Abstract
Aging is associated with frontal subcortical microangiopathy and executive cognitive dysfunction, suggesting that elderly individuals may have impaired metabolic activation of cerebral blood flow to the frontal lobes. We used transcranial Doppler (TCD) ultrasound to examine the cerebral blood flow response to executive control and visual tasks in the anterior and posterior cerebral circulations and to determine the effects of healthy aging on cerebral blood flow regulation during cognitive tasks. Continuous simultaneous anterior cerebral artery (ACA) and posterior cerebral artery (PCA) blood flow velocities (BFVs) and mean arterial pressure (MAP) were measured in response to word stem completion (WSC) and a visual search (VS) task in 29 healthy subjects (14 young, 30 ± 1.5 years; 15 old, 74 ± 1.4 years). We found that: (1) ACA and PCA blood flow velocities are both significantly increased during WSC and VS cognitive tasks, (2) ACA and PCA activations were task specific in our young volunteers, with ACA > PCA BFV during the WSC task and PCA > ACA BFV during the VS task, (3) while healthy elderly subjects also had PCA > ACA BFV during the VS task, they did not have ACA > PCA activation during the WSC task, and (4) healthy elderly subjects tend to have overall greater increases in BFV during both cognitive tasks. We conclude that TCD can be used to monitor cerebrovascular hemodynamics during the performance of cognitive tasks. Our data suggest that there is differential blood flow increase in the ACA and PCA in young versus elderly subjects during cognitive tasks.
Keywords: Cerebral blood flow velocity, Cognition, Aging, Transcranial Doppler ultrasound
1. Introduction
The association between aging, the development of subcortical microangiopathy, and executive cognitive dysfunction (Cabeza, 2001; Farkas and Luiten, 2001; van Gijn, 2000; Pugh and Lipsitz, 2002) suggests that elderly individuals may have an impaired cerebral blood flow response to executive function tasks that increase metabolic activity in the brain. Consistent with these findings, aging is associated with cortical volume losses that are particularly notable in the frontal cortices (Kemper, 1994; Madden and Hoffman, 1997; Raz et al., 1997). Indeed, some theories focus exclusively on frontal lobe changes to explain age-related cognitive decline (Nielson et al., 2002).
Neuroimaging techniques have shown decreased activation in some regions of the brain in elderly compared to young adults. This is often accompanied by increased activation in other, sometimes contralateral, areas (Nielson et al., 2002). These observations have lead to the hypothesis that older adults compensate for age-related neural changes by recruiting additional neural circuitry or by using alternative circuitry to assist with cognitive tasks (Nielson et al., 2002). Because the performance of cognitive tasks requires the delivery of adequate oxygen and glucose to specific regions of the brain, it has been assumed that region-specific blood flow should be related to enhanced metabolic activity in those regions engaged in task performance. However, this process relies on complex and poorly understood cerebrovascular regulatory mechanisms that may be altered in aging and disease. A common explanation of the mismatched cerebral blood flow and oxygen consumption during neuronal excitation holds that blood flow rises more than oxygen consumption to compensate for an absent oxygen reserve in brain mitochondria (Gjedde et al., 2005). Since positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) techniques do not provide direct measures of cerebral blood flow changes, the relationship between age-related changes in the cerebral circulation and cognitive decline remains unknown.
Functional transcranial Doppler ultrasound (fTCD) is a non-invasive method used to measure cerebral blood flow velocity (BFV) changes during the performance of cognitive tasks. Studies show that cerebral BFV is higher when subjects engage in cognitive activities compared to resting periods (Stroobant and Vingerhoets, 2000, 2001). Given that the diameter of the large cerebral arteries does not appear to change significantly under a variety of physiological stimuli (Serrador et al., 2000), cerebral BFV changes during mental stimuli are assumed to be related to volume flow changes. Hence, changes in cerebral BFV reflect changes in cerebral blood flow during cognitive activation. Studies utilizing fTCD have substantially contributed to the field of functional neuroimaging (for detailed reviews see Deppe et al., 2004; Stroobant and Vingerhoets, 2000, 2001). In an elegant study utilizing fTCD, Frauenfelder et al. (2004) examined cerebral BFV changes in young healthy adults during a task of executive function which involved separated phases of planning, execution and a control condition. They showed that cerebral BFV significantly differed among the three phases in both the anterior cerebral artery (ACA) and middle cerebral artery (MCA), but they did not observe any differences between the two vessels. They also did not include a cognitive task that minimized executive demands to determine whether these blood flow changes reflected the specific demands of the tasks used.
