Summary
In vivo and in vitro studies indicate that a sub-population of human marrow-derived stromal cells (MSCs, a.k.a. mesenchymal stem cells) has potential to differentiate into multiple cell types, including osteoblasts. In this study, we tested the hypotheses that there are intrinsic effects of age in human MSCs (17 to 90 years). We tested the effect of age on senescence-associated β-galactosidase (SA-β-gal), proliferation, apoptosis, p53 pathway genes, and osteoblast differentiation in confluent monolayers by alkaline phosphatase activity and osteoblast gene expression analysis. There were 4-fold more hMSCs positive for SA-β-gal in samples from older than younger subjects (p<0.001, n=17). Doubling time of hMSCs was 1.7-fold longer in cells from the older than the younger subjects and was positively correlated with age (p=0.002, n=19). Novel age-related changes were identified. With age, more cells were apoptotic (p=0.016, n=10). Further, there were age-related increases in expression of p53 and its pathway genes, p21 and BAX. Consistent with other experiments, there was a significant age-related decrease in generation of osteoblasts both in the STRO-1+ cells (p=0.047, n=8) and in adherent MSCs (p<0.001, n=10). In sum, there is an age-dependent decrease in proliferation and osteoblast differentiation and an increase in SA-β-gal-positive cells and apoptosis in hMSCs. Upregulation of the p53 pathway with age may have a critical role in mediating the reduction in both proliferation and osteoblastogenesis of hMSCs. These findings support the view that there are intrinsic alterations in human MSCs with aging that may contribute to the process of skeletal aging in humans.
Kew words: Aging, mesenchymal stem cells, marrow stromal cells, proliferation, apoptosis, p53, osteoblast differentiation
Introduction
In humans, peak bone mass is attained during the third decade of life. Subsequently, bone mass declines slowly with advancing age (Mautalen & Oliveri, 1999). Although there is currently intense activity to define the process of acute bone loss associated with sex steroid deficiency and development of osteoporosis, there is little information about the mechanism(s) by which the aging process influences bone loss. A better understanding of age-related changes in cells and tissues may offer new approaches to mitigate or avoid loss of bone with aging.
Recent findings suggest that a decline in the numbers or potential of stem cell populations in adult organs may contribute to human aging and age-related disease (Rao & Mattson, 2001; Van Zant & Liang, 2003; Carrington, 2005). Human adult bone marrow-derived mesenchymal stem cells, or marrow stromal cells (MSCs), have been shown to be precursors of several different cellular lineages, including osteoblast, chondrocyte, myoblast, adipocyte, and fibroblast (Prockop 1997; Pittenger et al., 1999; Krebsbach et al., 1999; Caterson et al., 2002). It has been shown with mouse marrow cells that there are age-associated differences in osteoblast differentiation and signaling (Kahn et al., 1995; Moerman et al., 2004). Less is known about the effects of age on the capacity of human marrow-derived cells for self-renewal and osteoblast differentiation. Some data from colony assays with marrow aspirates conflict about the effects of age on osteoblast potential of adult human marrow (Majors et al., 1997; Oreffo et al., 1998a&b; D’Ippolito et al., 1999; Nishida et al., 1999; Muschler et al., 2001; Stenderup et al., 2001; Justesen et al., 2002). Using molecular measures, we found an age-related decline in osteogenic potential of hMSCs from men (Mueller & Glowacki, 2001). In this study, we tested the hypotheses that there are intrinsic effects of aging on the biology of hMSCs. We tested the effect of age on proliferation, cell cycling, apoptosis, senescence-associated β-galactosidase (SA-β-gal), and osteoblast differentiation.
RESULTS
Yield of STRO-1+ Cells from Human Marrow Mononuclear Cells (MMCs)
Sixteen samples (42 to 75 years of age) of low-density MMCs were sorted for STRO-1+ cells by flow cytometry. The mean percent yield of STRO-1+ cells was 0.062% ± 0.038. There was no significant difference in % yield of STRO-1+ cells with age of the subjects (Spearman correlation, r=−0.297, p=0.242) (Fig. 1).
Figure 1.
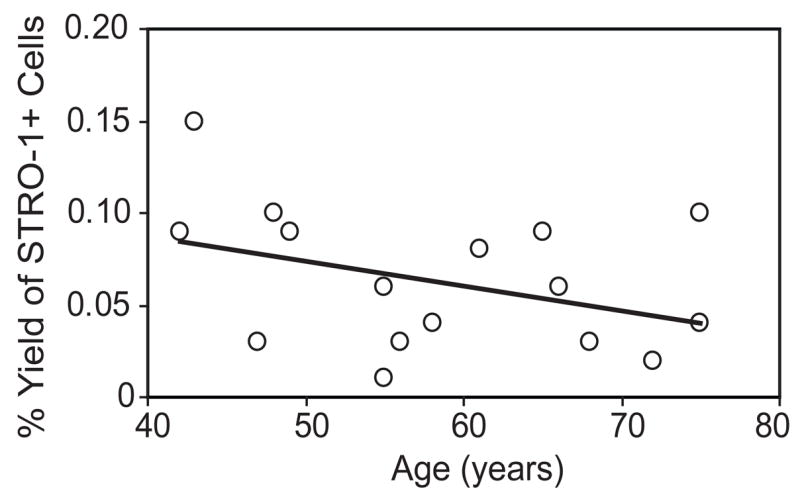
Yield of STRO-1+ cells in human marrow. Low-density mononuclear cells were sorted for STRO-1 by FACS. With the number available, there was no apparent relationship between percentage yield of STRO-1+ human Marrow Mononuclear Cells and age (n=16).
Effects of Age on Senescence-Associated β-Galactosidase in hMSCs
Seventeen samples of hMSCs (passage 2, 17 to 90-years old) were assayed for percent of cells positive for SA-β-gal (Fig. 2). These data suggest that there may not be a linear increase in positive cells with age, but rather a threshold age of its appearance. Frequency of hMSCs positive for SA-β-gal was 4-fold greater in the group ≥55 years (9.1±3.0, n=12) than in the group younger than 50 years (2.3 ± 1.8, n=5, p<0.001, non-parametric Mann-Whitney test) (Fig. 2 Inset).
Figure 2.
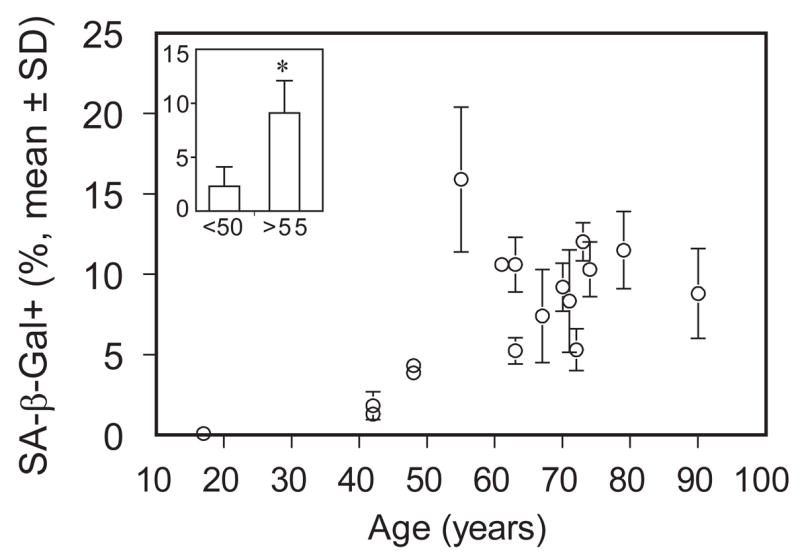
Effect of age on senescence-associated β-galactosidase (SA-β-gal)-positive human marrow stromal cells (hMSCs). hMSCs isolated from 17 subjects were stained for SA-β-gal. Value of percent positive cells is expressed for each subject as mean ± SD with 4 replicates. Inset: Frequency of hMSCs positive for SA-β-galactosidase was 4-fold higher in the group of subjects older than 55 years (n=12) than in the group younger than 50 years (n=5) (p<0.001, Mann-Whitney test).
