Abstract
Aim
To analyze data from a randomized clinical trial to determine the cost-effectiveness of using contingency management (CM) and motivational/skills building therapy (motivational enhancement therapy/cognitive-behavioral therapy: MET/CBT) to treat young adults with marijuana dependence.
Participants, design and measurements
A total of 136 marijuana-dependent young adults, all referred by the criminal justice system, were randomized to one of four treatment conditions: MET/CBT with CM, MET/CBT without CM, drug counseling (DC) with CM and DC without CM. Patient outcome measures include the longest duration of confirmed marijuana abstinence (LDA) during treatment and the total number of marijuana-free urine specimens provided during treatment. Costs were collected retrospectively from the provider and include the costs of therapy, patient drug testing, and those associated with the incentives component (value of vouchers, time to administer the voucher system).
Setting
Out-patient substance abuse clinic in New Haven, Connecticut, USA.
Findings
Which treatment is the most cost-effective depends on the threshold values of an additional week of LDA or an additional marijuana-free urine specimen. For example, the most effective treatment, MET/CBT with CM, was also the most cost-effective treatment at the highest threshold values, while the least effective treatment, DC, was the most cost-effective at the lowest values. Because consensus threshold values for these patient outcomes do not exist, results are presented showing the ranges of values over which each treatment would be considered cost-effective compared to the others. Acceptability curves are presented to show the decision uncertainty associated with these ranges. The results are shown to be robust to (i) sensitivity analyses on several key cost parameters and (ii) patient outcomes measured during the 6-month follow-up period.
Conclusions
This study uses incremental cost-effectiveness ratios and acceptability curves to shed light on the relative cost-effectiveness of four interventions for treating young adults with marijuana dependence. Given the relatively small and specialized nature of our study sample, and the fact that we examined a CM procedure with a single reinforcement schedule, additional studies are warranted to determine the reliability and generalizability of our results both to alternative marijuana-using populations and to CM procedures with alternative incentive parameters. Nevertheless, the relative durability of effects of MET/CBT compared to DC through the 6-month follow-up, and its cost-effectiveness over a comparatively wide range of threshold values, underscores the promise of this approach.
Keywords: Acceptability curves, contingency management, cost-effectiveness, marijuana dependence, motivational/skills building therapy, treatment, young adults
INTRODUCTION
Marijuana is the most commonly used illicit substance by young adults in the United States, with 16.1% of the young adult age group (18–25 years) reporting marijuana use during the month prior to the 2004 National Survey on Drug Use and Health [1]. Frequent marijuana use during young adulthood significantly increases the risk of life-time experiences with and greater involvement with other illicit drugs, earlier onset of substance dependence, poorer educational and occupational outcomes, multiple health and psychiatric problems, as well as higher levels of involvement with the criminal justice system [2–4]. Further, frequent marijuana use in early adulthood is the best predictor of persistent use, and initiation of drug use after age 29 is comparatively rare. Thus, providing effective interventions for marijuana disorders among young adults is a strategy of great potential significance in preventing progression to other drug use disorders as well as harmful consequences of continued marijuana use [2].
In a recent randomized clinical trial [5], both contingency management (CM) and motivational/skills building (motivational enhancement therapy/cognitive-behavioral therapy: MET/CBT) interventions were found to improve drug use outcomes, compared to traditional drug counseling (DC) alone, among young adults (18–25 years) with marijuana dependence. A total of 136 marijuana-dependent young adults, all referred by the criminal justice system, were randomized to one of four treatment conditions: MET/CBT with CM (incentives were contingent on session attendance or submission of marijuana-free urine specimens), MET/CBT without CM, DC with CM and DC without CM. These four interventions were chosen because, although several recent randomized trials have shown the efficacy and durability of behavioral approaches (e.g. MET, CBT, CM) for adult marijuana users [6,7], none of these trials focused on young adults or on those whose contact with the criminal justice system precipitated their involvement in treatment (an important and growing population that has proved difficult to engage in treatment [8–10], and who have markedly low rates of treatment retention [11]). Moreover, there has been a lack of research on combinations of different behavioral approaches that maximize outcomes.
Although the Carroll et al. study [5] demonstrated the effectiveness of both CM and MET/CBT in improving outcomes for marijuana-dependent young adults, little is known about the cost-effectiveness of either of these interventions [12]. Because both interventions can increase costs to a financially constrained treatment system, either directly through voucher payments (as with CM) or indirectly by increasing length of stay, a key issue is whether the additional costs of the interventions are justifiable. Without knowing the cost-effectiveness of using CM or MET/CBT to treat marijuana-dependent young adults, policy- and decision-makers have little guidance in determining whether the additional expenditures on either of these interventions are worthwhile investments. Policy- and decision-makers may be reluctant to adopt CM and MET/CBT interventions, despite their demonstrated effectiveness, without some information on the costs associated with implementing those treatments and whether those costs can be justified by the outcomes seen [12].
In this study, we present incremental cost-effectiveness ratios (ICERs) that define ranges of values over which each intervention would be considered cost-effective for improving each of two patient outcomes measured during treatment: longest duration of confirmed marijuana abstinence (LDA) and the total number of marijuana-free urine specimens provided. Acceptability curves are presented to illustrate the decision uncertainty in the ICERs. We also check the robustness of our results by (i) conducting sensitivity analyses on several key cost parameters and (ii) analyzing the longest duration of marijuana abstinence that occurred during the 6-month follow-up period. To our knowledge, no study has examined the cost-effectiveness of CM interventions targeted at marijuana users. Further, only two studies have examined either the cost-effectiveness or cost–benefit of MET/CBT interventions targeted at marijuana users, and both these studies focused on a relatively young age group (13–18 years) [13,14]. In addition to providing one of the first cost-effectiveness analyses of interventions for marijuana-dependent young adults, the present study is the first in the substance abuse treatment field to use acceptability curves in an application with more than two interventions. This study also adds to the growing literature on the cost-effectiveness of well-defined empirically validated treatments for substance use disorder, including CM and MET/CBT [15–22].
METHODS
Methods and results of the effectiveness study are described in the main report of study design and outcomes [5] and are thus summarized only briefly below, followed by a description of the analytical methods used for the cost-effectiveness analysis. Patient outcomes and resource utilization data for these analyses are taken from the effectiveness study [5]; to these we add cost data obtained from the clinic where the effectiveness study took place.
