Abstract
Study Objectives:
The aim of our study was to determine which muscle or combination of muscles (either axial or limb muscles, lower or upper limb muscles, or proximal or distal limb muscles) provides the highest rates of rapid eye movement (REM) sleep phasic electromyographic (EMG) activity seen in patients with REM sleep behavior disorder (RBD).
Setting:
Two university hospital sleep disorders centers.
Participants:
Seventeen patients with idiopathic RBD (n = 8) and RBD secondary to Parkinson disease (n = 9).
Interventions:
Not applicable.
Measurements and Results:
Patients underwent polysomnography, including EMG recording of 13 different muscles. Phasic EMG activity in REM sleep was quantified for each muscle separately. A mean of 1459.6 ± 613.8 three-second REM sleep mini-epochs were scored per patient. Mean percentages of phasic EMG activity were mentalis (42 ± 19), flexor digitorum superficialis (29 ± 13), extensor digitorum brevis (23 ± 12), abductor pollicis brevis (22 ± 11), sternocleidomastoid (22 ± 12), deltoid (19 ± 11), biceps brachii (19 ± 11), gastrocnemius (18 ± 9), tibialis anterior (right, 17 ± 12; left, 16 ± 10), rectus femoris (left, 11 ± 6; right, 9 ± 6), and thoraco-lumbar paraspinal muscles (6 ± 5). The mentalis muscle provided significantly higher rates of excessive phasic EMG activity than all other muscles but only detected 55% of all the mini-epochs with phasic EMG activity. Simultaneous recording of the mentalis, flexor digitorum superficialis, and extensor digitorum brevis muscles detected 82% of all mini-epochs containing phasic EMG activity. This combination provided higher rates of EMG activity than any other 3-muscle combination. Excessive phasic EMG activity was more frequent in distal than in proximal muscles, both in upper and lower limbs.
Conclusion:
Simultaneous recording of the mentalis, flexor digitorum superficialis, and extensor digitorum brevis muscles provided the highest rates of REM sleep phasic EMG activity in subjects with RBD.
Citation:
Frauscher B; Iranzo A; Högl B; Casanova-Molla J; Salamero M; Gschliesser V; Tolosa E; Poewe W; Santamaria J;. Quantification of electromyographic activity during REM sleep in multiple muscles in REM sleep behavior disorder. SLEEP 2008;31(5):724-731.
Keywords: REM sleep behavior disorder, phasic electromyographic activity, REM sleep
RAPID EYE MOVEMENT (REM) SLEEP BEHAVIOR DISORDER (RBD) IS A PARASOMNIA CHARACTERIZED BY DREAM-ENACTING BEHAVIORS ASSOCIATED WITH unpleasant dreams and loss of normal REM sleep muscle atonia.1 The International Classification of Sleep Disorders-2 established that the diagnosis of RBD requires demonstration of REM sleep without atonia by polysomnography,2 mainly because other sleep disorders can mimic the clinical features of RBD.3 The International Classification of Sleep Disorders-2 defined REM sleep without atonia as the “electromyographic (EMG) finding of excessive amounts of sustained or intermittent elevation of submental EMG tone or excessive phasic submental or (upper or lower) limb EMG twitching.”2 This definition has several limitations. First, a precise definition of “excessive amounts of tonic and phasic EMG activity” was not provided, since normal values of these measures are unknown. Second, it is not stated how the tonic and phasic EMG activity have to be measured. Third, it is unclear which muscle or combination of muscles of the body (either axial or extremity muscles, lower or upper extremity muscles, or proximal or distal extremity muscles) provides the highest rates of abnormal REM sleep EMG activity in RBD. Previous studies in RBD have evaluated the EMG activity in several combinations of muscles, including (1) exclusively the mentalis or the submentalis,4–12 (2) the mentalis or submentalis plus left and right anterior tibialis,13,14 and (3) mentalis or submentalis plus right and left tibialis anterior, and either brachioradialis,15,16 biceps brachii,17–19 extensor digitorum,20,21 or carpi radialis.22 The aim of our study was to determine which muscle or combination of muscles provides the highest rates of phasic EMG activity occurring during REM sleep in patients with RBD.
