, Cassone A, Fonzi WA. The pH of the host niche controls gene expression in and virulence of Candida albicans. Infect Immun. 1998;66:3317–3325. doi: 10.1128/iai.66.7.3317-3325.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sachs G. Physiology of the parietal cell and therapeutic implications. Pharmacotherapy. 2003;23:68S–73S. doi: 10.1592/phco.23.13.68s.31931. [DOI] [PubMed] [Google Scholar]
- 22.Brown D, Breton S. V-ATPase dependent luminal acidification in the kidney collecting duct and the epididymis/vas deferens. J Exp Biol. 2000;203:137–145. doi: 10.1242/jeb.203.1.137. [DOI] [PubMed] [Google Scholar]
- 23.Chandhoke PS, Packer RK, Knepper MA. Apical acidification by rabbit papillary surface epithelium. Am J Physiol. 1990;258:F893–F899. doi: 10.1152/ajprenal.1990.258.4.F893. [DOI] [PubMed] [Google Scholar]
- 24.Brisseau GF, Grinstein S, Hackam DJ, Nordstrom T, Manolson MF, Khine AA, Rotstein OD. IL-1 increases V-ATPase activity in murine peritoneal macrophages. J Biol Chem. 1996;271:2005–2011. doi: 10.1074/jbc.271.4.2005. [DOI] [PubMed] [Google Scholar]
- 25.Li YP, Chen W, Liang Y, Li E, Stashenko P. Atp6i-deficient mice exhibit severe osteopetrosis due to loss of osteoclast-mediated extracellular acidification. Nat Genet. 1999;23:447–451. doi: 10.1038/70563. [DOI] [PubMed] [Google Scholar]
- 26.Frattini A, Orchard PJ, Sobacchi C, Giliani S, Abinun M, Mattsson JP, Keeling DJ, Andersson AK, Wallbrandt P, Zecca L, Notarangelo LD, Vezzoni P, Villa A. Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat Genet. 2000;25:343–346. doi: 10.1038/77131. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Zaguilan R, Lynch R, Martinez G, Gillies R. Vacuolar-type H+-ATPases are functionally expressed in plasma membranes of human tumor cells. Am J Physiol. 1993;265:C1015–C1029. doi: 10.1152/ajpcell.1993.265.4.C1015. [DOI] [PubMed] [Google Scholar]
- 28.Wieczorek H, Grber G, Harvey WR, Huss M, Merzendorfer H, Zeiske W. Structure and regulation of the insect plasma membrane V-ATPase. J Exp Biol. 2000;203:127–135. doi: 10.1242/jeb.203.1.127. [DOI] [PubMed] [Google Scholar]
- 29.Nishi T, Forgac M. The vacuolar (H+)-ATPases—nature’s most versatile proton pumps. Nat Rev Mol Cell Biol. 2002;3:94–103. doi: 10.1038/nrm729. [DOI] [PubMed] [Google Scholar]
- 30.Gorodeski GI, Eckert RL, Utian WH, Rorke EA. Maintenance of in vivo-like keratin expression, sex steroid responsiveness and estrogen receptor expression in cultured human ectocervical epithelial cells. Endocrinology. 1990;126:399–406. doi: 10.1210/endo-126-1-399. [DOI] [PubMed] [Google Scholar]
- 31.Gorodeski GI, Romero MF, Hopfer U, Rorke E, Utian WH, Eckert RL. Human uterine cervical epithelial cells grown on permeable support—a new model for the study of differentiation and transepithelial transport. Differentiation. 1994;56:107–118. doi: 10.1046/j.1432-0436.1994.56120107.x. [DOI] [PubMed] [Google Scholar]
- 32.Gorodeski GI. The cultured human cervical epithelium: a new model for studying transepithelial paracellular transport. J Soc Gynecol Investig. 1996;3:267–280. doi: 10.1016/s1071-5576(96)00028-7. [DOI] [PubMed] [Google Scholar]
- 33.Gorodeski GI, Peterson D, De Santis BJ, Hopfer U. Nucleotide-receptor mediated decrease of tight-junctional permeability in cultured human cervical epithelium. Am J Physiol. 1996;270:C1715–C1725. doi: 10.1152/ajpcell.1996.270.6.C1715. [DOI] [PubMed] [Google Scholar]