The randomized clinical trial compared four interventions for marijuana-dependent young adults. Two different individual manual-guided psychotherapy conditions were contrasted: a motivational/skills building approach (MET/CBT) versus a manualized individual drug counseling approach (DC). In addition, participants were randomized to receive either voucher-based CM or no CM. Participants assigned to CM received vouchers contingent on session attendance or submission of marijuana-free urine specimens. Thus, the four treatment conditions were MET/CBT with CM, MET/CBT without CM, DC with CM and DC without CM.
All participants in the trial were between the ages of 18 and 25 years, referred for treatment for marijuana dependence by the Office of Adult Probation to the Substance Abuse Treatment Unit (SATU)—a state-supported out-patient facility in New Haven, Connecticut, USA—and met criteria for current marijuana dependence. The study intervention lasted 8 weeks. The final study sample comprised 136 individuals who were assigned randomly to one of the four treatment conditions. Of these 136 individuals, two were incarcerated and two dropped out prior to their first session, resulting in 132 individuals who were exposed to the study treatments.
Treatments
All treatments were manualized and delivered as individual weekly sessions over 8 weeks. All clinicians completed a 2-day didactic training seminar and at least one closely supervised training case. All treatment sessions were videotaped for supervision and assessment of fidelity to manual guidelines. Evaluators who were unaware of participants’ treatment assignment rated approximately half the session videotapes and found (i) the study treatments were highly discriminable in the expected direction and (ii) no significant differences by treatment condition in mean skill ratings of the counselors.
Individual drug counseling (DC)
This condition was intended as a standardized version of the counseling that is offered typically in community-based clinics. The treatment manual [23,24] places strong emphasis on achieving abstinence from marijuana and other drugs through utilization of self-help groups and concepts compatible with a Twelve-Step approach.
Motivational enhancement/skills training (MET/CBT)
This condition emphasized the development of motivation for change and the implementation of skills to bring about that change, using the manualized approach developed for the Marijuana Treatment Project [25]. Clinicians were encouraged to use an empathic therapeutic style associated with motivational interviewing to resolve ambivalence, heighten discrepancies about personal goals and marijuana use and elicit motivation to change. Once ambivalence about reducing marijuana use had been addressed via motivational interviewing, exposure to CBT techniques and skills training (e.g. understanding patterns of substance use, strategies for recognizing and coping with craving) was delivered using a therapeutic style compatible with motivational interviewing.
Contingency management (CM)
This condition used a two-track incentive system found to be effective in previous trials [26,27] in which participants received vouchers redeemable for goods or services for attending counseling sessions or submitting marijuana-free urine specimens. Specifically, participants received a voucher worth $25 for the first session attended, and the value of the vouchers increased in $5 increments for each consecutive session attended. In addition, participants received $50 in vouchers for the first marijuana-free urine specimen submitted, with increments of $5 for each consecutive marijuana-free urine thereafter. If the participant attended all eight sessions and submitted eight consecutive negative urines, they would earn a total of $880 worth of vouchers ($340 for session attendance and $540 for submission of negative urines). The value of the vouchers earned in the counseling track was reset to $25 if an individual missed a session, while the value of the vouchers earned in the urine track was reset to $50 if the individual submitted a marijuana-positive urine specimen (or failed to submit a specimen). The two tracks were independent in that if a participant submitted a marijuana-positive urine specimen but attended the scheduled counseling session, only the urine track was reset.
Assessments
Weekly assessments included urinalysis (Varian OnTrak Testcup 5 with temperature strip and adulterant checks), as well as self-reports of substance use collected via the time-line follow-back method (TLFB), a reliable and valid method for assessing substance use on a day-by-day basis [28,29]. Urine specimens were screened routinely for adulterants, and all subjects were required to leave bags, coats and excessive clothing behind before being escorted to the bathroom. In addition, urines were randomly supervised. Current and life-time psychiatric diagnoses were evaluated using the Structured Clinical Interview for DSM-IV (SCID) [30]. Psychosocial functioning was assessed using the Addiction Severity Index (ASI) [31].
Cost-effectiveness analysis
To calculate incremental cost-effectiveness ratios (ICERs), we first calculated the unit costs using cost data (e.g. average counselor salaries during the study period, typical amount of time to conduct a urinalysis, typical amount of time per week to administer the voucher system, cost of a urine testcup, etc.) obtained retrospectively from the clinic where the effectiveness study took place. Then, for each study participant, we multiplied the resources used by the unit costs. Data on resources used (e.g. number of sessions attended, number of urine tests) came from the original effectiveness study [5]. The total variable cost for each participant in each condition was then calculated, followed by the incremental cost of each successive condition, from the least costly to the most. All cost-effectiveness analyses are based on a sample of 129 participants; three of the 132 participants in the effectiveness study were excluded from the cost analyses due to missing data.
Incremental costs
Costs are calculated from the perspective of the clinic and include only those costs that vary by treatment condition. Such costs include those related to counseling sessions, urinalysis and the voucher system. All labor costs include fringe benefits (29%). Because implementing CM and/or MET/CBT may require additional staff in the long term, and these additional staff may, in turn, increase overheads (e.g. utilities, insurance, rent, etc.), all labor costs are multiplied by the clinic’s overhead rate (36.1%).
Unit counseling cost
The unit counseling cost measures the average cost of a counseling session and includes the time spent by the counselor both in treatment and in administration (e.g. taking notes before or after the session).
Unit testing cost
The unit testing cost measures the average cost per urine test and includes material costs (urine test cup and adulterant strip) and time spent by staff administering the test.
Unit voucher system costs
Voucher system costs comprise two components: the costs of the vouchers and the costs of administering the voucher system. The unit costs of the vouchers are straightforward. The costs of administering the voucher system are calculated by valuing the typical amount of time per week spent by staff filling out voucher-related paperwork, awarding vouchers and purchasing voucher items at counselor salary plus fringe benefits and overheads. Staff typically perform these administrative duties on a weekly basis, and the associated costs are shared by both of the conditions containing CM (i.e. DC with CM, MET/CBT with CM). Thus, there are several ways to apportion these administrative costs on a per participant basis. In the present study, the total administrative cost of running the voucher system was assigned to the two CM conditions in proportion to the total value of the vouchers earned by participants in each of the conditions. The administrative cost per participant was then determined by dividing each CM condition’s share of the total administrative cost of running the voucher system by the number of participants in that condition.