Our study was designed to characterize simultaneous cerebrovascular hemodynamic responses in the ACAs and posterior cerebral arteries (PCAs) during cognitive tasks designed to activate the frontal and occipital lobes, respectively, and to determine how these responses are affected by healthy aging. The word stem completion (WSC) task was chosen as a task requiring frontal lobe/ACA activation (Dhond et al., 2001) because it is technically compatible with the transcranial Doppler (TCD) technique and can be repeated during a short duration with a clear onset and termination time. A visual search (VS) task was chosen to study visual cortex/PCA activation. This task was chosen because it is technically similar to the WSC task and involves the same motor activity but was assumed to minimize effort, working memory and complex decision making and hence the requirement of frontal lobe involvement. Our hypothesis was that ACA and PCA blood flow velocities would increase during frontal and occipital lobe activation, respectively, and that aging would be associated with a relative decrease in ACA BFV during an executive function task.
2. Materials and methods
2.1. Subjects
Fourteen healthy young (8 men, 6 women) and 15 healthy older (5 men, 10 women) subjects volunteered to participate in the study. Subjects were recruited from laboratory personnel and members of the Harvard Cooperative Program on Aging subject registry. The young subjects were less than 40 years of age and the older subjects were greater than 60. All older subjects were carefully screened with a medical history, physical examination, carotid ultrasound and electrocardiogram (ECG) to exclude any acute or chronic medical conditions. Subjects were asked to refrain from caffeine, alcohol or nicotine for at least 12 h. The study was approved by the Hebrew Rehabilitation Center for Aged institutional review board, and followed institutional guidelines.
2.2. Experimental protocol
2.2.1. Instrumentation
Subjects reported to the cardiovascular laboratory in the postabsorptive state, at least 2 h after their last meal. Instrumentation for heart rate (HR), ECG and beat-to-beat mean arterial pressure (MAP, Finapress, Ohmeda Monitoring Systems, Englewood, CO) monitoring were as previously described (Lipsitz et al., 2000).
TCD ultrasonography (MultiDop X4, DWL-Transcranial Doppler Systems Inc., Sterling, VA) was used to measure simultaneous changes in ACA and PCA BFVs in response to: (1) WSC and VS tasks, (2) blood pressure (BP) changes during a thigh-cuff test, and (3) end-tidal CO2 changes (CO2 Analyzer, Vacumed, Ventura, CA). The left ACA and right PCA signals were identified according to the criteria of Aaslid et al. (1982) and recorded at a depth of 55-70 mm. The choice of left versus right for these vessels was based on what was most practical given the equipment set-up. A Mueller-Moll probe fixation device was used to stabilize the Doppler probes for the duration of the study. The mean frequency envelope of the velocity waveform, derived from a fast-Fourier analysis of the Doppler frequency signal, was digitized at 500 Hz, displayed simultaneously with the MAP, ECG, and end-tidal CO2 signals, and stored for later off-line analysis.
2.2.2. Frontal and visual cortex activation protocols
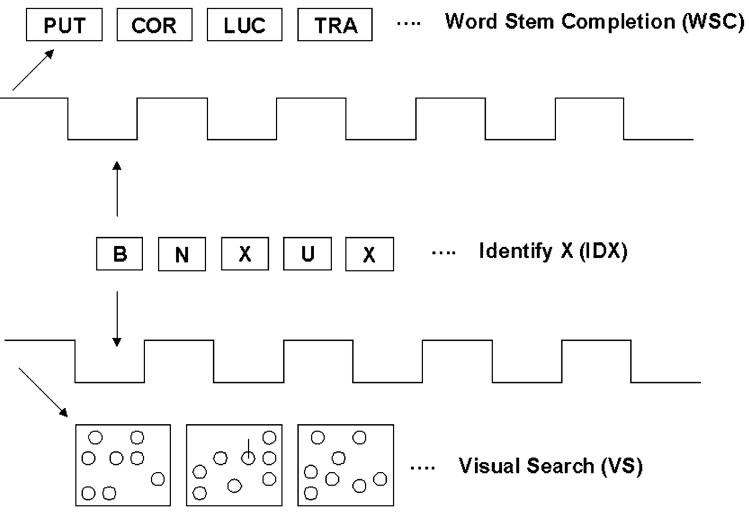
While subjects were resting in the supine position, they were asked to watch a computer display and complete the following three tasks (Fig. 1).
