Abstract
Dysfunctional Tregs have been identified in individuals with psoriasis. However, their role in the pathogenesis of the disease remains unclear. Here we explored the effect of diminished CD18 (β2 integrin) expression on the function of CD4+CD25+CD127– Tregs using the Cd18 hypomorphic (Cd18hypo) PL/J mouse model of psoriasis that closely resembles the human disease. We found that reduced CD18 expression impaired cell-cell contact between Tregs and DCs. This led to dysfunctional Tregs, which both failed to suppress the pathogenic T cells and promoted the onset and severity of the disease. This failure was TGF-β–dependent, as Tregs derived from Cd18hypo PL/J mice had diminished TGF-β1 expression. Adoptive transfer of Tregs expressing wild-type levels of CD18 into affected Cd18hypo PL/J mice resulted in a substantial improvement of the psoriasiform skin disease, which did not occur upon coinjection of the cells with TGF-β–specific neutralizing antibody. Our data indicate a primary dysfunction of Cd18hypo Tregs, allowing subsequent hyperproliferation of pathogenic T cells in the Cd18hypo PL/J mouse model of psoriasis. This study may provide a step forward in our understanding of the unique role of CD18 expression levels in avoiding autoimmunity.
Introduction
Psoriasis is a chronic disease affecting skin in 2%–3% of the general population (1). It presents with disfiguring erythematous lesions covered with white silvery scales and severely reduced quality of life (1). However, its pathogenesis is not understood in detail (2). An abnormal function of T lymphocytes has been proposed as a potential cause of psoriasis (3, 4). This notion is supported by many observations, including the fact that psoriasis can be induced in a SCID mouse xenograft model by injection of T cells (3) or develops spontaneously in xenotransplants from unaffected skin of psoriasis patients when grafted onto IFN-γ–deficient AGR mice (5). In addition, T cell immunosuppressants like cyclosporine, T cell depleting agents like denileukin (6), and antibodies either against CD4+, CD25+ T cells, or the α1β1 integrin, the last specifically suppressing the movement of pathogenic T cells from the dermis into the epidermis, lead to disease remission (5, 7, 8). The concept that deregulation of Tregs may play a role in the unrestrained generation of pathogenic T cells in psoriasis has recently been proposed (9). However, the causal proof is lacking. Despite careful execution of experiments, a confounding issue in this and other studies has been the reliance on Treg markers like CD25 and Foxp3, which can not consistently distinguish between Treg and activated effector cell subsets (7, 10–16). Recent identification of CD127, the α chain of IL-7 receptor, as a unique marker, which distinctly discriminates between Treg and effector cell subsets in human disease (17–19) and in mice (19), provided an important lead in the understanding of distinct T cell subsets in autoimmunity.
Given the importance of Tregs in preventing the development of autoimmune disease, including psoriasis, and their therapeutic potential, the molecular mechanisms governing CD4+CD25+CD127– Treg function are of great interest.
We previously reported on the Cd18 hypomorphic (Cd18hypo) PL/J mouse model, with reduced expression of the common chain of β2 integrins (CD11/CD18), which spontaneously develops a T cell–mediated psoriasiform skin disease in homozygous Cd18hypo PL/J mice (20–23). This murine psoriasis model strongly resembles human psoriasis clinically and histologically, in its T cell–dependent pathogenesis, its polygenic base, and its response to therapy (21). Depleting antibodies against CD4+ but not CD8+ T cells resulted in the complete resolution of this psoriasiform skin disease (22).
CD18 represents the common β2 chain of the β2 integrin family, with 4 heterodimeric molecules (CD11a/CD18, CD11b/CD18, CD11c/CD18, and CD11d/CD18) being exclusively expressed on hematopoietic cells. Among several possibilities, reduced CD18 expression may cause a disrupted formation of the immunological synapse, resulting in the generation and persistence of autoreactive T cells (24, 25). The pathogenic role of β2 integrins in human psoriasis and other inflammatory skin diseases is poorly understood (26–28).
Circumstantial evidence indicating that reduced CD18 expression may causally be involved in the development of the psoriasiform skin disease comes from the clinical observation that some patients suffering from leukocyte adhesion deficiency syndrome I, even with moderately reduced CD18 expression levels, can develop a psoriasiform skin disease (28). Linkage analysis of psoriasis families has identified a region on chromosome 17, which includes the ICAM-2 locus, an important ligand of the CD11/CD18 heterodimers (29). In addition, polymorphisms in the CD18 gene apparently predispose to autoimmune diseases (26, 27). CD18 acts as a costimulatory molecule in T cell activation, TCR signaling, and cytotoxic removal of target cells (30–32). Reduced CD18 expression may cause disturbance in the formation of the immunological synapse (21, 22, 25) for Treg priming and proliferation, with subsequent deregulated control of autoreactive T cells.
Here we explored the Cd18hypo PL/J psoriasis mouse model to study the effect of diminished CD18 expression on Treg function and the pathogenesis of psoriasiform skin disease. We found that reduced CD18 expression specifically impaired DC-Treg contact, resulting in dysfunctional Tregs, which through decreased expression of TGF-β1 failed to sufficiently suppress the proliferation of pathogenic T cells. This accelerates onset and severity of the psoriasiform skin disease. Our data indicate that the psoriasiform skin disease observed in Cd18hypo mice results primarily from a deficiency in Treg function with subsequent diminished production of TGF-β1. These findings support the concept that CD18 heterodimeric molecules involved in physical interaction of Tregs and DCs are essential for proper Treg function in PL/J mice avoiding psoriasiform skin disease.
Results
Adoptive transfer of Cd18wt Tregs into Cd18hypo PL/J mice results in resolution of the psoriasiform skin disease.
Hyperactivation of pathogenic T cells from affected Cd18hypo mice may be due to impaired Treg function in vivo. Investigation of this possibility was previously hampered as it was shown that CD25 antibodies cannot reliably discriminate Tregs from activated conventional T cells (17–19). In fact, we found neutralizing antibodies against CD25 resulted in the resolution of the psoriasiform phenotype, supporting the role of a CD4+CD25+ pathogenic T cell subset (Supplemental Figure 1 and Supplemental Results; supplemental material available online with this article; doi: 10.1172/JCI34916DS1). Here we have used a combination of CD4, CD25, and CD127 surface markers to reliably isolate Tregs from Cd18hypo mice, as CD127, the α-chain of the IL-7 receptor, is absent or found only at low levels on Tregs, whereas it is highly expressed on conventional T cells (17–19).
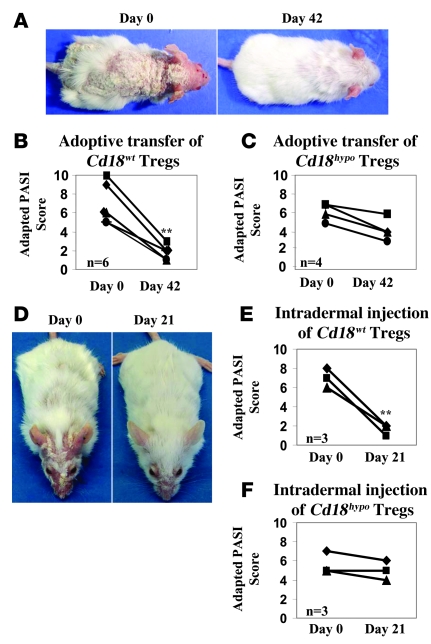
To investigate the function of Tregs in Cd18hypo PL/J mice with psoriasiform skin disease, 1 × 106 CD4+CD25+CD127– Tregs purified from the spleens of Cd18wt or Cd18hypo mice were transferred into syngeneic affected Cd18hypo recipients at day 0 and day 30. Notably, transfer of Cd18wt Tregs markedly reduced the psoriasiform phenotype compared with that before treatment as early as 42 days after transfer (Figure 1A). This was confirmed in 6 affected Cd18hypo mice by an adapted psoriasis activity and severity index (PASI) score. The adapted PASI score was 6.83 ± 2.14 before transfer of Cd18wt Tregs and decreased to 1.67 ± 0.82 (P = 0.0015) after 2 transfers of Cd18wt Tregs (Figure 1B), indicating substantial improvement of the psoriasiform skin disease. Notably, the transfer of Cd18hypo Tregs from healthy Cd18hypo PL/J mice into affected Cd18hypo PL/J mice did not result in any significant improvement. However, a mild salutary effect was observed in the adapted PASI score (Figure 1C). Interestingly, intradermal injection of 0.2 × 106 Tregs purified from the spleens of Cd18wt mice into the lesional back skin of affected Cd18hypo PL/J mice resulted in a resolution of the psoriasiform skin inflammation (Figure 1, D and E). In contrast, intradermal injection of Cd18hypo Tregs did not result in a significant improvement in all 3 affected Cd18hypo PL/J mice (Figure 1F). These results unequivocally show that the Cd18hypo mutation impairs the suppressive function of Tregs in vivo. Additionally, these data indicate that a primary hyperactivation of pathogenic T cells does not precede the lack of suppressive function, rather Cd18hypo Tregs are impaired, resulting in a lack of control of pathogenic T cells.