Resources used
In order to calculate the total variable costs, we multiplied the above calculated unit costs by the number of units of each resource used. Resource utilizations for each participant were obtained from the effectiveness study [5]. Data on the following were collected: the number of counseling sessions attended, the number of urinalysis tests, the value of vouchers earned due to session attendance and the value of vouchers earned due to submitting negative urine samples. Variable costs per participant were then estimated straightforwardly by multiplying unit costs by corresponding resource utilizations. Finally, the incremental costs among the four conditions were calculated by first sorting the conditions in ascending order of their respective average variable costs per participant, and then differencing the average variable costs per participant of successive treatment pairs, from the least to the most costly.
Incremental cost-effectiveness analysis
We conducted edincremental cost-effectiveness analyses (ICEA) [32,33] to answer the question of value per dollar spent on CM and MET/CBT over usual care (DC). The primary patient outcome used in the ICEA is the longest duration of abstinence (LDA) from marijuana. LDA is defined as the longest span of consecutive weeks in which all urine samples delivered under the once-weekly testing schedule indicated abstinence from marijuana.
The LDA was chosen as the primary patient outcome for the ICEA both because (i) the escalating nature of the vouchers in the CM conditions was designed specifically to reinforce long durations of abstinence and (ii) the longest duration of abstinence achieved during treatment is among the best predictors of improved outcomes at follow-up periods [34–36]. As a check on the robustness of our results, we also consider a secondary objective patient outcome measure: the number of marijuana-free urine samples submitted.
For each of the patient outcome measures, we calculated incremental cost-effectiveness ratios (ICERs). The ICER is defined as the incremental cost divided by the incremental effect. We used incremental costs estimated as described above and incremental effects obtained from the effectiveness study. The ICERs measure the incremental cost of using a given treatment, compared to the next-least-costly treatment, to produce an extra unit of effect for each of the patient outcomes.
Costs and effects for each treatment were bootstrapped (with 1000 replicates) to produce acceptability curves for each of the patient outcome measures [37]. Acceptability curves show the probability that each treatment is the most cost-effective, given the observed data, under different assumptions about the value of an extra unit of effect. Thus, acceptability curves illustrate the statistical uncertainty in our study due to our sample [37–39].
Finally, two additional analyses were performed to examine the robustness of the results. First, we conducted sensitivity analyses on several key cost parameters to assess how the ICERs and acceptability curves would probably change had the trial been implemented under alternative realistic conditions. Secondly, we analyzed the longest duration of marijuana abstinence that occurred during the 6-month follow-up period.
RESULTS
The effectiveness study found no statistically significant differences by treatment condition on any of the participant demographic, substance use or psychosocial functioning variables measured at baseline, with one exception—participants in the MET/CBT with CM group had a significantly higher level of baseline marijuana use than participants in the other three groups [5]. Accordingly, the effectiveness study used ordinary least squares (OLS) regression to adjust patient outcomes for baseline marijuana use, and we used the same approach here. Adjusting for baseline marijuana use, the average LDA during treatment was 3.90 weeks, 3.78 weeks, 3.08 weeks and 2.47 weeks among participants assigned to MET/CBT with CM, DC with CM, MET/CBT and DC, respectively, while the average number of marijuana-free urine samples submitted during treatment was 2.26, 2.00, 1.27 and 0.88 among participants assigned to MET/CBT with CM, DC with CM, MET/CBT and DC, respectively. Incremental effects between successively more effective treatment pairs were non-significant for both patient outcomes (due most probably to relatively small samples in each treatment group). Nevertheless, incremental cost-effectiveness analysis is still warranted, because (i) cost-effectiveness depends on the joint density of cost and effect differences (as opposed to individual differences in either cost or effect) and (ii) ‘absence of evidence is not evidence of absence’ (i.e. a focus on hypothesis testing leads to an overemphasis on type I errors at the expense of type II errors) [38,40,41]. Given that sample characteristics were similar across the four groups, observed differences in patient outcomes were probably associated with the interventions provided.
Unit costs were estimated following the methods described above using the cost data obtained from the treatment site in New Haven, Connecticut, USA. The cost of a 1-hour counseling session (including administrative time for notes) was $38.59. Each urinalysis cost an average of $20.00, of which slightly more than half ($10.35) was the material cost of the urine sample test cup and adulterant strip. The administrative cost to implement the voucher system was $422 and $516 per participant in the DC with CM and MET/CBT with CM groups, respectively.
Average resource utilizations per participant were obtained from the original effectiveness study and are summarized in Table 1. Table 2 presents the average variable cost per participant. As shown in Table 2, as would be expected, participants in the MET/CBT with CM condition had the highest average variable cost, followed by participants in the DC with CM, MET/CBT, and DC conditions. Both of the CM conditions had substantially higher per participant variable costs than either of the non-CM conditions, due almost entirely to the cost of the voucher system.
Table 1.
Average resources consumed per participant in each treatment arm.*
| DC (n = 32) | MET/CBT (n = 32) | DC and CM (n = 32) | MET/CBT and CM (n = 33) | |
|---|---|---|---|---|
| Counseling sessions (no.) | 4.38 (2.06) | 5.47 (2.26) | 5.72 (2.25) | 5.94 (2.57) |
| Tests (no.) | 3.63 (2.30) | 4.59 (2.26) | 5.03 (2.26) | 4.76 (2.50) |
| Vouchers ($)† | 0 | 0 | 335 (251) | 399 (279) |
DC = individual drug counseling; MET/CBT = motivational enhancement therapy/cognitive-behavioral therapy; CM = contingency management.
Values represent means and standard deviations (in parentheses).
Voucher earnings for attending counseling sessions averaged $191 and $223 for participants in DC and CM and MET/CBT and CM, respectively. Voucher earnings for submitting marijuana-free urine samples averaged $144 and $176 for participants in DC and CM and MET/CBT and CM, respectively.
Table 2.
Average variable cost per participant in each treatment arm—base case.*
| DC (n = 32) ($) | MET/CBT (n = 32) ($) | DC and CM (n = 32) ($) | MET/CBT and CM (n = 33) ($) | |
|---|---|---|---|---|
| Counseling | 170 (83) | 213 (89) | 220 (88) | 228 (101) |
| Testing | ||||
| Materials | 38 (24) | 48 (23) | 52 (23) | 49 (26) |
| Time | 35 (23) | 44 (22) | 49 (22) | 46 (25) |
| Subtotal | 73 (47) | 92 (46) | 101 (46) | 95 (50) |
| Vouchers | ||||
| Earnings | 0 | 0 | 335 (251) | 399 (279) |
| Administration | 0 | 0 | 422 (n/a) | 516 (n/a) |
| Subtotal | 0 | 0 | 757 (251) | 915 (279) |
| Total | 243 (124) | 305 (130) | 1078 (352) | 1238 (397) |
DC = individual drug counseling; MET/CBT = motivational enhancement therapy/cognitive-behavioral therapy; CM = contingency management; n/a = not applicable.