Effects of Age on Proliferation of hMSCs
Population kinetics were evaluated with short-term cultures of freshly isolated hMSCs obtained from young (<50-year-old, n=3) and older (≥55-year-old, n=16) subjects. Cell expansion was slower in cells from the older subjects (Fig. 3A). Cell population doubling time (CPDT) was 1.7-fold longer in cells from the older (76.1 ± 23.4 hrs) than the younger (44.0 ± 7.7 hrs) subjects (p=0.0021, Mann-Whitney test) (Fig. 3B inset) and was positively correlated with age (Spearman r = 0.62, p=0.005, n=19) (Fig. 3B).
Figure 3.
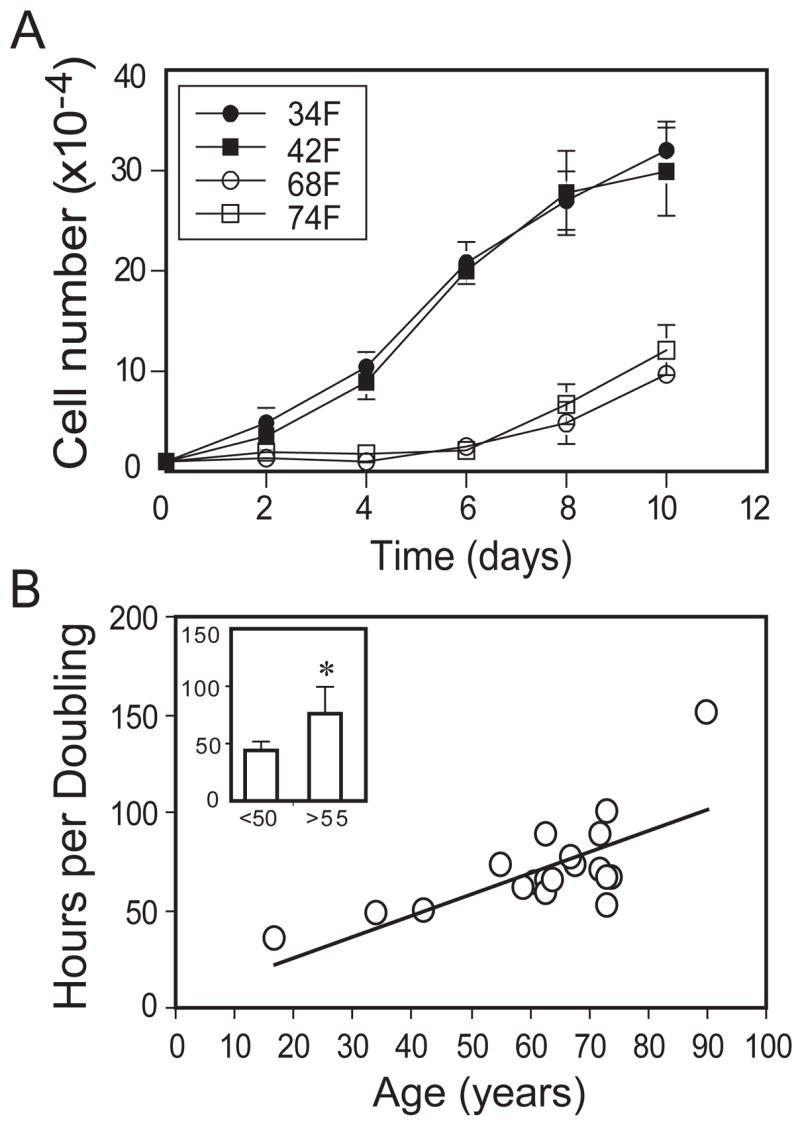
Effect of age on proliferation of human marrow stromal cells (hMSCs). (A): Proliferation rate was determined by counting cells with a hemacytometer. The hMSCs isolated from younger subjects (< 50 years) expanded more rapidly than hMSCs obtained from older (≥55 years) subjects (only 4 shown here for legibility). (B): Cell population doubling time (hours) of hMSCs obtained from all 19 subjects showed a significant correlation with age (r=0.62, p=0.005, Spearman rank order correlation). Inset: hMSCs isolated from 16 older subjects had a significantly longer cell doubling time than cells obtained from 3 younger subjects (p=0.0021, Mann-Whitney test).
Cell Cycle and Effect of Age on Apoptosis of hMSCs
To determine whether the age-related increase in doubling time results from disproportionately prolonged dwelling in a specific phase(s) of the cell cycle, we subjected cells from 10 subjects age 17 to 90 years to flow cytometric analysis of distribution in Go/G1, S, and G2/M phase. On average, 91.3 ± 0.5% of the cells were in G0/G1 with 6.2 ± 0.5% in S phase and 1.8 ± 0.2% of the cells in G2/M phase, with no effect of age apparent with the numbers available. These preliminary data suggest that with age there is a uniform prolongation of the duration of all phases of the cycle. There was a range of 0.2 to 4.2% apoptotic cells, with a significant increase with age (r=0.719, p=0.016, Spearman rank order correlation test) (Fig. 4).
Figure 4.
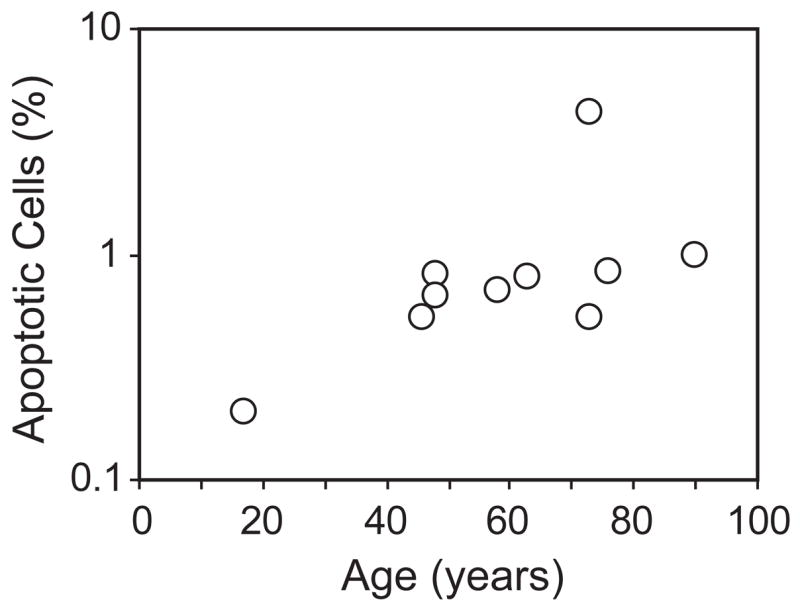
Effects of age on apoptosis of human marrow stromal cells. Cells from 10 subjects were analyzed with flow cytometric analysis for apoptotic cells. There was a significant increase of apoptotic cells with age (r=0.719, p = 0.0157, Spearman rank order correlation test).