Fig. 1.
Schematic of the experimental runs. Runs were set-up as a block design with a single trial consisting of five 30-40 sec blocks of the specific cognitive task alternating with five 30-40 sec blocks of the IDX. The cognitive tasks were - completing three letter word stems (WSC) and searching for a line bisecting a circle in a random array of circles (VS). Starting order of the task was counterbalanced between participants.
WSC - This is a relatively specific executive control task. Three letters were shown on the screen and subjects were asked to think of as many words as possible that begin with those letters and click the mouse with every word they generated.
Identify X (IDX) - This was the control condition, form which changes in blood flow during the executive and VS tasks were calculated. A series of single letters appeared in succession on the screen. Subjects were asked to click the mouse each time they saw the letter X.
VS - This was used as a visual task to activate the posterior circulation. A series of randomly distributed circles appeared in succession on the screen. Subjects were asked to quickly scan the screen and click the mouse each time they saw a circle with transecting line.
To confirm that subjects were participating in the tasks, they were asked to use the hand ipsilateral (left in all cases) to the insonated ACA to click a computer mouse each time they thought of a new word, saw the X, or saw a line bisecting a circle. The tasks were paired with IDX as either: (1) WSC and IDX or (2) VS and IDX and the order of each set was randomized. Each set of tasks was performed five times, with each task alternating with IDX every 30-40 sec. During each block of WSC and VS the subjects received 8 stems and 12 search screens per task period. The IDX task was inserted to standardize cognitive activity during baseline measurements. Blood flow activation was normalized to IDX to minimize any effects from differences in resting baseline cerebral BFVs which are known to decrease with aging. Supine testing was performed so that the data could be compared to fMRI data, which are also collected in the supine position.
2.2.3. Thigh-cuff protocol
Following the cognitive tasks, the subject remained in the supine position and a pair of thigh cuffs (Hokanson, Issaquah, WA) were inflated for 2 min to 20 mmHg above MAP then released to create a sudden drop in BP. The autoregulatory response to this transient hypotension was assessed by the autoregulatory index (ARI) using Tiecks’ method (Tiecks et al., 1995) as well as by the percent change in cerebrovascular resistance (CVR) at the MAP nadir.
2.2.4. CO2 reactivity protocol
BFV in the ACA and PCA was measured continuously while subjects inspired a gas mixture of 5% CO2, 21% O2, and balance nitrogen for 2 min and then mildly hyperventilated to an endtidal CO2 of approximately 25 mmHg for 2 min. Percent changes in ACA or PCA BFV were plotted against changes in CO2. Cerebral vasoreactivity (VR) was measured as the percent change in BFV per mmHg change in end-tidal CO2.
2.2.5. Data processing and analysis
All data were displayed and digitized in real time at 500 Hz with commercially available data acquisition software (WinDaQ, Dataq Instruments). Post processing was done using custom written MATLAB scripts. Beat-to-beat R-R intervals were determined from the R wave of the ECG. Systolic, diastolic and mean values for BP (reported as MAP) and cerebral BFV (reported as mean flow velocity) were determined from the associated waveforms.