Values represent means and standard deviations (in parentheses).
The incremental cost-effectiveness ratios (ICERs) were calculated using incremental costs derived from Table 2 and incremental effects as described above. Specifically, compared to usual care (i.e. DC), the incremental cost of using MET/CBT to lengthen the LDA by 1 week was $102 (i.e. $305–$243/3.08–2.47). Similarly, compared to MET/CBT, the incremental cost of using DC with CM to lengthen the LDA by 1 week was $1104; and, compared to DC with CM, the incremental cost of using MET/CBT with CM to lengthen the LDA by 1 week was $1333.
The corresponding ICERs for the patient outcome number of marijuana-free urine samples are $159, $1059 and $615, respectively. However, because the ICER for DC with CM ($1059) is higher than that of the next-most-costly alternative ($615), DC with CM is extended dominated by the combination of MET/CBT and MET/CBT with CM. Accordingly, the ICERs for the patient outcome number of marijuana-free urine samples were recalculated excluding the DC with CM condition. Specifically, compared to usual care, the incremental cost of using MET/CBT to obtain an additional marijuana-free urine sample was $159; and, compared to MET/CBT, the incremental cost of using MET/CBT with CM to obtain an additional marijuana-free urine sample was $942 (i.e. $1238–$305/2.26–1.27).
Acceptability curves are a relatively new approach that is used to illustrate the statistical uncertainty inherent in the ICERs due to the use of a single sample [37–39]. Figures 1 and 2 show the acceptability curves associated with the patient outcomes LDA and number of marijuana-free urines, respectively. Each acceptability curve shows the probability that a given intervention is the most cost-effective given the observed data [38]. Note that acceptability curves are a function of the threshold willingness-to-pay of the decision maker for an additional unit of outcome. For example, if the threshold value (perhaps determined by society’s willingness to pay) of extending the LDA by 1 week is $50, then the probability that DC, MET/CBT, DC with CM or MET/CBT with CM is the most cost-effective intervention is 82%, 18%, 0% and 0%, respectively. On the other hand, if the threshold value of extending the LDA by 1 week is $3000, then the probability that DC, MET/CBT, DC with CM or MET/CBT with CM is the most cost-effective intervention is 0%, 13%, 40% and 47%, respectively. Note that even though DC with CM was extended dominated for the outcome number of marijuana-free urines, it is included in the acceptability curves in Fig. 2. To exclude DC with CM in Fig. 2 on the basis of extended dominance would underestimate the uncertainty in the remaining treatments.
Figure 1.
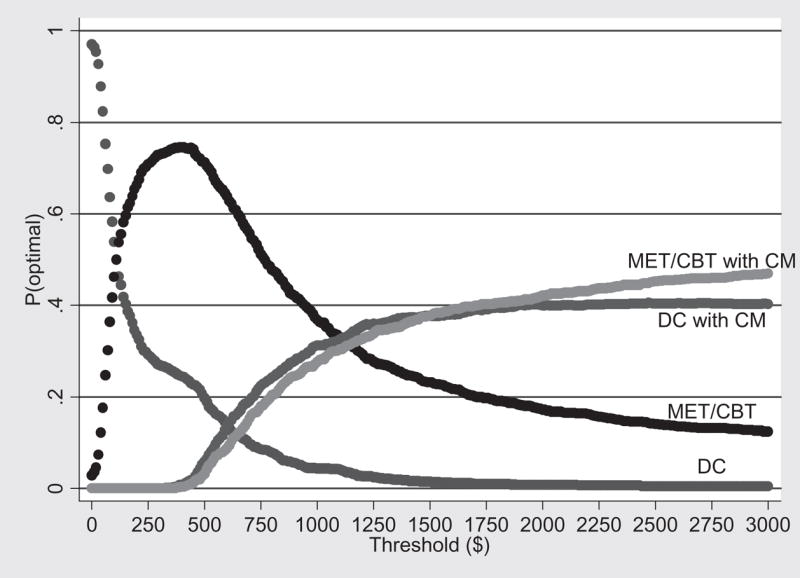
Acceptability curves for longest duration abstinent during treatment (LDA)—base case. DC = individual drug counseling; MET/CBT = motivational enhancement therapy/cognitive-behavioral therapy; CM = contingency management
Figure 2.
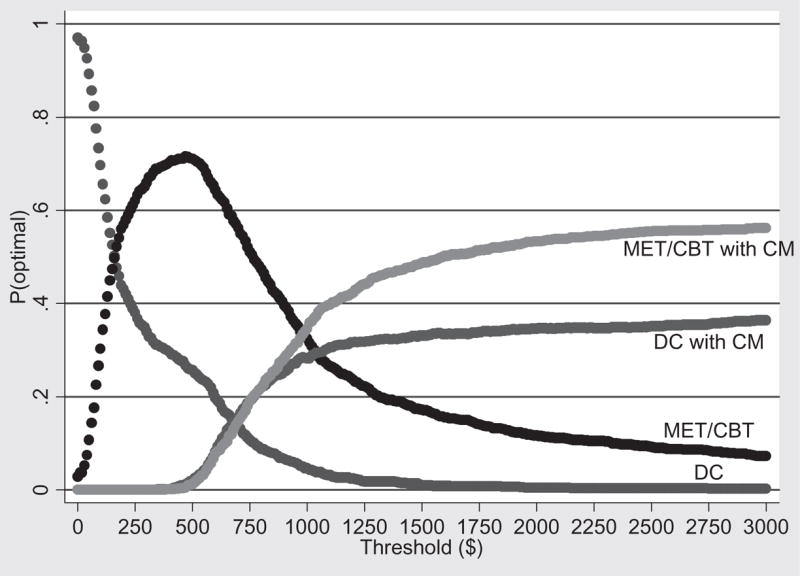
Acceptability curves for marijuana-free urine samples submitted during treatment—base case. DC = individual drug counseling; MET/CBT = motivational enhancement therapy/cognitive-behavioral therapy; CM = contingency management
Robustness checks: sensitivity analysis and LDA during follow-up
To determine how the ICERs and the acceptability curves would probably change had the trial been implemented under alternative realistic conditions, we conducted a sensitivity analysis in which we modeled an ‘alternative implementation scenario’ that made different assumptions from the base case about (i) the unit cost of urine sample test cups, (ii) the amount of overhead included in labor costs, (iii) the unit cost of administering the voucher system and (iv) the average salaries of staff conducting the urine tests and administering the voucher system.