Effects of Age on p53 Pathway in hMSCs
Human MSCs (passage 2) were cultured in MEM-α with 10% FBS-HI and antibiotics. Upon reaching 50% confluence, total RNA was isolated with TRIZOL reagents. To test the effects of age on p53 pathway gene expression, we examined the expression of p53, p21, and BAX by semi-quantitative RT-PCR for hMSCs from 12 subjects (17 to 90-years old) (Fig. 5A). There was significantly greater expression (Fig. 5B) of p53 (2.3-fold, p<0.01, Mann-Whitney test), p21 (2.1-fold, p<0.01), and BAX (2.2-fold, p<0.05) in hMSCs obtained from older subjects (n=9, ≥55-years) compared with younger subjects (n=3, <50-years). Analyses of correlations between p53 expression and each of those two target genes showed significance with p21 (Spearman r=0.846, p=0.0009) and BAX (Spearman r=0.587, p=0.049).
Figure 5.
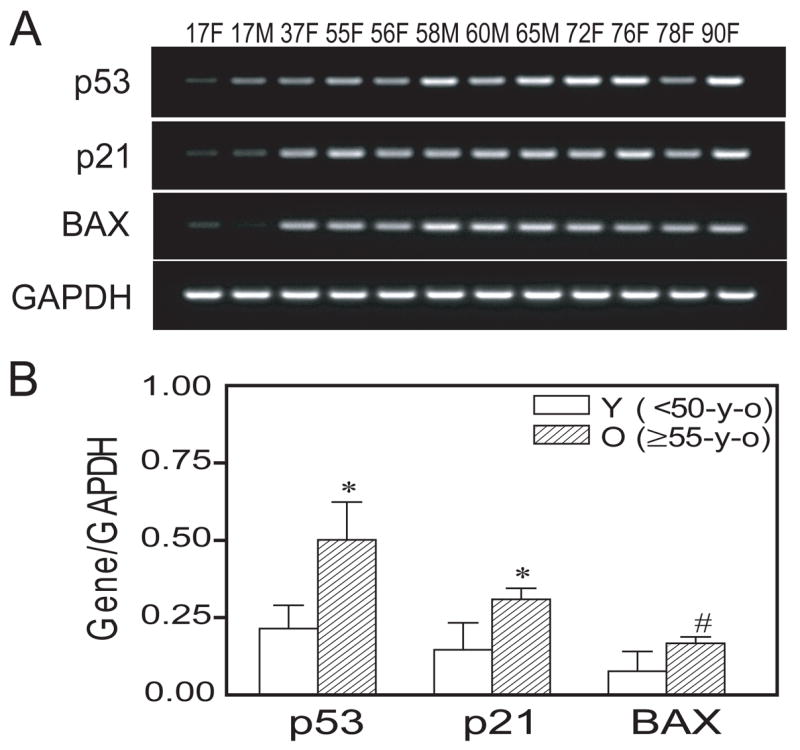
Effect of age on p53 pathway genes in human marrow stromal cells (hMSCs). (A): An age-related increase in p53, p21, and BAX gene expression was detected by RT-PCR. (B): Semi-quantitative RT-PCR analysis indicates significantly more expression of p53, p21, and BAX genes (normalized to internal control GAPDH) in hMSCs obtained from older subjects (n=9, ≥55-years), compared with hMSCs obtained from younger subjects (n=3, <50-years) (*p<0.01 and #p<0.05, Mann-Whitney test). Each lane indicates age and gender of the subject.
Effects of Age on Osteoblastogenesis in hMSCs and STRO-1+ MMCs
After hMSCs (n=10, 17 to 90-years) were cultured for 2 weeks in osteoblastogenic medium, alkaline phosphatase (AlkP) enzymatic activity was measured as an index of osteoblast differentiation. There was a significant decrease of AlkP activity with age (Spearman r= −0.82, p<0.001)(Fig. 6A). In addition, semi-quantitative RT-PCR analysis (Fig. 6B) showed significantly greater expression of osteoblast marker genes Cbfa1/Runx2, Osterix, AlkP, BSP (bone sialoprotein), and OC (Osteocalcin) in hMSCs obtained from younger subjects (n=3, <50-years), compared with hMSCs from older subjects (n=7, ≥55-years (p<0.05, Mann-Whitney test). There was no significant difference in COL I gene expression, a marker for fibroblast and osteoblast phenotypes.
Figure 6.
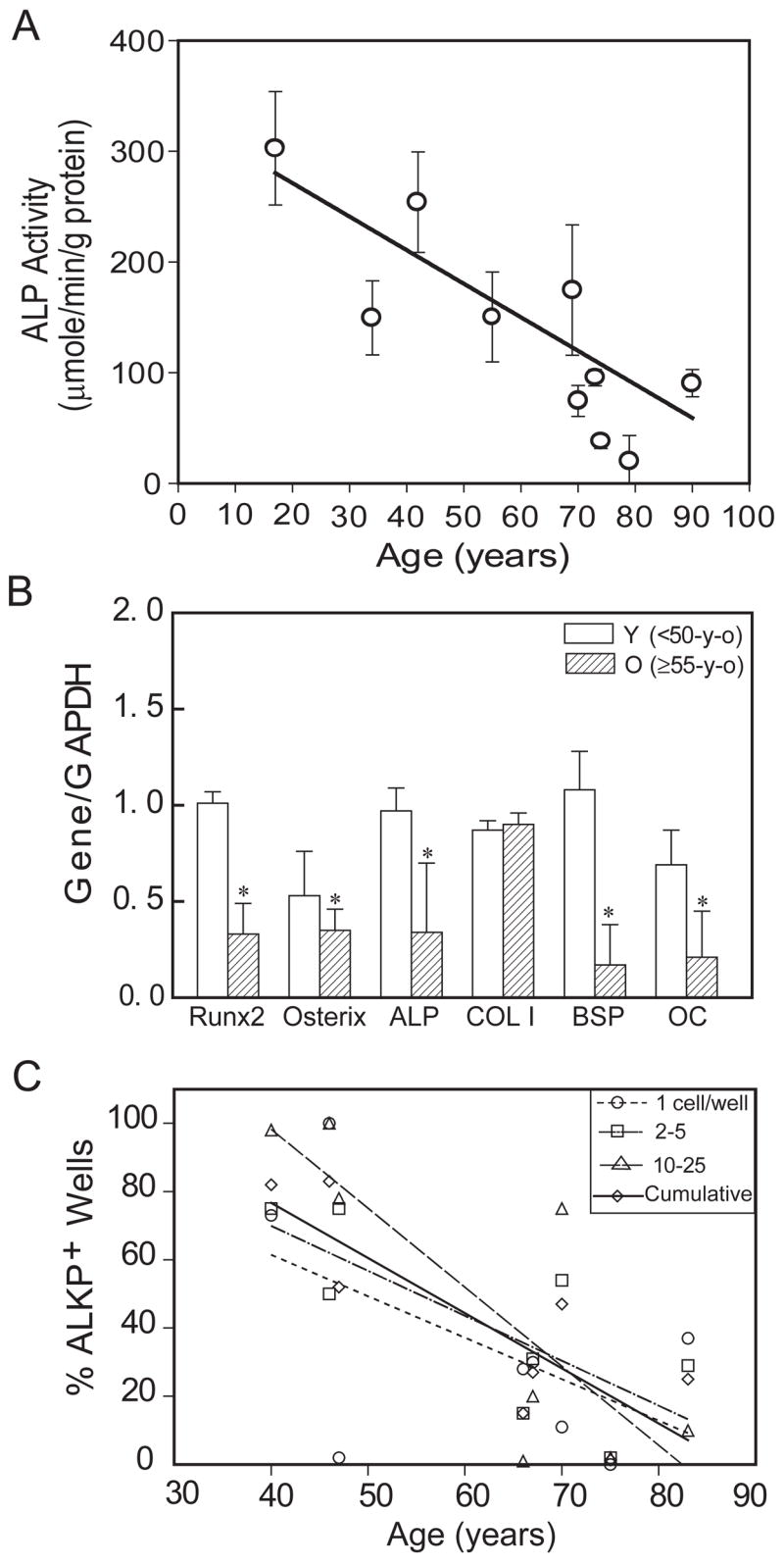
Effects of age on osteoblastogenesis. (A): Osteoblast differentiation of human marrow stromal cells (hMSCs) was assessed with AlkP enzyme activity assays, after 2 weeks’ culture in osteogenic medium. There was a significant decrease of AlkP activity with age (Spearman correlation, r= −0.82, p<0.001) in hMSCs obtained from 10 women. (B): Semi-quantitative RT-PCR showed that there is significantly less expression of Runx2, Osterix, AlkP, BSP, and OC genes (normalized to internal control GAPDH) in hMSCs obtained from old subjects (n=7, ≥55-year-old) compared with hMSCs obtained from younger subjects (n=3, <50-year-old) (p<0.05, Mann-Whitney test). (C): Osteoblast differentiation of STRO-1+ cells from 8 men was assessed by fluorescent immunoreactivity for Alkaline Phosphatase, 4 weeks after seeding at 1 to 50 cells per well in osteogenic medium. Data are presented as the % of wells at each seeding density with clear FITC signal that was above background and control levels. There was an age-dependent decrease in cells that differentiated into AlkP-positive osteoblasts (Spearman correlation, r= −0.714, p=0.0466, n=8 for cumulative data).
In addition, STRO-1+ human marrow mononuclear cells (n=8, 40 to 83-years old) were assessed for differentiation to osteoblasts 4 weeks after seeding at very low densities, from 1 to 50 cells per well. There was a striking age-dependent decrease in the percent of the sorted cells that differentiated into AlkP-positive osteoblastic cells (r= −0.714, p=0.047 for cumulative data)(Fig. 6C).
Discussion