Figure 1. Adoptive transfer of Cd18wt CD4+CD25+CD127– Tregs into affected Cd18hypo mice resolves the psoriasiform skin disease.
(A) Representative clinical pictures of a Cd18hypo mouse with severe psoriasiform skin disease before (left panel) and almost complete resolution 42 days after the first adoptive transfer with Tregs from Cd18wt mice (right panel). An adapted PASI score was used to assess the severity of the psoriasiform phenotype before and after adoptive transfer with Cd18wt Tregs (B) or Cd18hypo Tregs (C). Data are representative of 3 independent experiments. (D) An improvement of the psoriasiform skin disease in affected Cd18hypo mice was observed after intradermal injection of Cd18wt Tregs once weekly for 3 weeks. (E and F) The adapted PASI score of affected Cd18hypo mice treated with Cd18wt Tregs is significantly reduced compared with that of mice treated with Cd18hypo Tregs. **P < 0.01, using Student’s t test. Original magnification, × 1 (A and D).
CD4+CD25+CD127– Tregs are decreased in number in affected Cd18hypo PL/J mice.
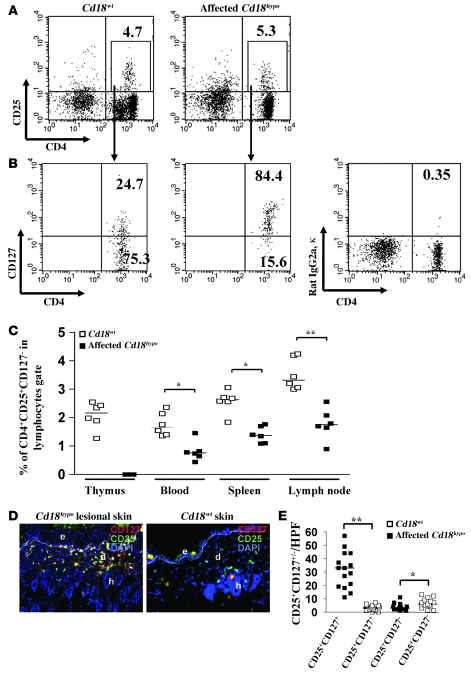
As impairment of Treg function may simply be conferred by reduction of absolute numbers, we set out to assess CD4+CD25+CD127– Treg counts in different organs and compartments. For this purpose, we first gated cells of Cd18wt or affected Cd18hypo PL/J mice for CD4 and CD25 expression (gated as in Figure 2A), and thereafter analyzed this fraction for CD127 expression (Figure 2B).
Figure 2. CD4+CD25+CD127– Tregs are decreased in numbers in affected Cd18hypo PL/J mice.
Lymphocytes were firstly gated for CD4+CD25+ T cells derived from spleens of either Cd18wt or Cd18hypo affected mice (A) and thereafter analyzed for CD127 expression (B). The percentage of CD127+ (top corner) and CD127– (bottom corner) T cells within CD4+CD25+ T cell gate is shown. The panel on the right shows isotype matched IgG control staining. (C) Percentage of CD4+CD25+CD127– T cells in thymus, blood, spleen, and DLNs of Cd18wt and affected Cd18hypo mice with psoriasiform skin disease (n = 6 for each genotype and organ). The experiment was done twice, the median is shown. (D) To investigate CD127 expression by CD25+ T cells in the skin of affected Cd18hypo mice, skin cryosections from Cd18wt and affected Cd18hypo mice were double stained with CD25–Alexa Fluor 488 (green) and CD127-PE (red). Cell nuclei (blue) were counterstained with DAPI (original magnification, ×20). Red cells indicate CD25–CD127+ T cells and green cells represent CD25+CD127– Tregs, while overlay (yellow) represents double-positive T cells. e, epidermis; d, dermis; h, hair follicle. The dotted line indicates the border between epidermis and dermis. (E) To quantify CD25+CD127+ or CD25+CD127– T cells in the skin of affected Cd18hypo and Cd18wt mice, the positively stained cells were counted. For all measurements, the median of specifically stained T cells counted in 15 high-power fields (HPFs) is presented (n = 5). *P < 0.05, **P < 0.01, using Student’s t test.
In splenocytes, 75.3% of CD4+CD25+ cells derived from Cd18wt mice (Figure 2B, left panel), while only 15.6% of CD4+CD25+ cells derived from affected Cd18hypo mice (Figure 2B, middle panel), fell within the CD4+CD127– gate, clearly indicating that the CD4+CD25+CD127– fraction of Tregs is substantially diminished in affected Cd18hypo mice as compared with Cd18wt mice.
Similarly, the percentage of CD4+CD25+CD127– Tregs derived from affected Cd18hypo mice was substantially diminished in blood, spleen, and lymph nodes when compared with Cd18wt mice (Figure 2C). As there is a complete thymic involution in Cd18hypo mice with severe psoriasiform skin disease, the numbers of CD4+CD25+CD127– Tregs cannot be analyzed in these thymi. Furthermore, we observed that 93.6% of CD4+CD25+CD127– Tregs expressed Foxp3 in Cd18wt mice. In contrast, 78.6% and 74.5% of CD4+CD25+CD127– Tregs were Foxp3+ in healthy and affected Cd18hypo mice, respectively (Supplemental Figure 2, A and B).
Interestingly, while most of the CD25+ T cells within the dermis of affected Cd18hypo mice stained positive for CD127 (yellow overlay), only a small fraction was CD25+CD127–, and some cells stained negative for CD25 and positive for CD127 (Figure 2, D and E). In contrast, in the skin of Cd18wt mice, only a small number of T cells were stained, most being positive for CD25 but negative for CD127.
In addition to the substantial increase in CD25+CD127+ T cells in the psoriasiform skin of Cd18hypo mice, depletion of CD25+ cells with antibodies against CD25 resulted in significant improvement of the psoriasiform skin disease (Supplemental Figure 1 and Supplemental Methods). These results indicate that the majority of CD25+ T cells are not Tregs but rather represent activated pathogenic T cells with high CD127 expression in the lesional skin of affected Cd18hypo PL/J mice.
Low CD18 expression reduces the proliferative response and the suppressive function of Tregs.
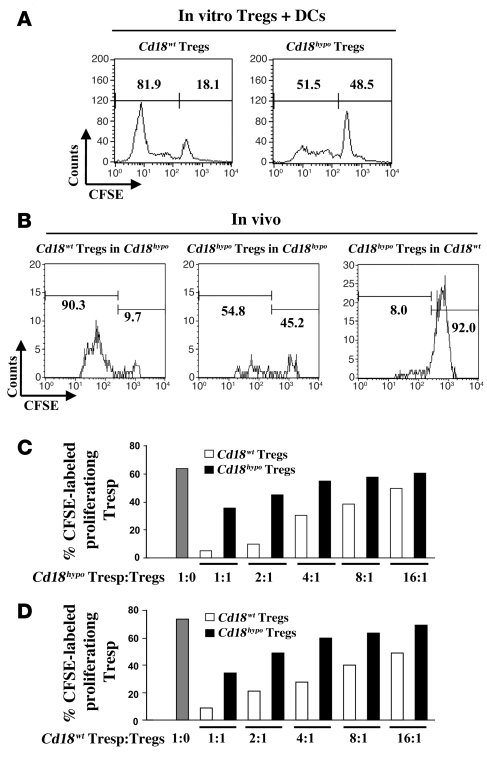
A classical feature of Tregs is that they are hyporesponsive upon TCR-mediated stimulation in vitro and in vivo (33, 34). However, allogeneic DCs are effective APCs, causing the proliferation of Tregs in the presence of recombinant IL-2 during the mixed leukocyte reactions (MLRs) in vitro (35), and moreover, self-peptides drive the peripheral expansion of Tregs in vivo (33, 36, 37). In order to study whether reduced CD18 expression impairs proliferation of alloantigen-specific Tregs in vitro, we performed MLRs with purified CD4+CD25+CD127– Tregs from Cd18hypo PL/J or Cd18wt (H-2u) PL/J mice together with irradiated bone marrow–derived allogeneic DCs from C57BL/6J (H-2b) mice in the absence and presence of 500 units/ml recombinant murine IL-2. Using CFSE, we found that in the presence of IL-2, the number of proliferative CD4+CD25+CD127– Tregs derived from Cd18hypo PL/J mice was markedly reduced to 51.5% (Figure 3A, right panel) compared with 81.9% of CD4+CD25+CD127– Tregs derived from Cd18wt PL/J mice (Figure 3A, left panel). As expected (35), proliferation did not occur in Cd18wt Tregs or Cd18hypo Tregs in the absence of either IL-2 or allogeneic DCs during MLRs (data not shown). To further investigate whether CD18 is required for homeostatic proliferation in vivo, MACS-purified CFSE-labeled syngeneic Tregs of Cd18wt and Cd18hypo mice were injected intravenously into affected Cd18hypo recipients. Seven days after adoptive transfer, 90.3% of the CD4+CD25+CD127– donor Tregs from Cd18wt mice had undergone substantial proliferation in the skin draining lymph nodes (DLNs) of the Cd18hypo recipients (Figure 3B, left panel). Notably, only 54.8% of Cd18hypo CD4+CD25+CD127– donor Tregs (Figure 3B, middle panel) revealed a proliferative response after adoptive transfer into Cd18hypo recipients. Only 8.0% of CD4+CD25+CD127– donor Tregs from Cd18hypo PL/J mice showed a minor proliferative response when transferred into Cd18wt PL/J recipients (Figure 3B, right panel). These data suggest that homeostatic expansion of Cd18hypo Tregs in affected Cd18hypo PL/J mice is substantially reduced, most likely contributing to the impaired control of pathogenic T cells.