The test cups used in the effectiveness study (i.e. the base case) cost $9.46 apiece and were capable of detecting amphetamine, methamphetamine, cocaine, tetrahydrocannabinol (THC), and morphine (Varian OnTrak Testcup 5). However, inasmuch as marijuana was the only target drug of the study, in the alternative scenario we assumed that less expensive test cups capable of detecting THC only and costing $1.72 apiece (Varian OnTrak Testcup) were used instead.
In the base case, labor costs were multiplied by the clinic overhead rate to account for long-term costs that might vary due to additional staff required by the CM and MET/CBT interventions. In practice, it is unlikely that all elements of overhead would vary proportionally with staff (e.g. clinic director, water for the lawn, etc.). In the alternative scenario, we assumed that labor costs were multiplied by only 75% (as opposed to 100%) of the overhead rate.
The costs of administering the voucher system (filling out voucher-related paperwork, purchasing voucher items selected by participants) were spread out over a relatively small number of participants. If the CM interventions had been implemented on a larger scale—only a small fraction of clinic patients were enrolled in the trial—then the clinic would be able to take advantage of economies of scale in running the voucher system, thereby reducing the ICERs of the two CM interventions. In the alternative scenario, we assumed that the unit voucher administration costs would be 25% lower than in the base case due to economies of scale from implementing CM on a larger scale. Finally, in the base case, all urine tests and voucher-related tasks were assumed to be done by the clinic counselors. In the alternative scenario, we assumed that the clinic would hire less-expensive personnel (with salaries of approximately two-thirds that of the counselors) to handle these activities.
Table 3 presents the average variable cost per participant in the alternative scenario. The ICERs in the alternative scenario are as follows: compared to usual care, the incremental cost of using MET/CBT to lengthen the LDA by 1 week was $77 (i.e. $238–$191/3.08–2.47); compared to MET/CBT, the incremental cost of using DC with CM to lengthen the LDA by 1 week was $766; and, compared to DC with CM, the incremental cost of using MET/CBT with CM to lengthen the LDA by 1 week was $942. The corresponding ICERs for the patient outcome number of marijuana-free urine samples are as follows: compared to usual care, the incremental cost of using MET/CBT to obtain an additional marijuana-free urine sample was $121; and, compared to MET/CBT, the incremental cost of using MET/CBT with CM to obtain an additional marijuana-free urine sample was $656 (DC with CM was still extended dominated for the outcome number of marijuana-free urine samples). Finally, Fig. 3 shows the acceptability curves for the patient outcome LDA in the alternative scenario.
Table 3.
Average variable cost per participant in each treatment arm—alternative scenario.*
| DC (n = 32) ($) | MET/CBT (n = 32) ($) | DC and CM (n = 32) ($) | MET/CBT and CM (n = 33) ($) | |
|---|---|---|---|---|
| Counseling | 159 (77) | 198 (83) | 205 (82) | 213 (94) |
| Testing | ||||
| Materials | 10 (6) | 12 (6) | 13 (6) | 12 (7) |
| Time | 22 (16) | 28 (16) | 29 (15) | 28 (17) |
| Subtotal | 32 (22) | 40 (21) | 42 (21) | 40 (23) |
| Vouchers | ||||
| Earnings | 0 | 0 | 335 (251) | 399 (279) |
| Administration | 0 | 0 | 192 (n/a) | 235 (n/a) |
| Subtotal | 0 | 0 | 527 (251) | 634 (279) |
| Total | 191 (96) | 238 (101) | 774 (326) | 887 (397) |
DC = individual drug counseling; MET/CBT = motivational enhancement therapy/cognitive-behavioral therapy; CM = contingency management.
Values represent means and standard deviations (in parentheses). n/a: not applicable.
Figure 3.
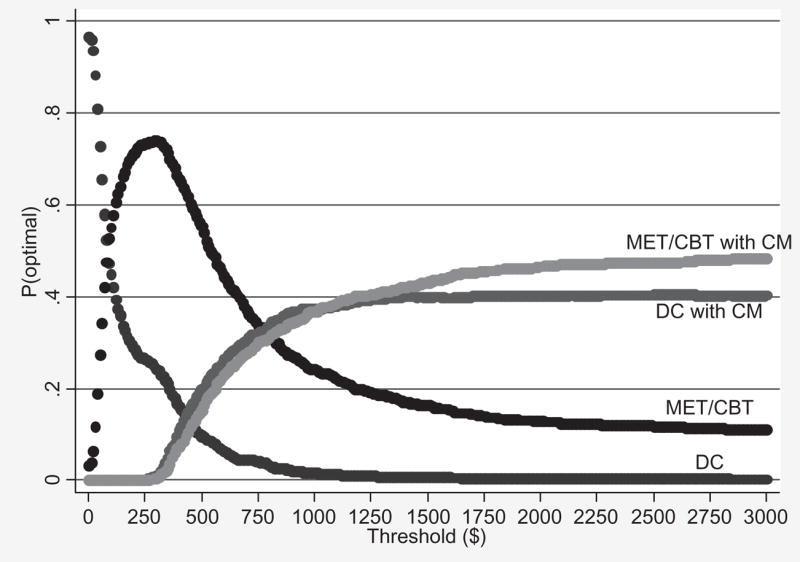
Acceptability curves for longest duration abstinent during treatment (LDA)—alternative scenario. DC = individual drug counseling; MET/CBT = motivational enhancement therapy/cognitive-behavioral therapy; CM = contingency management
To determine how the relative cost-effectiveness of the four interventions might change for patient outcomes measured beyond the 8-week treatment period, we calculated ICERs and acceptability curves for the longest duration of abstinence from marijuana that occurred during the 6-month follow-up period (LDA–FU). As reported in the effectiveness study [5], across outcomes, MET/CBT was associated with continued gains during the follow-up period while participants assigned to DC faired comparatively poorly. The LDA–FU was estimated using self-reports of daily marijuana use collected at 1, 3 and 6 months post-treatment using the TLFB, a reliable and valid method for assessing substance use on a day-to-day basis [28,29]. Eight of the 129 participants in the main cost-effectiveness study had no follow-up data, so analyses using LDA–FU were based on a sample of 121 participants.
