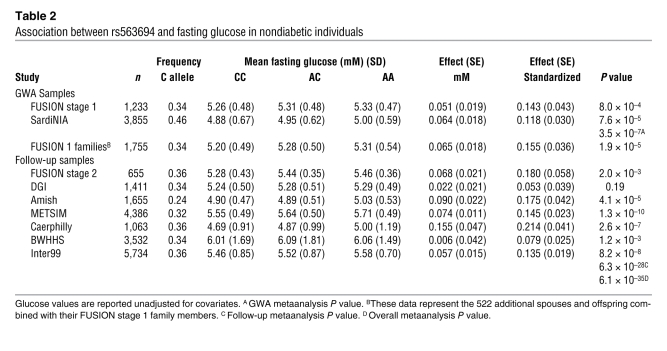
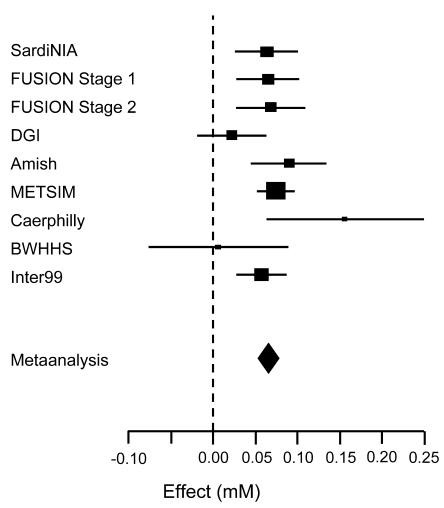
Wei-Min Chen
Wei-Min Chen
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
1,2,
Michael R Erdos
Michael R Erdos
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
3,
Anne U Jackson
Anne U Jackson
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
4,
Richa Saxena
Richa Saxena
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
5,
Serena Sanna
Serena Sanna
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
4,6,
Kristi D Silver
Kristi D Silver
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
7,
Nicholas J Timpson
Nicholas J Timpson
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
8,
Torben Hansen
Torben Hansen
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
9,
Marco Orrù
Marco Orrù
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
6,
Maria Grazia Piras
Maria Grazia Piras
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
6,
Lori L Bonnycastle
Lori L Bonnycastle
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
3,
Cristen J Willer
Cristen J Willer
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
4,
Valeriya Lyssenko
Valeriya Lyssenko
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
10,
Haiqing Shen
Haiqing Shen
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
7,
Johanna Kuusisto
Johanna Kuusisto
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
11,
Shah Ebrahim
Shah Ebrahim
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
12,
Natascia Sestu
Natascia Sestu
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
13,
William L Duren
William L Duren
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
4,
Maria Cristina Spada
Maria Cristina Spada
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
6,
Heather M Stringham
Heather M Stringham
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
4,
Laura J Scott
Laura J Scott
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
4,
Nazario Olla
Nazario Olla
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
6,
Amy J Swift
Amy J Swift
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
3,
Samer Najjar
Samer Najjar
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
13,
Braxton D Mitchell
Braxton D Mitchell
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
7,
Debbie A Lawlor
Debbie A Lawlor
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
8,
George Davey Smith
George Davey Smith
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
8,
Yoav Ben-Shlomo
Yoav Ben-Shlomo
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
14,
Gitte Andersen
Gitte Andersen
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
9,
Knut Borch-Johnsen
Knut Borch-Johnsen
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
9,15,16,
Torben Jørgensen
Torben Jørgensen
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
15,
Jouko Saramies
Jouko Saramies
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
17,
Timo T Valle
Timo T Valle
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
18,
Thomas A Buchanan
Thomas A Buchanan
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
19,20,
Alan R Shuldiner
Alan R Shuldiner
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
7,
Edward Lakatta
Edward Lakatta
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
13,
Richard N Bergman
Richard N Bergman
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
20,
Manuela Uda
Manuela Uda
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
6,
Jaakko Tuomilehto
Jaakko Tuomilehto
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
18,21,
Oluf Pedersen
Oluf Pedersen
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
9,16,
Antonio Cao
Antonio Cao
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
6,
Leif Groop
Leif Groop
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
10,
Karen L Mohlke
Karen L Mohlke
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
22,
Markku Laakso
Markku Laakso
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
11,
David Schlessinger
David Schlessinger
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
13,
Francis S Collins
Francis S Collins
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
3,
David Altshuler
David Altshuler
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
5,
Gonçalo R Abecasis
Gonçalo R Abecasis
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
4,
Michael Boehnke
Michael Boehnke
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
4,
Angelo Scuteri
Angelo Scuteri
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
23,24,
Richard M Watanabe
Richard M Watanabe
1Department of Public Health Sciences and
2Center for Public Health Genomics, University of Virginia, Charlottesville, Virginia, USA.
3Genome Technology Branch, National Human Genome Research Institute, Bethesda, Maryland, USA.
4Center for Statistical Genetics and Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
5Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
6Istituto di Neurogenetica e Neurofarmacologia, Consiglio Nazionale delle Ricerche, Cagliari, Italy.
7Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, Baltimore, Maryland, USA.
8MRC Centre for Causal Analyses in Translational Epidemiology, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
9Steno Diabetes Center, Gentofte, Denmark.
10Department of Clinical Sciences, Diabetes and Endocrinology, Lund University, University Hospital Malmö, Malmö, Sweden.
11Department of Medicine, University of Kuopio and Kuopio University Hospital, Kuopio, Finland.
12Department of Epidemiology and Population Health, Non-communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom.
13Gerontology Research Center, National Institute on Aging, Baltimore, Maryland, USA.
14Social Medicine Department, University of Bristol, Bristol, United Kingdom.
15Research Centre for Prevention and Health, Glostrup University Hospital, Glostrup, Denmark.
16Faculty of Health Sciences, University of Aarhus, Aarhus, Denmark.
17Savitaipale Health Center, Savitaipale, Finland.
18Diabetes Unit, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, and Department of Public Health, University of Helsinki, Helsinki, Finland.
19Department of Medicine, Division of Endocrinology, and
20Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
21South Ostrobothnia Central Hospital, Senäjoki, Finland.
22Department of Genetics, University of North Carolina, Chapel Hill, North Carolina, USA.
23Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, Maryland, USA.
24Unità Operativa Geriatria, Istituto Nazionale Ricovero E Cura Anziari, Rome, Italy.
25Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
20,25