Figure 3. Impaired homeostatic expansion and suppressive function of Cd18hypo CD4+CD25+CD127– Tregs.
(A) Representative CFSE dilution profiles of gated CFSE+CD4+CD25+CD127– Tregs from Cd18wt (left panel) and Cd18hypo mice (right panel). (B) Representative CFSE dilution profile of gated CFSE+CD4+CD25+CD127– Tregs from skin DLNs of Cd18wt (left panel) and Cd18hypo (middle panel) mice 7 days after adoptive transfer into affected Cd18hypo recipients or of gated CFSE+CD4+CD25+CD127– Tregs from Cd18hypo mice 7 days after adoptive transfer into Cd18wt recipients (right panel). Numbers on the top left of A and B indicate the percentage of CFSE-labeled proliferating cells. Numbers on the top right of A and B indicate the percentage of undivided CFSE-labeled cells. To investigate in vitro suppressive function of Tregs on Tresp cells, CD4+CD25+CD127– Tregs were pooled from spleens of Cd18wt or Cd18hypo mice (4 animals for each group) and cultured with CFSE-labeled CD4+CD25– Tresp cells derived from either affected Cd18hypo mice (C) or Cd18wt mice (D). A total of 1 × 105 Tresp cells were incubated alone (gray bar, ratio 1:0) or with decreasing numbers of Tregs from Cd18wt or affected Cd18hypo mice (the ratio of Tregs/Tresp cells was at 1:1, 2:1, 4:1, 8:1, and 16:1). After 3 days cells were harvested and analyzed by flow cytometry. Representative data are shown, which had been reproduced in 3 independent experiments.
To determine whether the reduced expression of CD18 on Tregs derived from Cd18hypo mice revealed impaired suppressive function, in vitro suppression assays with different ratios of either CFSE-labeled CD4+CD25– responder T (Tresp) cells derived from Cd18hypo PL/J mice (Figure 3C) or from Cd18wt mice (Figure 3D) were cocultured with Cd18wt PL/J Tregs or Cd18hypo PL/J Tregs. Compared with Cd18wt Tregs, purified CD4+CD25+CD127– Tregs from Cd18hypo PL/J mice showed a substantially reduced suppressive function independent of whether CD4+CD25– Tresp cells were derived from Cd18hypo (Figure 3C) or from Cd18wt (Figure 3D) mice.
Low CD18 expression disrupts cell-cell contacts between Tregs and DCs, resulting in reduced proliferation and impaired TGF-β1–dependent suppressive function.
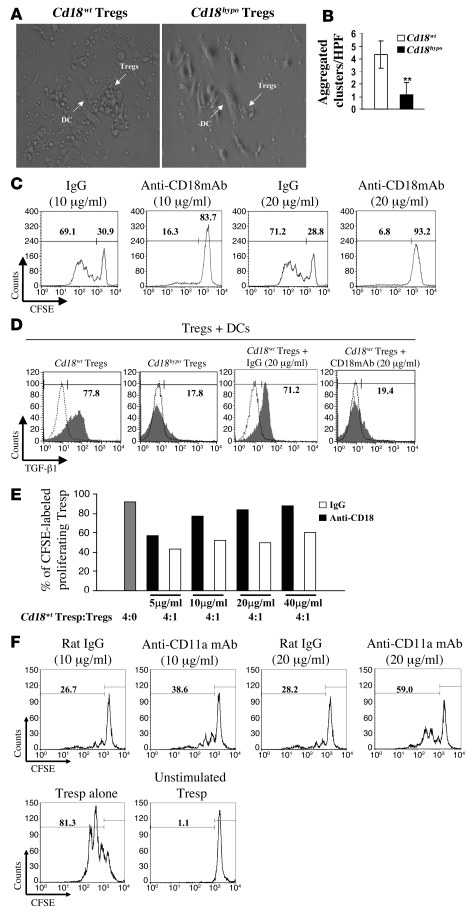
Persistent DC-Treg contact precedes the activation of Tregs and is required for their suppressive function on Tresp cells (38, 39). As CD18 is an essential molecule for cell-cell contact in a variety of inflammatory interactions (24, 40), we studied whether reduced CD18 expression impairs the DC-Treg contact and whether this is critical for the proliferation and the suppressive functions of alloantigen-specific Tregs. MLRs were performed with Tregs from Cd18wt PL/J or Cd18hypo PL/J (H-2u) mice together with allogeneic DCs from C57BL/6J (H-2b) mice. Diminished cell-cell contacts between allogeneic DCs and Cd18hypo Tregs occurred, and subsequently reduced cluster formation was seen when compared with Cd18wt Tregs (Figure 4, A and B). In addition, we used neutralizing antibody against CD18 in a complementary set of experiments. This neutralizing anti-CD18 mAb has earlier been shown to exclusively disrupt the binding of CD18 heterodimers to their ligands but has no known signaling effects. Notably, increasing concentrations of neutralizing antibodies against CD18 revealed a concentration-dependent decrease in cluster formation of Cd18wt Tregs (Supplemental Figure 3). Independent of whether cell-cell contact between DCs and Tregs was impaired due to decreased CD18 expression on Cd18hypo PL/J Tregs (Figure 4, A and B) or due to increasing concentrations of neutralizing antibody against CD18, proliferation of CFSE-labeled alloantigen-specific Tregs was reduced in a dose-dependent manner (Figure 4C). These data clearly indicate that Cd18wt levels are critical for DC-Treg contacts and subsequent expansion of allogenically stimulated Tregs.
Figure 4. Reduced CD18 function disrupts cell-cell contacts between DCs and Cd18hypo Tregs from PL/J mice, which impairs specific allogeneic Treg expansion and activation.
(A) Representative pictures of cluster formation of allogeneic DCs with Tregs derived from either Cd18wt mice or Cd18hypo mice are shown. Original magnification, ×40. (B) Cluster formation between allogeneic DCs and Tregs of different genotypes from Cd18wt mice and Cd18hypo mice was assessed by counting aggregated clusters/HPF in 100 randomly selected HPFs. Cluster formation with allogeneic DCs was substantially reduced for Tregs derived from Cd18hypo mice compared with Tregs from Cd18wt control mice. **P = 0.0029, using Student’s t test. (C) Increased neutralizing mAb against CD18 resulted in decreased proliferative response of specific allogeneic Tregs in MLRs. Numbers on the top left of C and F indicate the percentage of CFSE-labeled proliferating cells. Numbers on the top right of C and F indicate the percentage of undivided CFSE-labeled cells. (D) Increased TGF-β1 expression by Cd18wt Tregs was observed in MLRs. Neutralizing mAb against CD18 in MLRs resulted in a dramatic decrease in TGF-β1 expression compared with isotype-matched control antibody. Gray region, TGF-β1 expression; white region, normal goat IgG control for TGF-β1 staining. Numbers on the top of D indicate the percentage of CFSE-labeled proliferating cells. CD4+CD25+CD127– Tregs were purified from 4 pooled spleens of Cd18wt PL/J mice and cocultured with irradiated allogeneic DCs in the presence of 500 units/ml recombinant murine IL-2 and various concentrations of anti-CD18 mAb (E), or anti-mouse CD11a mAb (F), or isotype-matched IgG for 7 days. Tregs were then separated from allogeneic DCs by CD11c MACS beads, extensively washed 3 times with PBS, and mixed at a ratio of 1:4 with Cd18wt Tresp cells. After 3 days of culture, cells were harvested and analyzed by flow cytometry. One representative experiment out of 3 or 4 independent experiments is shown.