Several intrinsic properties of human MSCs show effects related to the age of the person from whom the cells were obtained. Stem and progenitor cells are located throughout the adult body and are believed to be involved in continuous maintenance and repair of tissues (Fehrer & Lepperdinger, 2005). Questions arise to what extent adult mesenchymal stem/stromal cells are either subject to, or causes of aging; and whether age-related changes in those cells are due to intrinsic factors or are induced by the extrinsic somatic environment, for example by declines in circulating hormones. Mesenchymal stem cells or marrow stromal cells (MSCs) derived from various sources such as bone marrow or fat can differentiate in vitro into osteoblasts, chondroblasts, adipocytes, myoblasts, and fibroblasts (Prockop 1997; Pittenger et al., 1999; Krebsbach et al., 1999; Caterson et al., 2002). The relationship between age-related skeletal diseases, such as osteoporosis and osteoarthritis, and aging of MSCs is not yet well documented.
To determine whether there are intrinsic, age-related changes of hMSCs in vivo, we evaluated either freshly-isolated marrow cells or stromal cells at very early passage. This approach was used to avoid changes in cell behaviors that are associated with prolonged culture, such as in vitro senescence or culture stress. Like other normal mammalian cells, such as human osteoblasts (Kassem et al., 1997), when cultured for many passages, MSCs display what is termed “in vitro senescence”, i.e. decreased proliferation, replicative quiescence, enlargement, increase in SA-β-gal activity, and erosion of telomeres (reviewed in Fehrer & Lepperdinger, 2005 and Sethe et al., 2006). There is controversy to what degree such in vitro changes recapitulate organismal aging (Bird et al., 2003; Herbig et al., 2006). The results obtained herein, however, should reflect the effects of in vivo aging because cells from young and old individuals were treated the same way and evaluated upon isolation or at early passage.
Age and proliferation
There were striking age-dependent decreases in proliferation and osteoblast differentiation and increases in SA-β-gal activity, apoptosis, and p53 pathway genes in hMSCs obtained from individuals aged 17 to 90 years. Understanding the normal processes of cell replacement may be crucial to understanding the imbalance between bone destruction and bone formation in the aging individual. Stem and progenitor cell dynamics may be the determinant of whether tissues show effects of aging that take place at the cellular level (Carrington, 2005). In this study of short-term culture of freshly isolated cells, the doubling time of hMSCs was longer for cells from the older than the younger subjects and was correlated with age. Theoretically, the prolonged doubling time could have been due, for example, to a prolonged G1 or G2 arrest with fewer numbers of cells entering S or M, respectively. Because there was no detectable difference in the distribution of cells in each phase of the cell cycle, however, those pilot data suggest that aging may uniformly increase the duration of all phases of cell cycle. The significant increase in apoptotic cells in MSCs obtained from older subjects suggests that loss of cells may contribute to the differences in population kinetics. Alternately, the cells from elders may have been less stimulated by, or more sensitive to harmful elements in fetal calf serum or to other stressful aspects of cell isolation or culture. Another study with marrow aspirates showed that MSCs from young subjects had more population doublings in long-term culture than did the MSCs from older subjects (Stenderup et al., 2003; Kassem, 2006).
Consistent with age-related decreased proliferation and increased apoptosis of hMSCs, there was an age-related increase in p53 and its targets p21 and BAX, which are known to mediate those cellular functions respectively. p53 is known to play critical roles in senescence and apoptotic responses to dysfunctional telomeres (Artandi & Attardi, 2005) and genotoxic stress (Helmbold et al., 2006). Once activated, p53 induces p21 and BAX, which mediate different aspects of p53 action on senescence and apoptosis, respectively (Vousden & Lu, 2002; Artandi & Attardi, 2005). Our data for age-related increases in p53, p21, and BAX gene expression in hMSCs suggest that upregulation of the p53/p21 pathway may regulate age-related decreases in proliferation, and the p53/BAX pathway may mediate age-related increases in apoptosis. Further, finding correlations between p53 and each of those target genes suggests constitutive relationships in this pathway even after isolation of the cells.