To determine group responses to ACA and PCA activation, BFV and BP waveforms during the cognitive tasks were resampled at 1 Hz using a MATLAB script. Beat-to-beat values for ACA or PCA BFV and MAP during the WSC and VS tasks were averaged across all trials for each individual. In order to identify blood flow changes specifically associated with the performance of each cognitive task (VS and WSC), mean percent change in BFV was calculated as the percent difference between the task of interest and its corresponding IDX control period. To allow BFV measures to stabilize after changing tasks, mean values were extracted for the 10-25 sec time window for each 30-sec task block. Specifically, all five task blocks for both the cognitive task and the IDX control period were averaged together and the mean percent change was calculated as a ratio of the difference between the BFV during the cognitive task and its corresponding IDX control period divided by BFV during 10-25 sec of performing IDX and multiplied by 100. These derived values were used in all the subsequent analysis of the cognitive tasks. Changes in BP during the performance of the cognitive tasks was analyzed in a similar way to that for BFV, namely BP was expressed as a percentage change from the IDX control task.
2.2.6. Statistical analysis
We first examined whether the expected pattern of percent BFV changes related to the cognitive tasks was evident in young participants using a 2 × 2 repeated measures ANOVA with location (ACA, PCA) and task (WSC, VS) as within subjects factors. Secondly, we examined whether the pattern of %BFV changes associated with each task was different between the groups. Each of these ANOVAs was performed utilizing a 2 × 2 mixed effects design on 14 young and 15 elderly participants,1 with group (young, elderly) as a between subjects factor and location (ACA, PCA) as a within subjects factor. Changes in MAP during the performance of each of the cognitive tasks was analyzed using a repeated measures ANOVA with group (elderly, young) as a between subjects factor and task (WSC, VS) as a within subjects factor. Linear regression was used to compute the slope of the relationship between end-tidal CO2 and BFV (a measure of VR). Student’s t-test was used to compare group mean VR, ARI, and percent change in CVR during thigh-cuff deflation.
3. Results
3.1. Subject characteristics
Characteristics of the young and older subject groups are displayed in Table 1. Young subjects had a significantly lower baseline MAP and a higher baseline BFV in the ACA. BFV was not significantly different in the PCA of the two groups. CVR in both vascular territories was higher in the older subjects. Cerebral VR and autoregulation were not significantly different between the groups.
Table 1.
Baseline subject characteristics
| Young | Old | p (young versus old) | |
|---|---|---|---|
| Number of subjects | 14 | 15 | |
| Age, y | 30 (1.5) | 74 (1.4) | |
| MAP, mmHg | 78 (3) | 94 (5) | .007 |
| HR, bpm | 62 (3) | 61 (2) | ns |
| ACA | |||
| BFV, cm/sec | 65 (3) | 51 (2.5) | .009 |
| CVR, (mmHg sec/cm) | 1.2 (0.04) | 2.0 (0.2) | .0001 |
| VR, (%ΔBFV/mmHg) | 1.7 (0.2) | 1.3 (0.2) | ns |
| ARI | 6.0 (0.8) | 6.0 (0.5) | ns |
| %ΔCVR | -14.5 (3.4) | -10.2 (3.1) | ns |
| PCA | |||
| BFV, cm/sec | 46 (6) | 37 (3.3) | ns |
| CVR, (mmHg sec/cm) | 2.0 (0.3) | 2.9 (0.3) | .03 |
| VR, (%ΔBFV/mmHg) | 1.6 (0.2) | 1.4 (0.3) | ns |
| ARI | 5.0 (0.6) | 6.0 (0.5) | ns |
| %ΔCVR | -14.5 (7.7) | -11.7 (3) | ns |
All values are means (SEM). %ΔCVR indicates the percent change in CVR during the thigh-cuff test and it is used as another measure of autoregulation in addition to ARI.
3.2. Cerebral BFV during cognitive tasks
Mean percent changes in BFV from the baseline control task (IDX) in the ACA and PCA during WSC and VS tasks for young and older subjects are summarized in Fig. 2. A 2 × 2 ANOVA performed on the data from young participants with task and location as within subjects factors revealed no main effect of task (F(1,10) = 1.17, not significant) nor location (F < 1) but a significant interaction between task and location (F(1,10) = 12.16, p < .01). This interaction reflected the fact that the WSC task was associated with greater ACA than PCA change in %BFV, while the reverse pattern was revealed for the visual task. Each task was examined separately between groups in order to determine if there were differences in this pattern as-sociated with aging. A 2 × 2 ANOVA on the WSC task (group × location) resulted in no main effect of group (F(1,27) = 1.70, ns) indicating no overall difference between young and old in the %BFV response to this task. There was also no main effect of location (F < 1) but there was an interaction (F(1,27) = 4.93, p < .05), consistent with the elderly not showing the expected pattern of greater ACA versus PCA %BFV change during this task. The 2 × 2 ANOVA testing on the results of the VS task revealed a marginal main effect of group (F(1,23) = 3.74, p < .07), indicating overall higher response during this task for the elderly. However, there was no main effect of location (F < 1), nor an interaction (F(1,23) = 1.57, ns), indicating that the overall pattern of greater PCA change during VS for the elderly did not differ between young and older participants.