Tregs exert their suppressive function on Tresp cells in part by TGF-β (34, 41). We studied the effect of impaired CD18 function with disrupted DC-Treg interactions on TGF-β1 expression by CD4+CD25+CD127– Tregs. Using cocultures of Tregs with allogeneic DCs in MLRs, we found that TGF-β1 expression by Tregs derived from Cd18hypo PL/J mice was severely decreased to 17.8% (Figure 4D, second panel from left) compared with a higher TGF-β1 expression of 77.8% in Tregs from Cd18wt PL/J mice (Figure 4D, left panel). Interestingly, neutralizing antibody against CD18 dramatically decreased TGF-β1 expression by Cd18wt Tregs in MLRs to 19.4% (Figure 4D, right panel) compared with 71.2% TGF-β1 expression of Cd18wt Tregs when treated with isotype-matched control antibody (Figure 4D, third panel from left).
To further confirm that CD18 is mandatory for the education of Tregs and their suppressive function during their physical interaction with DCs, we studied the suppressive function of Cd18wt Tregs after coculture with allogeneic DCs on Tresp cells. This experimental design allowed us to specifically address the role of CD18 in DC-dependent education of Tregs. For this purpose, Tregs were cocultured with allogeneic DCs in presence of various concentrations of neutralizing antibody against CD18 or isotype-matched IgG for 7 days. Thereafter, specific allogeneic Tregs were separated from allogeneic DCs, washed 3 times with PBS, and mixed at a ratio of 1:4 with Cd18wt Tresp cells. Cd18wt Tregs derived from the coculture in presence of anti-CD18 revealed a dose-dependent reduction of suppressor function on Tresp cells compared with Cd18wt Tregs derived from the coculture in presence of isotype-matched IgG (Figure 4E). Importantly, Cd18wt Tregs also demonstrated a dose-dependent reduction in their suppressor function on Tresp cells in the same experimental setting in the presence of anti-CD11a blocking antibody (Figure 4F). Furthermore, in line with a previous report (42), anti–TGF-β mAb (clone 1D11) reversed suppressor function of Cd18wt Tregs expanded by allogeneic DCs (Supplemental Figure 4). These data show that wild-type CD18 expression levels and function are mandatory for effective DC-Treg interaction and subsequent antigen-specific education for their TGF-β1–mediated suppressive function.
To our knowledge this is the first report that identifies CD18 as a critical molecule for the formation of DC-Treg contacts, which is crucial for homeostatic proliferation and TGF-β1–dependent suppressive Treg function.
Reduced expression of TGF-β1 by Cd18hypo Tregs contributes to the development of psoriasiform skin disease in vivo.
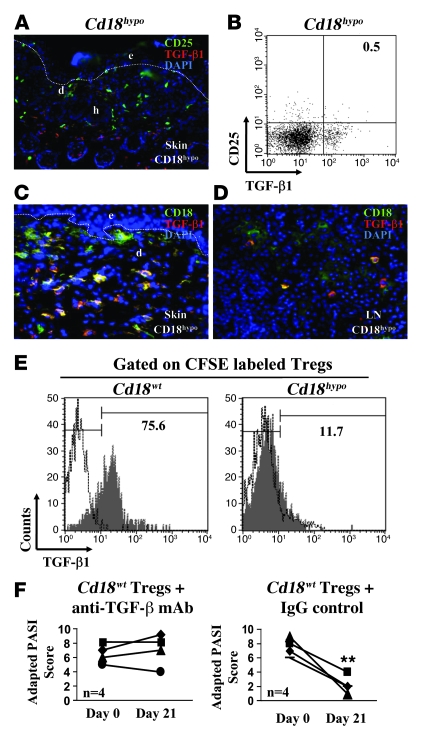
To confirm that a lack of TGF-β1, due to decreased CD18 expression on Tregs, is causal for the psoriasiform skin disease in vivo, we performed the following set of experiments. First, using immunohistology and FACS analysis, we did not observe any TGF-β1 expression by CD25+ T cells in the skin and DLNs (Figure 5, A and B) derived from Cd18hypo mice with psoriasiform skin disease. Second, after adoptive transfer of MACS-sorted CD4+CD25+CD127– Tregs derived from Cd18wt into affected Cd18hypo mice with severe psoriasiform phenotype, strong TGF-β1 expression of the adoptively transferred Cd18wt Tregs in the skin and in the skin DLN (yellow overlay) was observed (Figure 5, C and D). Third, we transferred CFSE-labeled CD4+CD25+CD127– Tregs purified from either Cd18wt or Cd18hypo mice into affected Cd18hypo mice. TGF-β1 production was analyzed 4 days after the injection of Tregs. Similar to our in vitro observation, 75.6% of Cd18wt Tregs produced TGF-β1 after adoptive transfer into affected Cd18hypo mice (Figure 5E, left panel). In contrast, only 11.7% of Cd18hypo Tregs expressed TGF-β1 in affected Cd18hypo mice (Figure 5E, right panel). Freshly isolated CD4+CD25+CD127– Tregs from naive Cd18wt mice express minimal TGF-β1 (data not shown). These data demonstrate that CD18 at wild-type levels is required for the induction of Tregs to express TGF-β1 in vivo. Furthermore, these data show that Cd18wt Tregs are able to migrate into the diseased skin to suppress pathogenic T cells, resulting in an almost complete resolution of the disease (Figure 1, A and B). In order to show that TGF-β1 expression of Tregs is causally involved in the suppression of pathogenic Tresp cells and the resolution of the psoriasiform skin disease, Tregs from Cd18wt mice were adoptively transferred into affected Cd18hypo mice. On subsequent intraperitoneal injection of 250-μg neutralizing mAb against TGF-β (1D11) or isotype IgG control antibody, we found that adoptively transferred Tregs of Cd18wt mice in the presence of neutralizing mAb against TGF-β were no longer able to resolve the psoriasiform skin disease as assessed by the adapted PASI score (Figure 5F, left panel). In contrast, as expected for adoptive transfer of Cd18wt Tregs into affected Cd18hypo mice, injection of isotype control IgG mAb still resulted in a significant improvement of the psoriasiform skin disease (P = 0.002) (Figure 5F, right panel).
Figure 5. TGF-β1 is causal for the suppressive function of Cd18wt Tregs in vivo.
(A) Cryosections from affected Cd18hypo mice were double stained with CD25-FITC and TGF-β1–Cy3 mAbs. Original magnification, ×20. (B) FACS analysis of pooled DLNs from affected Cd18hypo mice using TGF-β1 and CD25 mAbs. (C and D) Seven days after adoptive transfer of MACS-sorted Cd18wt Tregs into affected Cd18hypo mice, skin sections were stained with antibody against TGF-β1 and CD18. The overlay (yellow) of CD18-positive Cd18wt Tregs (green) and TGF-β1–expressing cells (red) indicate that most of the Cd18wt Tregs express TGF-β1 in the skin (C) and skin DLNs (D) after transfer into Cd18hypo mice. The dotted line indicates the border between epidermis and dermis. Three independent experiments were performed in total. (E) A total of 1 × 106 Tregs from either Cd18wt or Cd18hypo mice were labeled with CFSE and adoptively transferred into affected Cd18hypo mice. At day 4 after adoptive Tregs transfer, FACS analysis was performed to measure TGF-β1 expression of CFSE-labeled Cd18wt Tregs (left panel) or Cd18hypo Tregs (right panel) from skin DLNs of affected recipients. Gray region, TGF-β1 expression; white region, normal goat IgG control for TGF-β1 staining. Numbers on the top of B and E indicate the percentage of CFSE-labeled proliferating cells. (F) Following adaptive transfer of 1 × 106 Cd18wt Tregs, 250-μg TGF-β1–neutralizing mAb (left panel) or isotype control IgG (right panel) were injected intraperitoneally into affected Cd18hypo recipients. Repetitive injection of TGF-β neutralizing antibody or isotype control IgG after adoptive transfer of Cd18wt Tregs was performed till the end of treatment (21 days). Original magnification, ×40 (C and D). **P = 0.002, using Student’s t test.