In this study, there was a higher frequency of SA-β-gal positive cells in human MSCs isolated from older subjects, compared with younger ones. This marker is best know for skin fibroblasts; SA-β-gal positive fibroblasts accumulate with age in skin samples and have been shown to produce degradative enzymes and inflammatory cytokines, which can disrupt tissue organization and function (Campisi, 1998 & 2005). A previous small study with marrow aspirates showed no effect of age on SA-β-gal, although all specimens showed the expected increase with time in culture (Stenderup et al., 2003). In that study, SA-β-gal was measured after considerable expansion of the stromal cells, i.e., “to less than 50% of in vitro life-span”. Those cultures from young and older donors showed 17% and 16% SA-β-gal-positive cells, values much greater than reported here. It is possible that those findings reflect in vitro abolishment of detectable differences. Although SA-β-gal is widely used in studies of in vitro replicative senescence and of pathological tissues, finding an age-associated increase in this marker in early passage human MSCs extend its utility as a correlate for in vivo age. There is a potential theoretical limitation of using surgical discarded tissue for studying normal aging because of a possible systemic impact of the diagnosis of osteoarthritis, but this has been minimized by studying marrow from young and old subjects with similar stage of advanced joint disease and by excluding comorbidities and medications that may confound interpretation. We consider finding an age-related increase in Senescence-Associated β-galactosidase-positive cells in hMSCs as evidence in support of using orthopedic surgical discarded tissue for studies of aging. Scarcity of positive cells in samples from younger subjects (< 50 years of age) indicates that this marker is unrelated to the diagnosis of osteoarthritis and is related to age of the subject.
Age and osteoblast differentiation
There are apparently conflicting data about the effect of age on osteoblast potential of human marrow-derived cells (reviewed in Mueller & Glowacki, 2001). Finding either no effect or an age-related decline in osteoblast potential may in part be attributable to use of marrow aspirates, frozen cells, growth factor-supplemented media, and use of different assays for the number of colonies that stain for alkaline phosphatase. Colony assays have inherent inaccuracies regarding the threshold volume of each counted colony, intensity of stain, and problems with necrosis in cultures of longer duration. In addition, studies that use colony size or number have been criticized for inappropriate use of parametric statistical methods (Dobson et al., 1999). We previously used a 3-dimensional culture system and quantitative RT-PCR assays to measure generation of alkaline phosphatase-positive osteoblasts from human marrow from men and found a striking age-dependent decline (Mueller & Glowacki, 2001).
Human MSCs isolated from marrow aspirates by different sampling methods for small amounts of marrow may result in heterogeneity of the cell population, which affects in vitro cell behavior (Siddappa et al., 2007). A more recent review concluded that differences in isolation methods (whole marrow, low-density fraction, adherent stroma), sources (biopsies and necropsies), anatomical sites, colony assay protocols, and ambiguous terminology confound integration of the literature and resolution of apparently conflicting conclusions (Sethe et al., 2006).
The studies reported herein provide assessment of osteoblast potential without the confounders present with colony assays or due to expansion needed with small samples of cells. The use of all the bone discarded from each subject during orthopedic surgery ensures a large enough population of marrow cells to minimize sampling heterogeneity. Further, early evidence of osteoblastogenesis was measured. In addition, to avoid confounding effects of proliferation and differential responses to serum growth factors, osteoblast differentiation assays were always done with confluent, contact-inhibited cultures in osteogenic medium with low serum supplementation. Using the same conditions for culture, treatment, and analysis of hMSCs from groups of younger and older subjects, we report a significant age-related decrease of alkaline phosphatase activity and immunoreactivity as well as bone marker genes, such as Cbfa1/Runx2, Osterix, AlkP, BSP, and OC, in hMSCs after culture in osteogenic medium. Although there is heterogeneity in hMSC cultures (Phinney et al., 1999), our samples of bone marrow obtained from each subject’s whole femoral head provides large numbers of bone marrow mononuclear cells for testing reproducibility of the findings (as many at 800 million low-density cells).
Our experiments with microwell cultures of STRO-1+ marrow cells also showed a striking age-dependent decrease in differentiation into AlkP-positive osteoblastic cells. The observation that the number of STRO-1+ cells in the low-density mononuclear fraction of fresh marrow did not decline with age are supported by other studies of osteogenic stem cells (Stenderup et al., 2001) and muscle precursor cells (Conboy et al., 2003). Although the number of STRO-1+ cells may not decrease with age, their potential for osteoblastic differentiation does. Thus, our cumulative experiments with three different assays for osteoblastic differentiation, including a three-dimensional culture system (Mueller & Glowacki, 2001), all indicate that there is an age-related decline of osteoblastogenesis in human marrow cells. MSCs are characterized by the expression of numerous surface antigens, but none of them appears to be exclusively expressed on MSCs (Bobis et al., 2006). Antibodies against STRO-1 bind to stromal cell precursors and also to nucleated erythroid precursors (Simmons & Torok-Storb, 1991). An exclusive cell surface marker for MSCs will be critical for studies of age-related changes of MSCs in vivo.
Thus, some data reported here were obtained from 2D culture with assays of alkaline phosphatase enzyme activity and of osteoblast gene expression; other data were obtained from microwell cultures of STRO-1+ cells and fluorescence detection of antibodies against alkaline phosphatase. Use of those different methods strengthens the conclusions from the literature that support the view that human marrow-derived cells show an age-related decrease in osteoblast potential.
Very recent information from mouse studies indicates that p53 is a negative regulator of osteoblast differentiation. Unlike information with established cancer cell lines that show p53 suppression of proliferation and stimulation of differentiation, p53 is a negative regulator of osteoblast differentiation in vitro and in vivo (Wang et al., 2006; Lengner et al., 2006). Those observations with developing mice may apply more generally as our data with human MSCs show an age-related increase in p53 and decrease in osteoblast differentiation.
Conclusions
The age-related changes in human MSCs described here for proliferation, SA-β-gal, expression of p53, p21, and BAX, and osteoblast potential add to other age-related properties we previously reported for similar marrow samples. Marrow-derived hMSCs also show an age-related increase in constitutive in vitro secretion of the osteolytic cytokines interleukin-6 and -11 (Cheleuitte et al., 1998), increase in magnitude of interleukin-1β-stimulated secretion of those cytokines (Cheleuitte et al., 1998), increase in constitutive secretion of IGF-binding protein-3 (Rosen et al., 1997), and decrease in expression of the osteoclastogenesis-inhibitory factor, osteoprotegerin (Makhluf et al., 2000). Taken together with the finding of an age-related increase in osteoclastogenesis in human marrow cultures (Glowacki, 1995), those findings and the results reported herein support the hypothesis that human marrow cells and their products can contribute to skeletal aging by decreasing renewal of bone-forming osteoblasts and increasing the generation of bone-resorbing osteoclasts.
In conclusion, there were age-related decreases in proliferation and osteoblast differentiation in human MSCs and increases in apoptosis and in percent of SA-β-gal-positive cells. Upregulation of the p53 pathway with age may have a critical role in mediating the reduction in both proliferation and osteoblastogenesis of hMSCs. To our knowledge, this is the first report of age-related increases in p53, its targets p21 and BAX, and apoptosis in human MSCs. These findings open new avenues for research on the underlying mechanisms of human skeletal aging. Use of several different measures with monolayer cultures in these studies adds new evidence to the controversy about the effects of age on osteoblast differentiation; these studies reinforce the view that there is an age-related decline in osteoblast differentiation in human marrow stromal cells. These results support the conclusion that there are intrinsic alterations in human MSCs with aging that may explain skeletal cellular and tissue aging in humans.