Adjusting for baseline marijuana use, the average LDA–FU was 10.58 weeks, 9.56 weeks, 9.22 weeks and 7.73 weeks for participants assigned to MET/CBT with CM, MET/CBT, DC with CM and DC, respectively. Note that DC with CM was strictly dominated by MET/CBT for the outcome LDU–FU (i.e. MET/CBT was both less expensive and more effective than DC with CM). Accordingly, the ICERs for the outcome LDA–FU are as follows: compared to usual care, the incremental cost of using MET/CBT to lengthen the LDA–FU by 1 week was $34; and, compared to MET/CBT, the incremental cost of using MET/CBT with CM to lengthen the LDA–FU by 1 week was $915. Finally, Fig. 4 shows the acceptability curves for the patient outcome LDA–FU.
Figure 4.
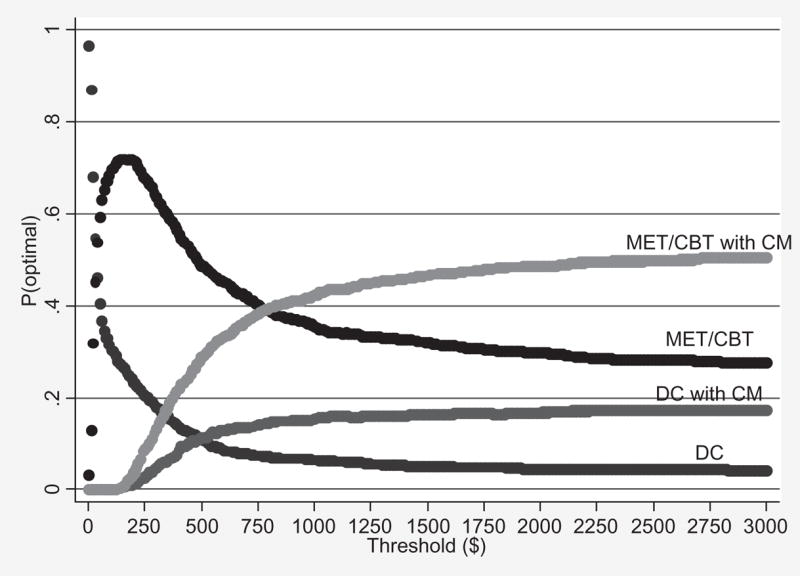
Acceptability curves for longest duration abstinent during 6-month follow-up (LDA–FU). DC = individual drug counseling; MET/CBT = motivational enhancement therapy/cognitive-behavioral therapy; CM = contingency management
DISCUSSION
The present study analyzed the cost-effectiveness of four interventions for treating young adults with marijuana dependence. The most cost-effective intervention is that intervention with the largest ICER that falls below the threshold value placed by decision makers on an additional unit of effect for a given patient outcome [38]. Thus, determining which intervention is the most cost-effective for improving patient outcomes depends on the existence of threshold values against which to compare the ICERs (one threshold value for each patient outcome). In the absence of consensus threshold values for substance use outcomes, we present ranges of values, defined by the ICERs for each patient outcome, over which each intervention would be considered cost-effective compared to the others. For example, we find that if the threshold value of extending the LDA by 1 week is less than $102, then DC is the most cost-effective of the interventions. However, if the value of extending the LDA by 1 week lies between $102 and $1104, between $1104 and $1333 or is greater than $1333, then the most cost-effective intervention is MET/CBT, DC with CM and MET/CBT with CM, respectively.
To illustrate the decision uncertainty in the cost-effectiveness analysis, we present acceptability curves (one set of curves for each patient outcome) that show the probability that each intervention is the most cost-effective for any given threshold value. Intuitively, as seen in Figs 1–4, as the threshold value of an additional unit of a given patient outcome increases, the treatment that produces the largest effect (i.e. MET/CBT with CM) becomes increasingly more likely to be the most cost-effective, even though it adds incremental costs. Similarly, as the threshold value of an additional unit of a given patient outcome decreases, the treatment that has the lowest cost (i.e. DC) becomes increasingly more likely to be the most cost-effective.
The results of the two robustness checks (i.e. the sensitivity analysis and the patient outcome LDA–FU) were consistent with our main findings. Specifically, the following general pattern of the relative cost-effectiveness of the four interventions emerged in all of the analyses: DC was the most cost-effective intervention for very low threshold values of an additional unit of effect for each of the patient outcomes, then MET/CBT was the most cost-effective intervention over a very wide range of threshold values, then DC with CM was either optimal over a very narrow range of threshold values or not at all, and finally MET/CBT with CM was the most cost-effective intervention for relatively high threshold values.
Our findings highlight the need for the substance abuse treatment field to develop consensus threshold values for policy relevant treatment outcomes. For example, although MET/CBT alone was somewhat less effective than either DC or MET/CBT delivered in combination with CM, it was also considerably less expensive than both of them. As a result, MET/CBT is likely to be the most cost-effective intervention over a wide range of threshold values for both an additional week of LDA ($102–$1104) and an additional marijuana-free urine specimen ($159–$942). On the other hand, given that marijuana is considered widely to be a gateway drug, if decision makers believe that improving treatment outcomes in young adults with marijuana dependence will impact long-term substance abuse significantly (e.g. several studies have shown that longer periods of continuous abstinence during treatment are associated with better long-term outcomes [34–36]), then they may be willing to pay thousands of dollars to extend the LDA by 1 week or to obtain an additional marijuana-free urine specimen. In this case, MET/CBT with CM is likely to be the most cost-effective intervention.
We acknowledge that developing such threshold values will be challenging, inasmuch as such an endeavor would require (i) identifying and quantifying the links between a given treatment outcome (e.g. an additional week of LDA) and the associated benefits to patients and society (e.g. increase in patient quality of life, reduction in crime, reduction in spread of disease, increase in work-place productivity, etc.) and (ii) monetizing the estimated benefits. Moreover, it is not yet obvious for which treatment outcomes consensus threshold values should be developed. For example, although quality adjusted life years (QALYs) are common in many health-care applications, they are not well-suited for research in substance abuse treatment because they ignore the large negative externalities associated with substance abuse (e.g. crime, spread of disease, welfare). Nevertheless, relevant consensus threshold values would be helpful in determining which interventions can be considered the most cost-effective in a given application.