- 34.Gorodeski GI, Pal D. Involvement of estrogen receptors α and β in the regulation of cervical permeability. Am J Physiol. 2000;278:C689–C696. doi: 10.1152/ajpcell.2000.278.4.C689. [DOI] [PubMed] [Google Scholar]
- 35.Gorodeski GI. Estrogen increases the permeability of the cultured human cervical epithelium by modulating cell deformability. Am J Physiol. 1998;275:C888–C899. doi: 10.1152/ajpcell.1998.275.3.C888. [DOI] [PubMed] [Google Scholar]
- 36.Mossman T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 37.Morgan DM. Tetrazolium (MTT) assay for cellular viability and activity. Methods Mol Biol. 1998;79:179–183. doi: 10.1385/0-89603-448-8:179. [DOI] [PubMed] [Google Scholar]
- 38.Gorodeski GI, Merlin D, De Santis BJ, Frieden KA, Hopfer U, Eckert RL, Romero MF. Characterization of paracellular permeability in cultured human cervical epithelium: regulation by extracellular ATP. J Soc Gynecol Investig. 1994;1:225–233. doi: 10.1177/107155769400100309. [DOI] [PubMed] [Google Scholar]
- 39.Gorodeski GI. Role of nitric oxide and cGMP in the estrogen regulation of cervical epithelial permeability. Endocrinology. 2000;141:1658–1666. doi: 10.1210/endo.141.5.7473. [DOI] [PubMed] [Google Scholar]
- 40.Gorodeski GI. Regulation of transcervical permeability by two distinct P2-purinergic receptor mechanisms. Am J Physiol. 2002;282:C75–C83. doi: 10.1152/ajpcell.2002.282.1.C75. [DOI] [PubMed] [Google Scholar]
- 41.Wang Q, Wang L, Feng YH, Li X, Zeng R, Gorodeski GI. P2X7-receptor-mediated apoptosis of human cervical epithelial cells. Am J Physiol Cell Physiol. 2004;287:C1349–C1358. doi: 10.1152/ajpcell.00256.2004. [DOI] [PubMed] [Google Scholar]
- 42.Lubman RL, Crandall ED. Regulation of intracellular pH in alveolar epithelial cells. Am J Physiol. 1992;262:L1–L14. doi: 10.1152/ajplung.1992.262.1.L1. [DOI] [PubMed] [Google Scholar]
- 43.Bowman EJ, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci USA. 1988;85:7972–9796. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gorodeski GI, Wenwu J, Hopfer U. Extracellular Ca2+ directly regulates tight junctional permeability in the human cervical cell line CaSki. Am J Physiol. 1997;272:C511–C524. doi: 10.1152/ajpcell.1997.272.2.C511. [DOI] [PubMed] [Google Scholar]
- 45.Gorodeski GI, Hopfer U, Wenwu J. Purinergic receptor induced changes in paracellular resistance across cultures of human cervical cells are mediated by two distinct cytosolic calcium related mechanisms. Cell Biochem Biophys. 1998;29:281–306. doi: 10.1007/BF02737899. [DOI] [PubMed] [Google Scholar]
- 46.Zhang J, Feng Y, Forgac M. Proton conduction and bafilomycin binding by the V0 domain of the coated vesicle V-ATPase. J Biol Chem. 1994;269:23518–23523. [PubMed] [Google Scholar]
- 47.Beutler JA, McKee TC. Novel marine and microbial natural product inhibitors of vacuolar ATPase. Curr Med Chem. 2003;10:787–796. doi: 10.2174/0929867033457827. [DOI] [PubMed] [Google Scholar]
- 48.Bowman EJ, Graham LA, Stevens TH, Bowman BJ. The bafilomycin/concanamycin binding site in subunit c of the V-ATPases from Neurospora crassa and Saccharomyces cerevisiae. J Biol Chem. 2004;279:33131–33138. doi: 10.1074/jbc.M404638200. [DOI] [PubMed] [Google Scholar]
- 49.Boron WF. Transport of H+ and of ionic weak acids and bases. J Membr Biol. 1983;72:1–16. doi: 10.1007/BF01870311. [DOI] [PubMed] [Google Scholar]
- 50.Aronson PS. Kinetic properties of the plasma membrane Na+-H+ exchanger. Annu Rev Physiol. 1985;47:545–560. doi: 10.1146/annurev.ph.47.030185.002553. [DOI] [PubMed] [Google Scholar]