In order to exclude for the possibility that the persistence of the psoriasiform skin disease upon combined treatment of adoptive transfer of Cd18wt Tregs and administration of mAb against TGF-β into affected Cd18hypo PL/J mice is simply due to apoptosis or reduced proliferation of the Tregs, we undertook a set of experiments. These control experiments strongly support the view that the persistence of the psoriasiform skin disease after adoptive transfer of Cd18wt Tregs into Cd18hypo affected mice in the presence of neutralizing anti–TGF-β mAb is not due to apoptosis of Tregs but is most likely due to the abrogation of their TGF-β–mediated suppressor function on Tresp cells (Supplemental Figure 5).
Discussion
The balance between regulatory and effector functions is important for maintaining efficient immune responses while avoiding autoimmunity. The inflammatory skin disease psoriasis is sustained by ongoing activation of pathogenic cells with as of yet unresolved mechanisms. Using the Cd18hypo murine psoriasis model, we found that reduced CD18 expression on CD4+CD25+CD127– Tregs is responsible for physical disruption of their cell-cell contact with DCs, with subsequent dysfunction of Tregs that fail to suppress pathogenic T cells and the psoriasiform skin disease in PL/J mice. This failure of the suppressive capacity of Tregs on pathogenic T cells is due to their diminished TGF-β1 expression. To our knowledge this is the first study on Treg function in a spontaneously occurring T cell–mediated murine model for psoriasis. The newly identified cell surface marker of the IL-7 receptor (CD127) was used as a unique tool for the reliable discrimination and purification of Treg and T effector cell subsets in this model.
The conclusion that Treg dysfunction plays a role in this psoriasis model is supported by the finding that Cd18wt Tregs, when adoptively transferred into affected Cd18hypo PL/J mice, led to the resolution of the psoriasiform skin phenotype. In the case of primary hyperactivation of pathogenic T cells being responsible, we would have expected the psoriasiform skin disease to have persisted after Cd18wt Treg transfer. Additionally, upon adoptive transfer of Tresp cells derived from affected Cd18hypo mice into Cd18wt mice, the psoriasiform skin phenotype did not develop (data not shown), which again means that primary hyperactivation of pathogenic T cells is not central. Finally, criss-cross experiments studying the suppressive capacity of Tregs of Cd18hypo and Cd18wt PL/J genotypes on defined Tresp cells unequivocally demonstrated that the reduced CD18 expression on Tregs derived from PL/J Cd18hypo mice is distinctly responsible for their impaired suppressive capacity.
Similar to previous reports on type 1 diabetes mellitus (10), multiple sclerosis (11), and rheumatoid arthritis (12), we did not observe any decrease in CD4+CD25+Foxp3+ Tregs in the murine Cd18hypo PL/J psoriasis model (data not shown). The identification of CD127 as reliable marker for Tregs facilitates an accurate definition of the Treg subset (17–19). We found that CD4+CD25+CD127– Tregs were decreased in affected Cd18hypo PL/J mice. Interestingly, Cd18wt Tregs revealed higher Foxp3 levels compared with Cd18hypo Tregs (Supplemental Figure 2, A and B), suggesting CD18 expression at wild-type levels may be essential for proper activation of Tregs. We did not find any significant difference in Foxp3 expression in Tregs derived from healthy Cd18hypo mice and affected Cd18hypo mice (78.6% versus 74.5%) (Supplemental Figure 2, A and B). However, in contrast to healthy Cd18hypo mice, a significant decrease in numbers of CD4+CD25+CD127– Tregs was observed in affected Cd18hypo mice (Supplemental Figure 2C). The observed small, beneficial effect on the modified PASI score after the transfer of 1 × 106 Cd18hypo Tregs into affected Cd18hypo mice (Figure 1C) may be due to the increase in Cd18hypo Treg numbers, which is not sufficient to resolve psoriasiform skin disease.
We show here that reduced CD18 expression levels critically impair the physical cell-cell interaction and signaling in Tregs. In fact, in vitro and in vivo expansion and/or activation of CFSE-labeled Cd18hypo PL/J Tregs in MLRs, as well as their suppressive function, was severely reduced. Consistent with previous results (43), homeostatic expansion and instruction for the suppressor function of Tregs depends on the cell-cell contact between DCs and Tregs (38, 39, 44).
Notably, the proliferation and suppressive function of Cd18wt Tregs can be abrogated by addition of anti-CD18 neutralizing mAb in vitro. These findings indicate a causal role of diminished CD18 expression in the disruption of DC-Treg contacts, with subsequent deficiency in Treg activation and suppressor function.
The physical contact between DCs and Tregs is essential, as observed in different experimental settings. Important adhesion/signaling molecules for these contacts are CD28/B7 as well as CD80 and CD86. Blocking of either the CD28/B7 or the CD80/CD86 pathway resulted in rapid loss of Tregs in vivo and subsequent loss of critical immune regulation (45–47). However, neither the CD28/B7 nor the CD80/CD86 molecules can compensate for the reduced CD18 expression on the Cd18hypo Tregs derived from PL/J mice. Hence, we have identified CD18 as an essential adhesion molecule in the formation of physical DC-Treg contacts, which if not expressed at appropriate concentrations on the cell surface of Tregs is responsible for the development of the psoriasiform skin disease. Low CD18 expression on Tregs from Cd18hypo PL/J mice may impair TCR activation, eventually leading to reduced proliferation, activation, and suppressor function of Tregs. In fact, injection of antibodies against CD3 into affected Cd18hypo mice, which is known to modulate the TCR activation, could fully restore Treg proliferation and their suppressor function, leading to a fast and persisting remission of the psoriasiform skin disease (our unpublished data). Notably, the presence of anti-CD11a antibodies in MLRs of allogeneic DCs and Tregs resulted in severely hampered suppressive function of Tregs, identical to the results in a Cd18hypo Treg and DC setting. These data may at least in part help to explain the pustular eruption that occurs occasionally in psoriasis patients under anti-CD11a antibody (efalizumab; Raptiva) treatment (48). Accordingly, when the efalizumab concentrations are decreased during treatment of psoriasis patients, activated pathogenic T cells, which have accumulated to excessive numbers in the circulation under saturating efalizumab concentrations, may rush into the skin and receive further activation by DCs. However, the suppressive function of Tregs is insufficient, resulting in a dramatic overactivation of pathogenic T cells in the skin and an acute pustular eruption. Even though the Cd18hypo PL/J model provides some mechanistic insight into how the acute pustular dermatitis in efalizumab-treated patients may develop, this pustular dermatitis is different from psoriasiform dermatitis in the Cd18hypo PL/J mouse model. Due to residual CD18 expression, extravasation of pathogenic T cells continuously occurs in the Cd18hypo mouse model. This is in line with the clinical and histological picture, which reflects a slowly developing chronic stationary plaque psoriasis in the Cd18hypo mouse model (21, 22) and not an acute pustular dermatitis as seen in the efalizumab-induced pustular dermatitis.
Our findings clearly show that reduction in CD18 expression differentially affects different immune synapses. While the DC-Tresp cell synapse does not need CD18 at wild-type expression levels, wild-type CD18 expression levels are absolutely mandatary for the TCR activation in DC-Treg synapse.
Interestingly, Sakaguchi and coworkers (49) reported a mouse strain that spontaneously develops a T cell–dependent inflammatory joint disease resembling human rheumatoid arthritis. Adoptive transfer of CD4+ T cells from this mouse strain bearing a point mutation in a gene encoding ZAP-70, a key signal-transducing molecule of TCR activation, could transfer the disease state in a variety of different mouse strains (49). In addition, 3 mutations involved in changes to the immune synapse function are linked to the development of autoreactive T cells (50).
Psoriasis may be improved by Tregs, IL-4 (51), or IL-10 (52). We could exclude a role for IL-10– and IL-4–dependent suppression of the psoriasiform inflammation (data not shown). In contrast, here we show a major role for the CD18-dependent suppressive function of Tregs via TGF-β1 in the resolution of the psoriasiform skin disease. The contribution of TGF-β1 to the suppressor function of Tregs in different models is still controversial. For example, TGF-β1 plays a nonredundant role in the control of intestinal inflammation and diabetes (53, 54), with these models being induced by TCR crosslinking with intravenously injected or orally administrated anti-CD3 antibodies (54, 55). However, TGF-β1 does not play a role in gastritis (41). A proportion of previously identified CD4+CD25+ T cell subsets express TGF-β1 on their surface, which has been implicated in their suppressor function in vitro (42, 56). In addition, a transient pulse of TGF-β1 in the pancreatic islets in an inducible transgenic mouse model is sufficient to inhibit diabetes onset, by promoting the regulatory T cell pool (57). In line with a pivotal role for TGF-β1 in immunosuppression is a multiorgan inflammatory disease that develops in TGF-β1–deficient mice (58, 59).