The present study has several strengths. First, it is based on a randomized clinical trial that relied on objective indicators of patient outcomes and paid careful attention to internal validity via use of manualized interventions and independent confirmation of the fidelity of treatment delivery [5]. Thus, the patient outcomes seen are likely to be related to the treatments delivered. Secondly, it relied on cost data collected from the clinic where the trial took place. Thirdly, in an advance over other cost-effectiveness studies in the substance abuse treatment field, we present ranges of values, for each patient outcome, over which each intervention would be considered cost-effective compared to the others. Fourthly, we rigorously address the statistical uncertainty inherent in the ICERs through the use of acceptability curves for each patient outcome that show the probability that each intervention is the most cost-effective for any given threshold value. Fifthly, we examined the robustness of the results by (i) conducting sensitivity analyses on several key cost parameters and (ii) analyzing the longest duration of marijuana abstinence that occurred during the 6-month follow-up period (i.e. LDA–FU). Finally, the ICERs estimated in the present study can be used as thresholds for future studies.
There are also several limitations. First, given the relatively small and specialized nature of our study sample (i.e. marijuana-dependent young adults, all referred by the criminal justice system) and the fact that we examined a CM procedure with a single reinforcement schedule, additional studies are warranted to determine the reliability and generalizability of our results. Thus, it would be premature to use data from this study to make policy decisions. Secondly, although the cost data were collected from the clinic where the trial took place, they were not collected simultaneously with the effectiveness measures. That is, we relied on a retrospective reporting of treatment costs. Thirdly, LDA–FU was based on self-reported data collected via the TLFB. Although TLFB is a reliable and valid method for assessing substance use on a day-to-day basis [28,29], participant abstinence estimated during the follow-up period (via TLFB) may not be as accurate as participant abstinence estimated during the treatment period (via urinalysis). Fourthly, we do not know whether the ICERs would change significantly had the treatment period extended beyond 8 weeks. Finally, our cost estimates include only those costs that vary by treatment condition. Hence, they do not measure the total cost of the interventions and are thus not useful for making comparisons to other ‘total cost’ estimates in the literature.
In conclusion, this study uses incremental cost-effectiveness ratios and acceptability curves to shed light on the relative cost-effectiveness of four interventions for treating young adults with marijuana dependence. As such, it adds to the growing literature on cost-effectiveness in substance abuse treatment by providing one of the first cost-effectiveness analyses of interventions for individuals with marijuana dependence, and by serving as a template for incorporating uncertainty into future cost-effectiveness studies of substance abuse trials comparing multiple treatment arms.
Acknowledgments
Support for this study was provided by NIDA grants K05-DA00457, P50-DA09241, R01-DA14471 and the US Department of Veterans Affairs VISN 1 Mental Illness Research, Education and Clinical Center (MIRECC). We wish to thank Karen Hunkele, Sally Vitolo and the clinical and research staff at the Substance Abuse Treatment Unit in New Haven, Connecticut for their help in implementing the trial and collecting the cost data.
References
- 1.Substance Abuse and Mental Health Services Administration. Results from the 2004 National Survey on Drug Use and Health: National Findings (Office of Applied Studies, NSDUH Series H-28, DHHS Publication No. SMA 05-4062) Rockville, MD: US Department of Health and Human Services; 2005. [Google Scholar]
- 2.Chen K, Kandel DB. The natural history of drug use from adolescence to the mid-thirties in a general population sample. Am J Public Health. 1995;85:41–7. doi: 10.2105/ajph.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellickson PL, Martino SC, Collins RL. Marijuana use from adolescence to young adulthood: multiple developmental trajectories and their associated outcomes. Health Psychol. 2004;23:299–307. doi: 10.1037/0278-6133.23.3.299. [DOI] [PubMed] [Google Scholar]
- 4.Windle M, Wiesner M. Trajectories of marijuana use from adolescence to young adulthood: predictors and outcomes. Dev Psychopathol. 2004;16:1007–27. doi: 10.1017/s0954579404040118. [DOI] [PubMed] [Google Scholar]
- 5.Carroll KM, Easton CJ, Nich C, Hunkele KA, Neavins TM, Sinha R, et al. The use of contingency management and motivational/skills-building therapy to treat young adults with marijuana dependence. J Consult Clin Psychol. 2006;74:955–66. doi: 10.1037/0022-006X.74.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MTP Research Group. Brief treatments for cannabis dependence: findings from a randomized multisite trial. J Consult Clin Psychol. 2004;72:455–66. doi: 10.1037/0022-006X.72.3.455. [DOI] [PubMed] [Google Scholar]
- 7.Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. J Consult Clin Psychol. 2000;68:1051–61. doi: 10.1037//0022-006x.68.6.1051. [DOI] [PubMed] [Google Scholar]
- 8.Commission on Adolescent Substance and Alcohol Abuse. Treatment of substance use disorders. In: O’Brien CP, editor. Treating and Preventing Adolescent Mental Health Disorders: What We Know and What We Don’t Know. New York: Oxford University Press; 2005. pp. 391–410. [Google Scholar]
- 9.Deas D, Thomas SE. An overview of controlled studies of adolescent substance abuse treatment. Am J Addict. 2001;10:178–89. doi: 10.1080/105504901750227822. [DOI] [PubMed] [Google Scholar]
- 10.Santisteban DA, Coatsworth JD, Perez-Vidal A, Mitrani V, Jean-Gilles M, Szapocznik J. Engaging behavior problem/drug abusing youth and their families in treatment: a replication and further exploration of the factors that contribute to differential effectiveness. J Fam Psychol. 1996;10:35–44. [Google Scholar]
- 11.Sinha R, Easton C, Kemp K. Substance abuse treatment characteristics of probation-referred young adults in a community-based outpatient program. Am J Drug Alcohol Abuse. 2003;29:585–97. doi: 10.1081/ada-120023460. [DOI] [PubMed] [Google Scholar]