- 51.Ediger TR, Kraus WL, Weinman EJ, Katzenellenbogen BS. Estrogen receptor regulation of the Na+/H+ exchange regulatory factor. Endocrinology. 1999;140:2976–2982. doi: 10.1210/endo.140.7.6885. [DOI] [PubMed] [Google Scholar]
- 52.Zhou Q, Clarke L, Nie R, Carnes K, Lai LW, Lien YH, Verkman A, Lubahn D, Fisher JS, Katzenellenbogen BS, Hess RA. Estrogen action and male fertility: roles of the sodium/hydrogen exchanger-3 and fluid reabsorption in reproductive tract function. Proc Natl Acad Sci USA. 2001;98:14132–14137. doi: 10.1073/pnas.241245898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sterling D, Casey JR. Bicarbonate transport proteins. Biochem Cell Biol. 2002;80:483–497. doi: 10.1139/o02-152. [DOI] [PubMed] [Google Scholar]
- 54.Potter CPS, Harris AL. Diagnostic, prognostic and therapeutic implications of carbonic anhydrases in cancer. Br J Cancer. 2003;89:2–7. doi: 10.1038/sj.bjc.6600936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Breton S, Hammar K, Smith PJ, Brown D. Proton secretion in the male reproductive tract: involvement of Cl+-independent HCO3− transport. Am J Physiol. 1998;257:C1134–C1142. doi: 10.1152/ajpcell.1998.275.4.C1134. [DOI] [PubMed] [Google Scholar]
- 56.Limlomwongse L, Piyachaturawat P. Inhibition of gastric H+ secretion, K+-ATPase and K+-phosphatase by estradiol in vivo and in vitro. Can J Physiol Pharmacol. 1982;60:680–684. doi: 10.1139/y82-093. [DOI] [PubMed] [Google Scholar]
- 57.Van Dyke RW, Root KV. Ethinyl estradiol decreases acidification of rat liver endocytic vesicles. Hepatology. 1993;18:604–613. [PubMed] [Google Scholar]
- 58.Fisher JS, Pastor-Soler N, Sharpe RM, Breton S. Modulation of the onset of postnatal development of H+-ATPase-rich cells by steroid hormones in rat epididymis. Biol Reprod. 2002;67:1106–1114. doi: 10.1095/biolreprod67.4.1106. [DOI] [PubMed] [Google Scholar]
- 59.Weiss DJ, Gurpide E. Non-genomic effects of estrogens and antiestrogens. J Steroid Biochem. 1988;31:671–676. doi: 10.1016/0022-4731(88)90017-9. [DOI] [PubMed] [Google Scholar]
- 60.Chen Y, Schindler M, Simon SM. A mechanism for tamoxifen-mediated inhibition of acidification. J Biol Chem. 1999;274:18364–18373. doi: 10.1074/jbc.274.26.18364. [DOI] [PubMed] [Google Scholar]
- 61.Chatterjee M, Harris AL. Reversal of acquired resistance to Adriamycin in CHO cells by tamoxifen and 4-hydroxy tamoxifen: role of drug interaction with α1 acid glycoprotein. Br J Cancer. 1990;62:712–717. doi: 10.1038/bjc.1990.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Love RR, Mazess RB, Barden HS, Epstein S, Newcomb PA, Jordan VC, Carbone PP, DeMets DL. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med. 1992;326:852–856. doi: 10.1056/NEJM199203263261302. [DOI] [PubMed] [Google Scholar]
- 63.Zhang JJ, Jacob TJ, Valverde MA, Hardy SP, Mintenig GM, Sepulveda FV, Gill DR, Hyde SC, Trezise AE, Higgins CF. Tamoxifen blocks chloride channels. A possible mechanism for cataract formation. J Clin Invest. 1994;94:1690–1697. doi: 10.1172/JCI117514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams JP, Blair HC, McKenna MA, Jordan SE, McDonald JM. Regulation of avian osteoclastic H+-ATPase and bone resorption by tamoxifen and calmodulin antagonists. Effects independent of steroid receptors. J Biol Chem. 1996;271:12488–12495. doi: 10.1074/jbc.271.21.12488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