Experimental Procedures
Subjects
Bone marrow samples were obtained with IRB approval as femoral tissue discarded during primary hip arthroplasty for osteoarthritis, i.e., erosion of the articular cartilage that results in pain and reduced joint mobility. Although the prevalence of osteoarthritis increases with age, there are younger people with this diagnosis who require the same surgery, in which femoral bone and marrow are removed in order for the surgeon to implant the femoral component of the joint prosthesis. Thus, we identified from the surgical schedule a wide range of ages of subjects with similar stage of advanced hip osteoarthritis in order to minimize any possible impact of the joint disease on effects of age on marrow. Criteria for exclusion are rheumatoid arthritis, cancer, and other comorbid conditions that may influence skeletal metabolism, i.e. renal insufficiency, alcoholism, active liver disease, malabsorption, hyperthyroidism, ankylosing spondylitis, aseptic necrosis, hyperparathyroidism, morbid obesity, and diabetes. Also excluded were patients who were taking medications that may influence skeletal metabolism (e.g. thyroid hormone, glucocorticoids, NSAIDs, and bisphosphonates). A total 57 subjects, 33 women and 24 men, age from 17 to 90-year-old was included in this study. Of them, 17 subjects were classified for convenience as younger (≤50-years, mean 41±10 years) and 40 were classified as older (≥55-years, mean 67±8 years). Not all specimens could be included in every experiment, due to the surgical schedule and numbers of cells needed for each assay. In each experiment, standardized conditions were used for all samples, e.g. early cell passage, identical medium, serum, and regents. For cell cycle, RNA, and alkaline phosphatase assays, cells obtained from different subjects were cultured, treated, and stored for analysis at the same time to avoid technical differences between assays.
Preparation of hMSCs
Low-density marrow mononuclear cells were isolated by density centrifugation on Ficoll/Histopaque 1077 (Sigma, MO) (Cheleuitte et al., 1998; Zhou et al., 2005b). This procedure enriches for undifferentiated cells. Low-density marrow mononuclear cell preparations include a population of non-adherent hematopoietic cells and a fraction capable of adherence and differentiation into musculoskeletal cells. Adherent human MSCs were expanded in 2-D monolayer culture with phenol red-free α-MEM medium, 10% Fetal Bovine Serum-Heat Inactivated (FBS-HI), 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen, Carlsbad, CA).
STRO-1+ human bone MMCs
For some experiments, STRO-1+ cells were isolated from the low-density mononuclear cell suspensions with monoclonal anti-STRO-1 primary antibody (Mouse IgM anti-human marrow stromal progenitor cells obtained from Dr. Paul J. Simmons, Adelaide, Australia) (Simmons & Torok-Storb, 1991) and flow cytometric sorting. Samples from 12 men and 4 women between 40 and 83 years of age, containing more than approximately 10 million cells were divided into three groups (unstained control or for incubation with either STRO-1 antibody or isotype-matched control antibody, Mouse IgM, BD Pharmingen, San Diego, CA, USA) for 15 minutes at 4° C. Following 2 washes with phosphate buffered saline (PBS) with 2% FBS, the samples were incubated with FITC-labeled goat-anti-mouse IgM antibody (Jackson Immuno Research Labs, Inc, Westgrove, PA) for an additional 15 minutes at 4° C. The STRO-1+ cells were isolated with a Mo-Flo high-speed sorter (DAKO, Fort Collins, CO). Positive populations were enumerated by morphology (forward scatter for size and side scatter for granularity) and by level of fluorescence greater than autofluorescence of unstained cells and than nonspecific fluorescence of the isotype matched control as described (Simmons & Torok-Storb, 1991). Percent STRO-1 yield was calculated for each sample. For assays of osteoblast potential, STRO-1+ human bone marrow mononuclear cells from 8 men were plated in 1% gelatin coated Terasaki microwell plates (72-well plates) at densities ranging from 1 to 50 cells per well. Cells were cultured for 4 weeks in medium supplemented with 10 nM dexamethasone, 5 mM β-glycerophosphate, and 50 μg/mL ascorbate-2-phosphate. On day 28, viable cells in all wells were counted and stained for alkaline phosphatase as follows. After wells were rinsed, 7μL of monoclonal anti-bone alkaline phosphatase (B4–78 hybridoma, DSHB, University of Iowa) or NS-1 normal serum (negative control) was added and incubated for 20 minutes. Cells were rinsed thrice with PBS and 2% BSA and were incubated with goat anti-mouse FITC-conjugated secondary antibody (Cappel, Solon, OH) for 20 minutes. Following three rinses with PBS and 2% BSA, the cells were scored using fluorescence microscopy. Wells were scored as alkaline phosphatase-positive if they contained cells with clear FITC signal that was above background and control levels. Data are normalized as percent of numbers of wells with viable cells.
Staining of cells for SA-β-gal activity
Human MSCs from 17 subjects (17 to 90 years old; 14 women, 3 men) at passage 2 were seeded at 4×104 cells in each of four 35-mm dishes (5,000 cells/cm2). After 4 days in α-MEM with 10% FBS-HI, cells were stained for senescence-associated β-galactosidase (Dimri et al., 1995). In brief, the cells were rinsed twice with PBS solution, fixed for 5 minutes in 3% formaldehyde, and rinsed with PBS. Two mL staining solution was added to each 35 mm dish. After overnight incubation at 37°C, the staining solution was removed, the cells were rinsed with PBS, and a 25-mm coverslip was placed in each dish with glycerol vinyl alcohol mounting solution (Zymed, South San Francisco, CA). The number of stained cells and the total number of cells were enumerated with bright light and phase microscopy, respectively. Values are expressed as percentages of cells that were stained blue.
Proliferation
Human MSCs at passage 2 were seeded at 1×104 cells in each of three 35-mm dishes for each time point (0, 2, 4, 6, 8, 10 days) in α-MEM with 10% FBS-HI. Cells were suspended with 0.5 ml of 0.05% trypsin-EDTA (Invitrogen) and cell number was determined by hemacytometer. Cell population doubling time (CPDT) was calculated with the formula, CPDT=(t-t0)×log2×(log[N/N0])−1, with t as time and N as cell number.
Cell cycle and apoptosis analysis
Cell cycle distribution was measured by flow analysis for DNA content (Epperly et al., 1999). Cells (hMSCs, passage 2) at approximately 80% confluence were trypsinized, combined with the floating cells, centrifuged for 10 min at 1500 rpm, washed 3 times in PBS, and resuspended in 1 mL of ice cold 70% ethanol added drop-wise with mixing. The cells were stored for at least 24 hours at −20° C. The cells were centrifuged for 5 min at 3000 rpm and the supernatant was partially decanted, with approximately 200 μL of 70% ethanol retained with the pellet. The cells were vortexed and 1 ml of staining solution (50 μg/ml propidium iodide, 0.1 mg/mL RNase A in a solution of 1 g glucose in 1000 mL PBS) was added. The cells were incubated at room temperature for 30 min and stored at 4°C until analysis with a Mo-Flo high-speed sorter (DAKO, Fort Collins, CO). Modfit Software (Verity Software House, Topsham, ME) was used to express the data as the percent of cells in phases of the cell cycle, G0/G1, S, and G2/M, and cells that were apoptotic.