Fig. 2.
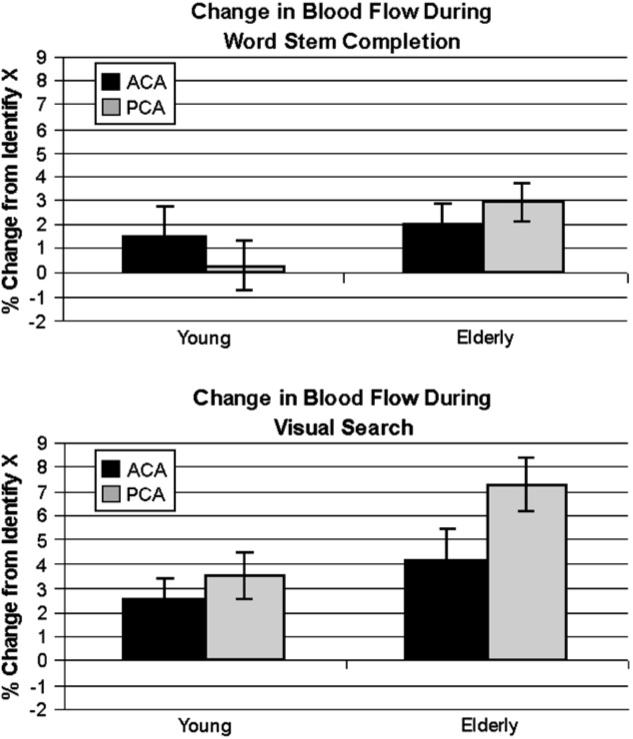
The bars reflect the mean percent change in BFV relative to an “IDX” baseline task in ACA and PCA territories in young and elderly participants. Error bars represent the standard error of the mean. The top panel shows changes during the WSC task where participants are asked to generate as many completions to a given three letter stem as possible and indicate with a button press whether a completion came to mind. There was no main effect of group or location, but there was a significant interaction. The bottom panel shows changes during the VS task where participants were asked to indicate with a button press the presence of a line bisecting a circle among a random array of circles. There was a trend towards a main effect of group, but no effect of location or interaction.
The results of the BP analysis indicated no group effect (F(1,23) < 1), task effect (F(1,23) < 1), or group by task interaction (F(1,23) = 1.58, ns). These results support the conclusion that the observed changes in blood flow velocities were not due to changes in BP during performance of the tasks.
4. Discussion
Our study demonstrates that ACA and PCA blood flow velocities are significantly increased during cognitive tasks and that these changes can be simultaneously monitored with TCD. Moreover, we show that increases in blood flow velocities are task specific. In normal young participants, we see a differential pattern of activation in ACA and PCA regions, with relatively greater ACA activation during WSC and relatively greater PCA activation during VS. Older participants showed a similar pattern of response during the VS task. However, as we hypothesized, older participants did not show the pattern of frontal greater than posterior activation during WSC that was seen in the young participants, supporting the contention that frontal responsiveness is altered as part of the aging process.
In this study both young and elderly participants demonstrated similar ACA blood flow changes for WSC and a trend towards greater responsiveness in the elderly during VS. This may reflect the fact that we did not require subjects to make overt verbal responses in the WSC, so that attentional demands and effort were determined by the participant. In contrast, the stimuli in the VS task were controlled by the experimenter and required an overt response by the subject, hence requiring greater attention than was anticipated. Previous studies have shown that attentional resources may be under utilized in older adults in the absence of fixed attentional demands (Logan et al., 2002). Moreover, while there were same numbers of trials during WSC and VS, there were significantly more visual stimuli than word stems per block (12 versus 8). This may also contribute to more PCA activation.