- 12.Carroll KM, Rounsaville BJ. Bridging the gap between research and practice in substance abuse treatment: a hybrid model linking efficacy and effectiveness research. Psychiatr Serv. 2003;54:333–9. doi: 10.1176/appi.ps.54.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dennis M, Godley SH, Diamond G, Tims FM, Babor T, Donaldson J, et al. The Cannabis Youth Treatment (CYT) Study: main findings from two randomized trials. J Subst Abuse Treat. 2004;27:197–213. doi: 10.1016/j.jsat.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 14.French MT, Roebuck MC, Dennis ML, Godley SH, Liddle HA, Tims FM. Outpatient marijuana treatment for adolescents: economic evaluation of a multisite field experiment. Eval Rev. 2003;27:421–59. doi: 10.1177/0193841X03254349. [DOI] [PubMed] [Google Scholar]
- 15.Olmstead T, Sindelar J, Petry N. Cost-effectiveness of prize-based incentives for stimulant abusers in outpatient psychosocial treatment programs. Drug Alcohol Depend. 2007;87:175–82. doi: 10.1016/j.drugalcdep.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sindelar J, Elbel B, Petry N. What do we get for our money? Cost-effectiveness of adding contingency management. Addiction. 2007;102:309–16. doi: 10.1111/j.1360-0443.2006.01689.x. [DOI] [PubMed] [Google Scholar]
- 17.Barnett PG, Zaric GS, Brandeau ML. The cost-effectiveness of buprenorphine maintenance therapy for opiate addiction in the United States. Addiction. 2001;96:1267–78. doi: 10.1046/j.1360-0443.2001.96912676.x. [DOI] [PubMed] [Google Scholar]
- 18.French MT, Mausopf JA, Teague JL, Roland J. Estimating the dollar value of health outcomes from drug abuse interventions. Med Care. 1996;34:890–910. doi: 10.1097/00005650-199609000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Sindelar JL, Jofre-Bonet M, French MT, McLellan TA. Cost-effectiveness analysis of treatments for illicit drug dependence: paradoxes with multivariate outcomes. Drug Alcohol Depend. 2004;73:41–50. doi: 10.1016/j.drugalcdep.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Jofre-Bonet M, Sindelar JL. Creating an aggregate outcome index: cost-effectiveness analysis of substance abuse treatment. J Behav Health Serv Res. 2004;31:229–41. doi: 10.1007/BF02287287. [DOI] [PubMed] [Google Scholar]
- 21.Cartwright WS. Cost benefit and cost-effectiveness analysis of drug abuse treatment services. Eval Rev. 1998;22:609–36. doi: 10.1177/0193841X9802200503. [DOI] [PubMed] [Google Scholar]
- 22.Cartwright WS. Cost-benefit analysis of drug treatment services: review of the literature. J Ment Health Policy Econ. 2000;3:11–26. doi: 10.1002/1099-176x(200003)3:1<11::aid-mhp66>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Baker SM. Twelve Step Facilitation Therapy for Drug Abuse and Dependence. New Haven: Yale University PDC; 1998. [Google Scholar]
- 24.Mercer DE, Woody GE. An Individual Drug Counseling Approach to Treat Cocaine Addiction: The Collaborative Cocaine Treatment Study Model. Rockville, MD: NIDA; 1999. [Google Scholar]
- 25.Steinberg K, Roffman RA, Carroll KM, McRee B, Babor TF, Miller M, et al. Brief Counseling for Marijuana Dependence: A Manual for Treating Adults. Rockville, Maryland: Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration; 2005. [Google Scholar]
- 26.Carroll KM, Ball SA, Nich C, O’Connor PG, Eagan D, Frankforter TL, et al. Targeting behavioral therapies to enhance naltrexone treatment of opioid dependence: efficacy of contingency management and significant other involvement. Arch Gen Psychiatry. 2001;58:755–61. doi: 10.1001/archpsyc.58.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carroll KM, Sinha R, Nich C, Babuscio T, Rounsaville BJ. Contingency management to enhance naltrexone treatment of opioid dependence: a randomized clinical trial of reinforcement magnitude. Exp Clin Psychopharmacol. 2002;10:54–63. doi: 10.1037//1064-1297.10.1.54. [DOI] [PubMed] [Google Scholar]
- 28.Babor TF, Steinberg K, Anton RF, Del Boca FK. Talk is cheap: measuring drinking outcomes in clinical trials. J Stud Alcohol. 2000;61:55–63. doi: 10.15288/jsa.2000.61.55. [DOI] [PubMed] [Google Scholar]
- 29.Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J Consult Clin Psychol. 2000;68:134–44. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- 30.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV, Patient Edition. Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- 31.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 32.Drummond MF, O’Brien B, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programs. 2. Oxford, UK: Oxford University Press; 1997. [Google Scholar]
- 33.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. Oxford, UK: Oxford University Press; 1996. [Google Scholar]
- 34.Higgins ST, Badger GJ, Budney AJ. Initial abstinence and success in achieving longer-term cocaine abstinence. Exp Clin Psychopharmacol. 2000;8:377–86. doi: 10.1037//1064-1297.8.3.377. [DOI] [PubMed] [Google Scholar]
- 35.Higgins ST, Sigmon SC, Wong CJ, Heil SH, Badger GJ, Donham R, et al. Community reinforcement therapy for cocaine-dependent outpatients. Arch Gen Psychiatry. 2003;60:1043–52. doi: 10.1001/archpsyc.60.9.1043. [DOI] [PubMed] [Google Scholar]
- 36.Carroll KM, Rounsaville BJ, Nich C, Gordon LT, Wirtz PW, Gawin FH. One year follow-up of psychotherapy and pharmacotherapy for cocaine dependence: delayed emergence of psychotherapy effects. Arch Gen Psychiatry. 1994;51:989–97. doi: 10.1001/archpsyc.1994.03950120061010. [DOI] [PubMed] [Google Scholar]
- 37.Briggs A. Handling uncertainty in economic evaluation and presenting the results. In: Drummond M, McGuire A, editors. Economic Evaluation in Health Care: Merging Theory with Practice. Oxford, UK: Oxford University Press; 2001. pp. 172–214. [Google Scholar]
- 38.Fenwick E, Claxton K, Schulpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ. 2001;10:779–87. doi: 10.1002/hec.635. [DOI] [PubMed] [Google Scholar]
- 39.Lothgren M, Zethraeus N. Definition, interpretation and calculation of cost-effectiveness acceptability curves. Health Econ. 2000;9:623–30. doi: 10.1002/1099-1050(200010)9:7<623::aid-hec539>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 40.Briggs A, O’Brien B. The death of cost-minimization analysis? Health Econ. 2001;10:179–84. doi: 10.1002/hec.584. [DOI] [PubMed] [Google Scholar]
- 41.Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford, UK: Oxford University Press; 2007. pp. 198–202. [Google Scholar]