Conditions for osteoblast differentiation
For each sample to be assayed for Alkaline Phosphatase activity, 2×104 cells/well were seeded in triplicate in 12-well-plates in α-MEM with 10% FBS-HI until confluence; this required different times depending upon rates of proliferation. Therafter, cultures were changed to osteogenic medium (α-MEM with 1% FBS-HI, 100 U/mL penicillin, 100 μg/mL streptomycin plus 10 nM dexamethasone, 5 mM β-glycerophosphate, 50 μg/mL ascorbate-2-phosphate) for 14 days. Reduction of serum to 1% for differentiation was designed to minimize possible differences in proliferation that could confound interpretation of effects of age on osteoblastogenesis. For each sample to be assayed for osteogenic gene expression by RT-PCR, cells were cultured in 100-mm dishes in α-MEM with 10% FBS-HI. Upon confluence, medium was changed into osteogenic medium for 14 days.
RNA isolation and RT-PCR
Total RNA was isolated from human MSCs with Trizol reagent (Invitrogen). For RT-PCR, 2 μg of total RNA was reverse-transcribed into cDNA with SuperScript II (Invitrogen), following the manufacturer’s instructions. One-tenth or one-twentieth of the cDNA was used in each 50 μL PCR reaction (30–40 cycles of 94° C for 1 minute, 55–60° C for 1 minute, and 72° C for 2 minutes) as described (Zhou et al., 2005b). The gene-specific primers for human p53 (Vakifahmetoglu et al., 2006); p21 (Lohr et al., 2003); BAX (Tirado et al., 2005); Cbfa1/RUNX2, Osterix, and bone sialoprotein (D’Ippolito et al., 2006); AlkP (Winn et al., 1999); COL I and Osteocalcin (Lomri et al., 1999) were used for amplification. Gene expression levels were measured by semi-quantitative PCR. PCR products were quantitated by densitometry of captured gel images with KODAK Gel Logic 200 Imaging System and measured by KODAK Molecular Imaging Software, following the manufacturer’s instructions (KODAK, Molecular Imaging Systems, New Haven, CT, USA). Quantitative data were expressed by normalizing the densitometric units to GAPDH (internal control).
AlkP enzyme assay
Alkaline phosphatase (AlkP) enzyme activity was measured in 10 samples (9 women, 1 man; 17 to 90-years old. Dishes were rinsed with PBS, 200 μL of lysis buffer was added, and the plates were stored at −80° C until assays could be done together (Zhou et al., 2005a)
Statistical analyses
All experiments were performed at least in triplicate. Group data are presented as mean values ± SD. Quantitative data were analyzed with non-parametric tools, either the Mann-Whitney test for group comparisons or Spearman correlation test. A value of p<0.05 was considered significant.
Acknowledgments
This study was presented in part at the 27th ASBMR annual meeting, 2005, in Nashville, TN and 2006 AIMM/ASBMR meeting at Snowmass, CO. The authors greatly appreciate help from I. Amato, K. D. Johnson, N. A. Glass, A. Tilt, and Dr. S. Mizuno for aspects of these experiments. This study was supported by grants from the National Institutes of Health R01 AG 025015 and R01 AG 028114. The discarded marrow was obtained and studied with approval and annual review from the Partners Human Research Committee.
References
- Artandi SE, Attardi LD. Pathways connecting telomeres and p53 in senescence, apoptosis, and cancer. Biochem Biophys Res Commun. 2005;331:881–890. doi: 10.1016/j.bbrc.2005.03.211. [DOI] [PubMed] [Google Scholar]
- Bobis S, Jarocha D, Majka M. Mesenchymal stem cells: characteristics and clinical applications. Folia Histochem Cytobiol. 2006;44:215–230. [PubMed] [Google Scholar]
- Bird J, Ostler EL, Faragher RG. Can we say that senescent cells cause ageing? Exp Gerontol. 2003;38:1319–1326. doi: 10.1016/j.exger.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Campisi J. The role of cellular senescence in skin aging. J Investig Dermatol Symp Proc. 1998;3:1–5. [PubMed] [Google Scholar]
- Campisi J. Senescent cells, tumor suppression, and organismal aging: Good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Carrington JL. Aging bone and cartilage: cross-cutting issues. Biochem Biophys Res Commun. 2005;328:700–708. doi: 10.1016/j.bbrc.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Caterson EJ, Nesti LJ, Danielson KG, Tuan RS. Human marrow-derived mesenchymal progenitor cells: isolation, culture expansion, and analysis of differentiation. Mol Biotech. 2002;20:245–256. doi: 10.1385/MB:20:3:245. [DOI] [PubMed] [Google Scholar]
- Cheleuitte D, Mizuno S, Glowacki J. In vitro secretion of cytokines of human bone marrow: effects of age and estrogen status. J Clin Endo Metab. 1998;83:2043–2051. doi: 10.1210/jcem.83.6.4848. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- Dimri GP, Lee Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campisi J. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115–1122. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- D’Ippolito G, Diabira S, Howard GA, Roos BA, Schiller PC. Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone. 2006;39:513–522. doi: 10.1016/j.bone.2006.02.061. [DOI] [PubMed] [Google Scholar]
- Dobson K, Reading L, Scutt A. A cost-effective method for the automatic quantitative analysis of fibroblastic colony-forming units. Calcif Tissue Int. 1999;65:166–172. doi: 10.1007/s002239900677. [DOI] [PubMed] [Google Scholar]
- Epperly MW, Bray JA, Carlos TM, Prochownik E, Greenberger JS. Biology of Marrow Stromal Cell lines Derived from Long-Term Bone Marrow Cultures of Trp53-Deficient Mice. Radiation Research. 1999;152:29–40. [PubMed] [Google Scholar]
- Fehrer C, Lepperdinger G. Mesenchymal stem cell aging. Exp Gerontol. 2005;40:926–930. doi: 10.1016/j.exger.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Glowacki J. Influence of age on human marrow. Calcif Tissue Int. 1995;56S:50–51. [Google Scholar]
- Helmbold H, Deppert W, Bohn W. Regulation of cellular senescence by Rb2/p130. Oncogene. 2006;25:5257–5262. doi: 10.1038/sj.onc.1209613. [DOI] [PubMed] [Google Scholar]
- Herbig U, Ferreira M, Condel L, Carey D, John M, Sedivy JM. Cellular Senescence in Aging Primates. Science. 2006;311:1257. doi: 10.1126/science.1122446. [DOI] [PubMed] [Google Scholar]
- Kahn A, Gibbons R, Perkins S, Gazit D. Age-related bone loss. A hypothesis and initial assessment in mice. Clin Orthop Relat Res. 1995;313:69–75. [PubMed] [Google Scholar]
- Kassem M, Ankersen L, Eriksen EF, Clark BF, Rattan SI. Demonstration of cellular aging and senescence in serially passaged long-term cultures of human trabecular osteoblasts. Osteoporos Int. 1997;7:514–524. doi: 10.1007/BF02652556. [DOI] [PubMed] [Google Scholar]
- Kassem M. Stem cells: potential therapy for age-related diseases. Ann N Y Acad Sci. 2006;1067:436–442. doi: 10.1196/annals.1354.062. [DOI] [PubMed] [Google Scholar]