Our proposal that a decrease in the TGF-β–mediated suppressive function of Tregs results from diminished CD18 expression levels and impaired DC-Treg interactions in PL/J mice is supported by several lines of evidence in this study. First, antibody directed against CD18 decreased cluster formation between DCs and Tregs, with subsequent diminished expansion/activation and TGF-β1 expression of Cd18wt Tregs when cultured with allogeneic DCs. Second, a major prerequisite for in vivo suppression of Tresp cells is an identical homing and migration pattern of Tregs and Tresp cells (39). As antigenic stimulation and its potential suppression begins in the antigen DLNs and thereafter is continued in the target organs (39), we studied the expression of TGF-β1 on Tregs derived from both Cd18wt and Cd18hypo mice after adoptive transfer into affected Cd18hypo mice. TGF-β1 production was minimal by Cd18hypo Tregs in severely affected Cd18hypo recipients, whereas a large amount of TGF-β1 was found for Cd18wt Tregs transferred into affected Cd18hypo PL/J mice. Notably, adoptive transfer of Cd18wt Tregs into affected Cd18hypo PL/J mice revealed a clear TGF-β1 staining on Cd18wt Tregs in the skin and skin DLNs of Cd18hypo recipients with a substantial improvement of the psoriasiform skin disease. Third, the resolution of psoriasiform skin disease in the identical setting of adoptively transferred Cd18wt Tregs into affected Cd18hypo mice could be abolished by intraperitoneal injection of neutralizing mAb to TGF-β (55).
We have excluded the possibility that neutralizing TGF-β function resulted in enhanced Treg apoptosis, as TGF-β1 has been suggested to sustain Treg proliferation in other experimental models (60). Sequential skin biopsies derived from the above mentioned experiments did not reveal any significant difference in Treg numbers in affected Cd18hypo mice after adoptive transfer of Cd18wt Tregs in the presence or absence of neutralizing anti–TGF-β mAb (Supplemental Figure 5). Also, FACS analysis using mAb against annexin V, a marker for apoptosis, did not show any increase in apoptotic Tregs in skin DLNs (Supplemental Figure 5).
Collectively, our results indicate that CD18 at wild-type expression levels leads to effective DC-Treg contacts for the induction of sufficient TGF-β1 expression by Tregs, while decreased CD18 expression promotes the psoriasiform skin disease in the Cd18hypo PL/J mouse model.
Our results led us to suggest a sequence of pathogenic events in the development of the psoriasiform skin disease where a decrease in CD18 expression impairs DC-Treg interaction, with diminished suppressive function and subsequent hyperactivation of effector T cells (21, 22). These activated pathogenic effector T cells contribute to the recruitment and activation of macrophages that subsequently overproduce the proinflammatory cytokine TNF-α, leading to the overall amplification of the inflammatory process and thus the psoriasiform skin disease (23). In fact, preliminary data from psoriasis patients suggest that CD4+CD25+CD127– Tregs reveal a decrease in their suppressive function compared with matched healthy controls (data not shown).
It remains to be further studied whether polymorphisms or mutations contributing to immune synapse and/or TCR activation in DC-Treg contacts may be responsible for the observed lack of suppression of autoimmune diseases in humans.
In conclusion, our data identified what we believe to be a previously unrecognized role for reduced CD18 expression on Tregs in a murine model of psoriasis, and thus hints at a central role for CD18 in TCR signaling in Tregs, which if defective may contribute to autoimmunity in general. This study highlights the critical role of CD18 in the function of Tregs that controls development of the psoriasiform skin disease and possibly other autoimmune diseases.
Methods
Mice.
PL/J mice with a hypomorphic mutation of the CD18 gene (Cd18hypo) were genotyped by Southern analysis (20). CD18+/+ littermates (Cd18wt) resulting from heterozygote crosses served as wild-type controls. Affected Cd18hypo PL/J mice with a strong psoriasiform phenotype were used. All mice were kept under specific pathogen-free conditions. All procedures were done in accordance with the guidelines for animal experimentation approved by the Regierungsprasidium, Tubingen, Germany.
Assessment of the psoriasiform skin disease.
To evaluate the severity of the psoriasiform phenotype, an adapted PASI score (22) was used for affected Cd18hypo mice before and after adoptive transfer of CD4+CD25+CD127– Tregs into affected Cd18hypo mice. For Cd18hypo mice, the PASI score was modified accordingly: 0, no symptoms; 1, slight erythema of the ears; 2, strong erythema of the ears; 3, slight hair loss on the head; 4, extensive hair loss, including the trunk; 5, slight hair loss, isolated scaling; 6, extensive hair loss, isolated scaling; 7, extensive hair loss, widespread slight scaling; 8, moderate scaling of a large area of the body; 9, widespread hair loss, strong scaling of a few smaller areas; 10, extensive hair loss, extensive scaling of a large area of the body.
FACS analysis.
The following mAbs were used for flow cytometry: FITC-conjugated rat anti-mouse CD25 (clone 7D4; BD Biosciences — Pharmingen), APC- conjugated or PerCP-conjugated rat anti-mouse CD4 (clone RM4-5; BD Biosciences — Pharmingen), PE-conjugated rat anti-mouse CD25 (clone PC61; eBioscience), PE-conjugated or APC-conjugated rat anti-mouse CD127 (clone A7R34; eBioscience), and biotinylated anti-human LAP (TGF-β1) (clone BAF246; R&D Systems). Cells isolated from thymus, blood, spleen, and skin DLNs of Cd18wt and Cd18hypo mice were processed for FACS analysis as previously described (23). To monitor Foxp3, cells were first stained for surface markers with anti-CD4, anti-CD25, and/or anti-CD127 mAbs (BD Biosciences — Pharmingen). After fixation and permeabilization, intracellular Foxp3 was measured using PE anti-mouse/rat Foxp3 Staining Set (eBioscience) according to the manufacturer’s protocols. To detect TGF-β1 expression on mouse Tregs, cultured Tregs with allogenic DCs were stained with anti-human LAP mAb, which was previously used to measure mouse TGF-β1 production by FACS analysis (61). TGF-β1 staining was performed for FACS analysis as previously described (62). In some experiments anti-human LAP was also employed to stain for TGF-β1 in CFSE-labeled Cd18wt Tregs that had been adoptively transferred into affected Cd18hypo mice 4 days before immunostaining (61). Isotype IgG were used as controls for all experiments.
Cell isolation.
CD4+CD25+CD127– Tregs were isolated from spleen of Cd18wt and Cd18hypo PL/J mice using the CD4+CD25+ Regulatory T Cell Isolation kit (Miltenyi Biotec) for in vivo injections and in vitro experiments. For CD4+CD25+CD127– Treg isolation from Cd18wt mice, the manufacturer’s protocol was used without any modification. For CD4+CD25+CD127– Treg isolation from Cd18hypo mice, we used a modified protocol in which CD127-PE (eBioscience) and GR-1-PE (Miltenyi Biotec) mAbs were added to negatively deplete CD127+ and GR-1+ cells using LD columns. Thereafter, CD25-PE mAb (Miltenyi Biotec) was used for a positive selection of CD4+CD25+CD127– Tregs. The MACS-sorted CD4+CD25+CD127– Treg population was more than 94% pure as determined by FACS. For in vitro suppression assays, we purified CD4+CD25– Tresp cells from Cd18wt or Cd18hypo mice with MACS beads (Miltenyi Biotec). The purity of CD4+CD25– Tresp cells was more than 94%.
Suppression assays.