These findings are in line with prior functional imaging data which have shown that during some cognitive tasks older healthy volunteers appear to have a more extensive neuronal activation than younger subjects to achieve an accuracy that equals young subjects (Grossman et al., 2002; Rypma et al., 2001; Milham et al., 2002; Schacter et al., 1996). PET studies of perception (Madden et al., 1996; Grady et al., 1994) have suggested that aging is associated with a weaker activity in the visual cortex, which is compensated by a stronger activity in the prefrontal cortex allowing older individuals to maintain accuracy at the expense of reaction times (for detailed review see Cabeza, 2001; Cabeza et al., 2004). In further support of this model, several studies have shown that during tasks of working memory, episodic retrieval, perception, and episodic encoding, prefrontal cortex activity was lateralized in young adults, but bilateral in older adults, leading to formulation of the Hemispheric Aging Reduction in Old Adults (HAROLD) theory (Cabeza et al., 1997). These observations have led to the hypothesis that older subjects exhibit additional activation beyond that of young subjects in order to compensate for age-related neural changes. They may need to recruit additional neural circuitry or use alternative circuitry to assist with cognitive tasks (Nielson et al., 2002). In support of these findings, the current study revealed greater blood flow changes in aging compared to young. However, this conclusion is limited by different blood flow velocities in the baseline condition in elderly versus young subjects. Moreover, since this study did not record bilaterally, no conclusions about the laterality of changes can be made. Future studies will need to examine both these issue.
In summary, we show that TCD can be used to monitor cerebrovascular hemodynamics during the performance of cognitive tasks in healthy young and older volunteers. Our results provide preliminary support for our hypothesis that ACA and PCA blood flow velocities increase during frontal and occipital lobe activation, respectively, and that aging is associated with a relative decrease in ACA BFV during an executive function task. Future studies measuring bilateral BFVs during cognitive tasks in healthy older subjects as well as older subjects with vascular disease and cognitive impairment, will allow us to extend our knowledge of the association of aging, cognitive decline and cerebral blood flow activation.
Acknowledgements
We thank Margaret Gagnon for her help with subject recruitment. We also thank Ike Iloputaife and Mitul Vyas for their assistance in data collection and MATLAB Programming.
This work was supported by a generous donation from Mr. and Mrs. Robert Krakoff at the Hebrew Rehabilitation Center for Aged and by grants AG04390, AG08812, and AG05134 from the National Institute on Aging, Bethesda, MD. Dr. Sorond is the recipient of Mentored Clinical Scientist K12 Award (AG00294) from the National Institute on Aging. Dr. Lipsitz holds the Irving and Edyth S. Usen and Family Chair in Geriatric Medicine.
Footnotes
One elderly and two young participants did not complete the VS task. When possible, these additional participants were used in testing of the WSC task.
REFERENCES
- Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. Journal of Neurosurgery. 1982;57:769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Cognitive neuroscience of aging: contributions of functional neuroimaging. Scandinavian Journal of Psychology. 2001;42:277–286. doi: 10.1111/1467-9450.00237. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cerebral Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, Mcintosh AR, Tulving E, Kapur S, Jennings JM, Houle S, Craik FI. Age-related differences in neural activity during memory encoding and retrieval: A positron emission tomography study. Journal of Neuroscience. 1997;17:391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppe M, Ringelstein EB, Knecht S. The investigation of functional brain lateralization by transcranial Doppler sonography. Neuroimage. 2004;21:1124–1146. doi: 10.1016/j.neuroimage.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Dhond RP, Buckner RL, Dale AM, Marinkovic K, Halgren E. Spatiotemporal maps of brain activity underlying word generation and their modification during repetition priming. Journal of Neuroscience. 