- Krebsbach PH, Kuznetsov SA, Bianco P, Robey PG. Bone marrow stromal cells: characterization and clinical application. Crit Rev Oral Biol Med. 1999;10:165–181. doi: 10.1177/10454411990100020401. [DOI] [PubMed] [Google Scholar]
- Lengner CJ, Steinman HA, Gagnon J, Smith TW, Henderson JE, Kream BE, Stein GS, Lian JB, Jones SN. Osteoblast differentiation and skeletal development are regulated by Mdm2-p53 signaling. J Cell Biol. 2006;172:909–921. doi: 10.1083/jcb.200508130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löhr K, Möritz C, Contente A, Dobbelstein M. p21/CDKN1A mediates negative regulation of transcription by p53. J Biol Chem. 2003;278:32507–32516. doi: 10.1074/jbc.M212517200. [DOI] [PubMed] [Google Scholar]
- Lomri A, Fromigue O, Hott M, Marie PJ. Genomic insertion of the SV-40 large T oncogene in normal adult human trabecular osteoblastic cells induces cell growth without loss of the differentiated phenotype. Calcif Tissue Int. 1999;64:394–401. doi: 10.1007/pl00005821. [DOI] [PubMed] [Google Scholar]
- Justesen J, Stenderup K, Eriksen EF, Kassem M. Maintenance of osteoblastic and adipocytic differentiation potential with age and osteoporosis in human marrow stromal cell cultures. Calcif Tissue Int. 2002;71:36–44. doi: 10.1007/s00223-001-2059-x. [DOI] [PubMed] [Google Scholar]
- Majors AK, Boehm CA, Nitto H, Midura RJ, Muschler GF. Characterization of human bone marrow stromal cells with respect to osteoblastic differentiation. J Orthop Res. 1997;15:546–557. doi: 10.1002/jor.1100150410. [DOI] [PubMed] [Google Scholar]
- Makhluf HA, Mueller SM, Mizuno S, Glowacki J. Age-related decline in osteoprotegerin expression by human bone marrow cells cultured in three-dimensional collagen sponges. Biochem Biophys Res Comm. 2000;268:669–672. doi: 10.1006/bbrc.2000.2182. [DOI] [PubMed] [Google Scholar]
- Mautalen CA, Oliveri B. Densitometric manifestations in age-related bone loss. In: Rosen C, Glowacki J, Bilezikian JP, editors. The Aging Skeleton. San Diego: Academic Press; 1999. pp. 263–276. [Google Scholar]
- Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004;3:379–89. doi: 10.1111/j.1474-9728.2004.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SM, Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem. 2001;82:583–590. doi: 10.1002/jcb.1174. [DOI] [PubMed] [Google Scholar]
- Muschler GF, Nitto H, Boehm CA, Easley KA. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res. 2001;19:117–125. doi: 10.1016/S0736-0266(00)00010-3. [DOI] [PubMed] [Google Scholar]
- Nishida S, Endo N, Yamagiwa H, Tanizawa T, Takahashi HE. Number of osteoprogenitor cells in human bone marrow markedly decreases after skeletal maturation. J Bone Miner Metab. 1999;17:171–177. doi: 10.1007/s007740050081. [DOI] [PubMed] [Google Scholar]
- Oreffo RO, Bord S, Triffitt JT. Skeletal progenitor cells and ageing human populations. Clin Sci. 1998a;94:549–555. doi: 10.1042/cs0940549. [DOI] [PubMed] [Google Scholar]
- Oreffo RO, Bennett A, Carr AJ, Triffitt JT. Patients with primary osteoarthritis show no change with ageing in the number of osteogenic precursors. Scand J Rheumatol. 1998b;27:415–424. doi: 10.1080/030097498442235. [DOI] [PubMed] [Google Scholar]
- Oreffo RO. Growth factors for skeletal reconstruction and fracture repair. Curr Opin Investig Drugs. 2004;5:419–423. [PubMed] [Google Scholar]
- Phinney DG, Kopen G, Righter W, Webster S, Tremain N, Prockop DJ. Donor variation in the growth properties and osteogenic potential of human marrow stromal cells. J Cell Biochem. 1999;75:424–436. [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Rao MS, Mattson MP. Stem cells and aging: expanding the possibilities. Mech Ageing Dev. 2001;122:713–734. doi: 10.1016/s0047-6374(01)00224-x. [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Verault D, Steffens C, Cheleuitte D, Glowacki J. Effects of age and estrogen status on the skeletal IGF regulatory system: Studies with human marrow. Endocrine. 1997;7:77–80. doi: 10.1007/BF02778068. [DOI] [PubMed] [Google Scholar]
- Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev. 2006;5:91–116. doi: 10.1016/j.arr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Siddappa R, Licht R, van Blitterswijk C, de Boer J. Donor variation and loss of multipotency during in vitro expansion of human mesenchymal stem cells for bone tissue engineering. J Orthop Res. 2007;25:1029–1041. doi: 10.1002/jor.20402. [DOI] [PubMed] [Google Scholar]
- Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- Stenderup K, Justesen J, Eriksen EF, Rattan SI, Kassem M. Number and proliferative capacity of osteogenic stem cells are maintained during aging and in patients with osteoporosis. J Bone Miner Res. 2001;16:1120–1129. doi: 10.1359/jbmr.2001.16.6.1120. [DOI] [PubMed] [Google Scholar]
- Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–926. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Tirado OM, Mateo-Lozano S, Notario V. Rapamycin induces apoptosis of JN-DSRCT-1 cells by increasing the Bax: Bcl-xL ratio through concurrent mechanisms dependent and independent of its mTOR inhibitory activity. Oncogene. 2005;24:3348–3357. doi: 10.1038/sj.onc.1208471. [DOI] [PubMed] [Google Scholar]
- Vakifahmetoglu H, Olsson M, Orrenius S, Zhivotovsky B. Functional connection between p53 and caspase-2 is essential for apoptosis induced by DNA damage. Oncogene. 2006;25:5683–5692. doi: 10.1038/sj.onc.1209569. [DOI] [PubMed] [Google Scholar]
- Van Zant G, Liang Y. The role of stem cells in aging. Exp Hematol. 2003;31:659–672. doi: 10.1016/s0301-472x(03)00088-2. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- Wang X, Kua H-Y, Hu Y, Guo K, Zeng Q, Wu Q, Ng HH, Karsenty G, de Crombrugghe B, Yeh J, Li B. p53 functions as a negative regulator of osteoblasogenesis, osteoblast-dependent osteoclastogenesis, and bone remodeling. J Cell Biol. 2006;172:115–125. doi: 10.1083/jcb.200507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn SR, Randolph G, Uludag H, Wong SC, Hair GA, Hollinger JO. Establishing an immortalized human osteoprecursor cell line: OPC1. J Bone Miner Res. 1999;14:1721–1733. doi: 10.1359/jbmr.1999.14.10.1721. [DOI] [PubMed] [Google Scholar]
- Zhou S, Yates KE, Eid K, Glowacki J. Demineralized bone promotes chondrocyte or osteoblast differentiation of human marrow stromal cells cultured in collagen sponges. Cell Tissue Bank. 2005a;6:33–44. doi: 10.1007/s10561-005-4253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Lechpammer S, Greenberger SJ, Glowacki J. Hypoxia Inhibition of adipocytogenesis in human bone marrow stromal cells requires TGFβ/Smad3 signaling. J Biol Chem. 2005b;280:22688–22696. doi: 10.1074/jbc.M412953200. [DOI] [PMC free article] [PubMed] [Google Scholar]