CD4+CD25+CD127– Tregs and CD4+CD25– Tresp cells were both purified from the spleen of Cd18wt or Cd18hypo mice using MACS beads. In some experiments, purified Cd18wt Tregs were cocultured with allogeneic DCs in the presence of various concentrations of neutralizing antibody against CD18 or neutralizing antibody against CD11a (clone M17/4; BD Biosciences — Pharmingen) or isotype-matched IgG, with or without 500 units/ml recombinant murine IL-2 for 7 days. Thereafter, Tregs were separated from allogeneic DCs using CD11c microbeads (Miltenyi Biotec), extensively washed 3 times with PBS, and mixed at a ratio of 1:4 with Cd18wt Tresp cells. Tresp cells were labeled in complete medium with 2-μM CFSE for 8 minutes at 37°C in the dark and then washed 3 times with PBS. Tregs of Cd18wt and Cd18hypo PL/J mice were assessed for their ability to suppress proliferation by coculture with CFSE-labeled Tresp cells (1 × 105 cells/well) derived from either Cd18wt or affected Cd18hypo PL/J mice at different ratios. Tregs and Tresp cells were cocultured in the presence of plate-bound anti-CD3 (clone 145-2C11; BD Biosciences — Pharmingen) and soluble anti-CD28 (clone 37.51; BD Biosciences — Pharmingen) mAbs or in presence of 1 μg/ml soluble anti-CD3 and 1 × 105 T cell–depleted splenocytes (irradiated at 30 Gy) at 37°C and 5% CO2 for 3 days. In the indicated experiments, neutralizing antibodies against TGF-β1 (clone 1D11) were added to the Tregs and Tresp cells coculture. Dilution of CFSE labeling of Tresp cells was assessed as a measure of Tresp cell proliferation as previously described (63).
Mixed lymphocyte reaction.
To detect antigen-induced activation of Tregs by allogeneic irradiated DCs (irradiated with 30 Gy) of C57BL/6J mice, MACS-sorted CD4+CD25+CD127– Tregs derived from Cd18wt and Cd18hypo T cells were labeled with CFSE and cocultured in U-bottom 96-well plates (NUNC) with DCs in the presence of 500 units/ml recombinant murine IL-2 (Cell Concepts) for 7 days (35). Cells were then stained with CD4, CD25, and anti-human LAP (TGF-β1) mAbs and analyzed by FACS. DCs were prepared as previously described (23).
Adoptive transfer.
A total of 1 × 106 MACS-sorted CD4+CD25+CD127– Tregs from Cd18wt or Cd18hypo mice were transferred intravenously into affected Cd18hypo PL/J mice on both day 0 and day 30, with a total observation period of 42 days after the first injection. In a different set of experiments, 0.2 × 106 Cd18wt Tregs in 200 μl 1× PBS or 0.2 × 106 Cd18hypo Tregs in 200 μl 1× PBS (control) were injected intradermally at 4 sites (50 μl/site) into lesional skin on the back and/or neck of each mouse. Intradermal injections of Tregs were done once every week for a period of 21 days. Disease severity was determined by assessment of the clinical picture using an adapted PASI score as previously described (22). To detect TGF-β1 in Tregs derived from Cd18wt PL/J mice after adoptive transfer into affected Cd18hypo PL/J mice, 1 × 106 Cd18wt Tregs were injected intravenously into affected Cd18hypo mice, and the recipients were sacrificed 4 or 7 days after transfer for FACS analysis or immunostaining of TGF-β1. As antibody directed against CD18 only detects CD18 wild-type expression but not Cd18hypo expression levels in skin sections, adoptively transferred Cd18wt Tregs and concomitant TGF-β1 expression can be reliably identified by double staining with anti-CD18 (green fluorescence) and anti-mature TGF-β1 (red fluorescence) (MBL International Corp.), which results in yellow overlay. Two hundred and fifty micrograms of TGF-β neutralizing mAb (clone 1D11; BioExpress) or isotype control IgG was injected intraperitoneally into recipient mice 1 day after a transfer of 1 × 106 Cd18wt Tregs for 3 consecutive days. Subsequently, injections were performed every 3 days for a period of total 21 days.
Immunohistochemical analysis.
Immunostaining of sections from skin or skin DLNs was performed using a previously described protocol (23). Frozen cryosections of skin or DLNs were fixed in ice-cold acetone for 10 minutes before staining. To specifically show TGF-β1 expression of transferred Cd18wt Tregs in affected Cd18hypo PL/J mice, we used Alexa Fluor 488–conjugated CD18 mAb (clone GAME-46; BD Biosciences — Pharmingen) that was specific for the identification of Cd18wt Tregs, as low expression of CD18 on Cd18hypo T cells cannot be detected by immunostaining with this antibody. This allowed us to specifically trace transferred Cd18wt Tregs. We stained for TGF-β1 expression by Cd18wt Tregs with polyclonal anti-mature TGF-β1 Ab (MBL International Corp.) conjugated with Alexa Fluor 555. DAPI (Sigma-Aldrich) was used to stain nuclei. All antibodies were diluted in antibody diluent (catalog no. S3022; Dako) according to manufacturer’s protocols. Immunostainings were analyzed with a fluorescence microscope (Axioskop 2 plus; Zeiss).
Statistics.
Quantitative results are presented as mean ± SD. Mean values were tested by a 2-tailed Student’s t test. P values of less than 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We are grateful to Heidi Hainzl for technical assistance in the immunohistochemistry. This work was supported by the German Research Foundation (DFG) within the SFB 497 “Signals and Signal Processing during Cellular Differentiation” and the individual research grant SCHA 411/12-1. Work in X.Z. Yu’s lab was supported by NIH grants AI-063553 and CA-118116.
Footnotes
Nonstandard abbreviations used: Cd18hypo, Cd18 hypomorphic mutation; DLN, draining lymph node; MLR, mixed leukocyte reaction; PASI, psoriasis activity and severity index; Tresp cell, responder T cell.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:2629–2639 (2008). doi:10.1172/JCI34916
References
- 1.Schon M.P., Boehncke W.H. Psoriasis. N. Engl. J. Med. 2005;352:1899–1912. doi: 10.1056/NEJMra041320. [DOI] [PubMed] [Google Scholar]
- 2.Gottlieb A.B., Bos J.D. Recombinantly engineered human proteins: transforming the treatment of psoriasis. Clin Immunol. 2002;105:105–116. doi: 10.1006/clim.2002.5289. [DOI] [PubMed] [Google Scholar]
- 3.Schon M.P., Detmar M., Parker C.M. Murine psoriasis-like disorder induced by naive CD4+ T cells. Nat. Med. 1997;3:183–188. doi: 10.1038/nm0297-183. [DOI] [PubMed] [Google Scholar]
- 4.Sano S., et al. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat. Med. 2005;11:43–49. doi: 10.1038/nm1162. [DOI] [PubMed] [Google Scholar]
- 5.Conrad C., et al. Alpha1beta1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nat. Med. 2007;13:836–842. doi: 10.1038/nm1605. [DOI] [PubMed] [Google Scholar]
- 6.Gottlieb S.L., et al. Response of psoriasis to a lymphocyte-selective toxin (DAB389IL-2) suggests a primary immune, but not keratinocyte, pathogenic basis. Nat. Med. 1995;1:442–447. doi: 10.1038/nm0595-442. [DOI] [PubMed] [Google Scholar]
- 7.Owen C.M., Harrison P.V. Successful treatment of severe psoriasis with basiliximab, an interleukin-2 receptor monoclonal antibody. Clin. Exp. Dermatol. 2000;25:195–197. doi: 10.1046/j.1365-2230.2000.00612.x. [DOI] [PubMed] [Google Scholar]
- 8.Morel P., et al. Anti-CD4 monoclonal antibody therapy in severe psoriasis. J. Autoimmun. 1992;5:465–477. doi: 10.1016/0896-8411(92)90006-C. [DOI] [PubMed] [Google Scholar]
- 9.Sugiyama H., et al. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J. Immunol. 2005;174:164–173. doi: 10.4049/jimmunol.174.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brusko T., et al. No alterations in the frequency of FOXP3+ regulatory T-cells in type 1 diabetes. Diabetes. 2007;56:604–612. doi: 10.2337/db06-1248. [DOI] [PubMed] [Google Scholar]
- 11.Putheti P., Pettersson A., Soderstrom M., Link H., Huang Y. Circulating CD4+CD25+ T regulatory cells are not altered in multiple sclerosis and unaffected by disease-modulating drugs. . J. Clin. Immunol. 2004;24:155–161. doi: 10.1023/B:JOCI.0000019780.93817.82. [DOI] [PubMed] [Google Scholar]
- 12.Ehrenstein M.R., et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J. Exp. Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baecher-Allan C., Brown J.A., Freeman G.J., Hafler D.A. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 14.Sakaguchi S., et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol. Rev. 2001;182:18–32. doi: 10.1034/j.1600-065X.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 15.Fontenot J.D., Gavin M.A., Rudensky A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 16.Seddiki N., et al. Persistence of naive CD45RA+ regulatory T cells in adult life. Blood. 2006;107:2830–2838. doi: 10.1182/blood-2005-06-2403. [DOI] [PubMed] [Google Scholar]