2001;21:3564–3571. doi: 10.1523/JNEUROSCI.21-10-03564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Progress in Neurobiology. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- Frauenfelder BA, Schuepbach D, Baumgartner RW, Hell D. Specific alterations of cerebral hemodynamics during a planning task: A transcranial Doppler sonography study. Neuroimage. 2004;22:1223–1230. doi: 10.1016/j.neuroimage.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Johannsen P, Cold GE, Ostergaard L. Cerebral metabolic response to low blood flow: Possible role of cytochrome oxidase inhibition. Journal of Cerebral Blood Flow and Metabolism. 2005 doi: 10.1038/sj.jcbfm.9600113. [DOI] [PubMed] [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, Pietrini P, Wagner E, Haxby JV. Age-related changes in cortical blood flow activation during visual processing of faces and location. Journal of Neuroscience. 1994;14:1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Cooke A, Devita C, Alsop D, Detre J, Chen W, Gee J. Age-related changes in working memory during sentence comprehension: An fMRI study. Neuroimage. 2002;15:302–317. doi: 10.1006/nimg.2001.0971. [DOI] [PubMed] [Google Scholar]
- van Gijn J. White matters: Small vessels and slow thinking in old age. Lancet. 2000;356:612–613. doi: 10.1016/S0140-6736(00)02599-X. [DOI] [PubMed] [Google Scholar]
- Kemper R. Neuroanatomical and neuropathological changes in normal aging and in dementia. In: Albert M, Knoepfel EE, editors. Clinical Neurology of Aging. Oxford University Press; New York: 1994. pp. 3–67. [Google Scholar]
- Lipsitz LA, Mukai S, Hamner J, Gagnon M, Babikian V. Dynamic regulation of middle cerebral artery blood flow velocity in aging and hypertension. Stroke. 2000;31:1897–1903. doi: 10.1161/01.str.31.8.1897. [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: Dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Hoffman JM. Application of positron emission tomography to age-related cognitive changes. In: Krishman KRR, Doraiswamy PM, editors. Brain Imaging in Clinical Psychiatry. Marcel Dekker; New York: 1997. [Google Scholar]
- Madden DJ, Turkington TG, Coleman RE, Provenzale JM, Degrado TR, Hoffman JM. Adult age differences in regional cerebral blood flow during visual world identification: Evidence from h215o pet. Neuroimage. 1996;3:127–142. doi: 10.1006/nimg.1996.0015. [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, Cohen NJ. Attentional control in the aging brain: Insights from an fMRI study of the stroop task. Brain and Cognition. 2002;49:277–296. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Langenecker SA, Garavan H. Differences in the functional neuroanatomy of inhibitory control across the adult life span. Psychology and Aging. 2002;17:56–71. doi: 10.1037//0882-7974.17.1.56. [DOI] [PubMed] [Google Scholar]
- Pugh KG, Lipsitz LA. The microvascular frontal-subcortical syndrome of aging. Neurobiology of Aging. 2002;23:421–431. doi: 10.1016/s0197-4580(01)00319-0. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, Mcquain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: Differential vulnerability of the prefrontal gray matter. Cerebral Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V, Desmond JE, Gabrieli JD. Age differences in prefrontal cortical activity in working memory. Psychology and Aging. 2001;16:371–384. doi: 10.1037//0882-7974.16.3.371. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Savage CR, Alpert NM, Rauch SL, Albert MS. The role of hippocampus and frontal cortex in age-related memory changes: A pet study. Neuroreport. 1996;7:1165–1169. doi: 10.1097/00001756-199604260-00014. [DOI] [PubMed] [Google Scholar]
- Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke. 2000;31:1672–1678. doi: 10.1161/01.str.31.7.1672. [DOI] [PubMed] [Google Scholar]
- Stroobant N, Vingerhoets G. Transcranial doppler ultrasonography monitoring of cerebral hemodynamics during performance of cognitive tasks: A review. Neuropsychology Review. 2000;10:213–231. doi: 10.1023/a:1026412811036. [DOI] [PubMed] [Google Scholar]
- Stroobant N, Vingerhoets G. Test-retest reliability of functional transcranial doppler ultrasonography. Ultrasound in Medicine and Biology. 2001;27:509–514. doi: 10.1016/s0301-5629(00)00325-2. [DOI] [PubMed] [Google Scholar]
- Tiecks FP, Lam AM, Aaslid R, Newell DW. Comparison of static and dynamic cerebral autoregulation measurements. Stroke. 1995;26:1014–1019. doi: 10.1161/01.str.26.6.1014. [DOI] [PubMed] [Google Scholar]