- 17.Seddiki N., et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu W., et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartigan-O’Connor D.J., Poon C., Sinclair E., McCune J.M. Human CD4+ regulatory T cells express lower levels of the IL-7 receptor alpha chain (CD127), allowing consistent identification and sorting of live cells. J. Immunol. Methods. 2007;319:41–52. doi: 10.1016/j.jim.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Wilson R.W., et al. Gene targeting yields a CD18-mutant mouse for study of inflammation. . J. Immunol. 1993;151:1571–1578. [PubMed] [Google Scholar]
- 21.Bullard D.C., et al. A polygenic mouse model of psoriasiform skin disease in CD18-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 1996;93:2116–2121. doi: 10.1073/pnas.93.5.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kess D., et al. CD4+ T cell-associated pathophysiology critically depends on CD18 gene dose effects in a murine model of psoriasis. J. Immunol. 2003;171:5697–5706. doi: 10.4049/jimmunol.171.11.5697. [DOI] [PubMed] [Google Scholar]
- 23.Wang H., et al. Activated macrophages are essential in a murine model for T cell-mediated chronic psoriasiform skin inflammation. J. Clin. Invest. 2006;116:2105–2114. doi: 10.1172/JCI27180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hynes R.O. Integrins: a family of cell surface receptors. Cell. 1987;48:549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- 25.Griffiths C.E., Railan D., Gallatin W.M., Cooper K.D. The ICAM-3/LFA-1 interaction is critical for epidermal Langerhans cell alloantigen presentation to CD4+ T cells. Br. J. Dermatol. 1995;133:823–829. doi: 10.1111/j.1365-2133.1995.tb06911.x. [DOI] [PubMed] [Google Scholar]
- 26.Gencik M., et al. The association of CD18 alleles with anti-myeloperoxidase subtypes of ANCA-associated systemic vasculitides. Clin. Immunol. 2000;94:9–12. doi: 10.1006/clim.1999.4811. [DOI] [PubMed] [Google Scholar]
- 27.Meller S., et al. Novel SNPs in the CD18 gene validate the association with MPO-ANCA+ vasculitis. Genes Immun. 2001;2:269–272. doi: 10.1038/sj.gene.6363781. [DOI] [PubMed] [Google Scholar]
- 28.van de Kerkhof P.C., Weemaes C.M. Skin manifestations in congenital deficiency of leucocyte-adherence glycoproteins (CDLG). Br. J. Dermatol. 1990;123:395–401. doi: 10.1111/j.1365-2133.1990.tb06301.x. [DOI] [PubMed] [Google Scholar]
- 29.Tomfohrde J., et al. Gene for familial psoriasis susceptibility mapped to the distal end of human chromosome 17q. Science. 1994;264:1141–1145. doi: 10.1126/science.8178173. [DOI] [PubMed] [Google Scholar]
- 30.Van Seventer G.A., Shimizu Y., Horgan K.J., Shaw S. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J. Immunol. 1990;144:4579–4586. [PubMed] [Google Scholar]
- 31.Shier P., et al. Impaired immune responses toward alloantigens and tumor cells but normal thymic selection in mice deficient in the beta2 integrin leukocyte function-associated antigen-1. J. Immunol. 1996;157:5375–5386. [PubMed] [Google Scholar]
- 32.Damle N.K., Klussman K., Aruffo A. Intercellular adhesion molecule-2, a second counter-receptor for CD11a/CD18 (leukocyte function-associated antigen-1), provides a costimulatory signal for T-cell receptor-initiated activation of human T cells. J. Immunol. 1992;148:665–671. [PubMed] [Google Scholar]
- 33.Gavin M.A., Clarke S.R., Negrou E., Gallegos A., Rudensky A. Homeostasis and anergy of CD4+CD25+ suppressor T cells in vivo. Nat. Immunol. 2002;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi T., et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 35.Yamazaki S., et al. Effective expansion of alloantigen-specific Foxp3+ CD25+ CD4+ regulatory T cells by dendritic cells during the mixed leukocyte reaction. Proc. Natl. Acad. Sci. U. S. A. 2006;103:2758–2763. doi: 10.1073/pnas.0510606103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamazaki S., et al. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J. Exp. Med. 2003;198:235–247. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cozzo C., Larkin J., 3rd, Caton A.J. Cutting edge: self-peptides drive the peripheral expansion of CD4+CD25+ regulatory T cells. J. Immunol. 2003;171:5678–5682. doi: 10.4049/jimmunol.171.11.5678. [DOI] [PubMed] [Google Scholar]
- 38.Kretschmer K., et al. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 39.Tang Q., et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat. Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson D.C., Miller L.J., Schmalstieg F.C., Rothlein R., Springer T.A. Contributions of the Mac-1 glycoprotein family to adherence-dependent granulocyte functions: structure-function assessments employing subunit-specific monoclonal antibodies. J. Immunol. 1986;137:15–27. [PubMed] [Google Scholar]
- 41.Piccirillo C.A., et al. CD4+CD25+ Regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J. Exp. Med. 2002;196:237–246. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakamura K., et al. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J. Immunol. 2004;172:834–842. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 43.Marski M., Kandula S., Turner J.R., Abraham C. CD18 is required for optimal development and function of CD4+CD25+ T regulatory cells. . J. Immunol. 2005;175:7889–7897. doi: 10.4049/jimmunol.175.12.7889. [DOI] [PubMed] [Google Scholar]
- 44.Tarbell K.V., Yamazaki S., Olson K., Toy P., Steinman R.M. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J. Exp. Med. 2004;199:1467–1477. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salomon B., et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/S1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 46.Tang Q., et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J. Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 47.Judkowski V., et al. Identification of MHC class II-restricted peptide ligands, including a glutamic acid decarboxylase 65 sequence, that stimulate diabetogenic T cells from transgenic BDC2.5 nonobese diabetic mice. J. Immunol. 2001;166:908–917. doi: 10.4049/jimmunol.166.2.908. [DOI] [PubMed] [Google Scholar]
- 48.Gaylor M.L., Duvic M. Generalized pustular psoriasis following withdrawal of efalizumab. J. Drugs Dermatol. . 2004;3:77–79. [PubMed] [Google Scholar]
- 49.Sakaguchi N., et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 2003;426:454–460. doi: 10.1038/nature02119. [DOI] [PubMed] [Google Scholar]
- 50.Alarcon-Riquelme M.E. A RUNX trio with a taste for autoimmunity. Nat. Genet. 2003;35:299–300. doi: 10.1038/ng1203-299. [DOI] [PubMed] [Google Scholar]
- 51.Ghoreschi K., et al. Interleukin-4 therapy of psoriasis induces Th2 responses and improves human autoimmune disease. Nat. Med. 2003;9:40–46. doi: 10.1038/nm804. [DOI] [PubMed] [Google Scholar]
- 52.Asadullah K., et al. IL-10 is a key cytokine in psoriasis. Proof of principle by IL-10 therapy: a new therapeutic approach. J. Clin. Invest. 1998;101:783–794. doi: 10.1172/JCI1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Read S., Malmstrom V., Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. . J. Exp. Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Belghith M., et al. TGF-[beta]-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat. Med. 2003;9:1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 55.Ochi H., et al. Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4+ CD25– LAP+ T cells. . Nat. Med. 2006;12:627–635. doi: 10.1038/nm1408. [DOI] [PubMed] [Google Scholar]
- 56.Nakamura K., Kitani A., Strober W. Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T Cells is mediated by cell surface-bound transforming growth factor beta. . J. Exp. Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peng Y., Laouar Y., Li M.O., Green E.A., Flavell R.A. TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4572–4577. doi: 10.1073/pnas.0400810101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shull M.M., et al. Targeted disruption of the mouse transforming growth factor-[beta]1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kulkarni A.B., et al. Transforming growth factor beta1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl. Acad. Sci. U. S. A. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghiringhelli F., et al. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J. Exp. Med. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ishikawa H., et al. Inhibition of autoimmune diabetes by oral administration of anti-CD3 monoclonal antibody. Diabetes. 2007;56:2103–2109. doi: 10.2337/db06-1632. [DOI] [PubMed] [Google Scholar]
- 62.Garba M.L., Frelinger J.A. Intracellular cytokine staining for TGF-[beta]. J. Immunol. Methods. 2001;258:193–198. doi: 10.1016/S0022-1759(01)00491-4. [DOI] [PubMed] [Google Scholar]
- 63.Venken K., et al. A CFSE based assay for measuring CD4+CD25+ regulatory T cell mediated suppression of auto-antigen specific and polyclonal T cell responses. J. Immunol. Methods. 2007;322:1–11. doi: 10.1016/j.jim.2007.01.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.