PATIENTS AND METHODS
Patient Selection
Seventeen consecutive patients from the sleep laboratories of the departments of neurology of Innsbruck Medical University, Austria (n = 12), and Hospital Clinic de Barcelona, Spain (n = 5) with the diagnosis of idiopathic RBD and secondary RBD due to Parkinson disease (PD) were included. All patients granted written informed consent for this study. Diagnosis of RBD required (1) history of dream-enacting behaviors and (2) nocturnal video-polysomnographic demonstration of prominent tonic and/or phasic EMG activity associated with abnormal behaviors and absence of electroencephalographic epileptiform activity during REM sleep.2 Exclusion criteria were current treatment with clonazepam, history of bruxism, and an apnea-hypopnea index more than 10 per hour. Diagnosis of idiopathic RBD required absence of cognitive and motor complaints and normal neurologic examination. The diagnosis of PD was made according to the United Kingdom PD Society Brain Bank criteria.23 Patients' demographic and clinical data, concomitant central nervous system active medications, and daily levodopa equivalent dose24 were recorded.
Nocturnal Video-Polysomnography
All patients underwent 2 consecutive full-night video-polysomnographic studies. The first night was used for adaptation to the sleep laboratory, to confirm the diagnosis of RBD, and to exclude patients with artifacts in the EMG channels due to snoring, bruxism, or apnea-induced arousals that would interfere with the EMG quantification. The second night was used for analysis of the EMG activity in REM sleep.
Polysomnography was performed with a digital polygraph (Innsbruck: Schwarzer Brainlab, software version 3.00, Munich, Germany; Barcelona: Deltamed, software version 1998, Paris, France) and consisted of vertical and horizontal electrooculography, electroencephalography (O2-A1, O1-A2, C4-A1, C3-A2), surface EMG of 13 muscles, electrocardiography, nasal and oral air flow, thoracic and abdominal respiratory effort, oxygen saturation, microphone and synchronized digital videography. Sleep stages were scored according to Rechtschaffen and Kales criteria,25 with allowance to score REM sleep despite sustained EMG activity in the mentalis channel. The occurrence of the first REM in the electrooculographic channel was used to determine the onset of a REM sleep period. The end of a REM sleep period was determined either when no REMs were detected in 3 consecutive minutes or when an awakening, K complexes, or spindles were observed.7 Scoring of sleep stages and quantification of phasic EMG activity in REM sleep were done by different investigators.
Analysis of EMG Activity
Phasic EMG activity of 13 muscles in different localizations of the human body was quantified during REM sleep. We evaluated cranial nerve-innervated muscles (mentalis and sternocleidomastoid), trunk (thoraco-lumbar paraspinal muscles), proximal upper limb (deltoid and biceps brachii), distal upper limb (flexor digitorum superficialis and abductor pollicis brevis), proximal lower limb (rectus femoris), and distal lower limb (tibialis anterior, gastrocnemius, and extensor digitorum brevis) muscles. Rectus femoris and tibialis anterior activity was recorded bilaterally. Because of channel-number limitation, the remaining muscles were evaluated on 1 side of the body. The left side was chosen because, in case of unilateral movements during sleep, these movements have been reported to be more frequent in the nondominant limb.26 Bipolar surface electrodes were attached to the skin with each electrode spaced 2 to 3 cm apart. The EMG activity was analyzed using amplification at 5 microvolts per mm, low-frequency filter at 10 Hz, high-frequency filter at 100 Hz, and a sampling rate of 256 Hz. Impedances of surface EMG electrodes had to be lower than 10 kΩ.
In each patient, phasic EMG activity was evaluated in mini-epochs of 3 seconds in all REM sleep periods. Quantification of phasic EMG activity was done visually. A phasic EMG event was defined as any burst of EMG activity lasting 0.1 to 5 seconds with an amplitude exceeding twice the background EMG activity.4 Each EMG channel was scored separately on the computer screen. After finishing the scoring of 1 EMG channel, the process was repeated in the remaining muscles. Every mini-epoch in each EMG channel was scored as “0” when phasic EMG activity was not present, “1” when phasic EMG activity was present, or “3” when an artifact prevented optimal scoring. Artifact scores were excluded from analysis (0.4% of all possible observations). A scoring consensus between the investigators of the 2 centers participating in this study was reached by reviewing together several polysomnographic studies. In addition, to calculate the interrater reliability, a sample of 100 mini-epochs belonging to different REM sleep episodes of different patients were scored independently by each sleep center. κ coefficients were calculated for each muscle. The mean κ coefficient was 0.872 (range, 0.72–1). The scores were entered into a database for analysis. In each muscle, the mean phasic EMG activity was expressed as the percentage of the 3-second mini-epochs containing phasic EMG events in REM sleep. For aggregate analysis, phasic EMG activity in each mini-epoch was calculated for the whole limb (upper or lower) and for proximal and distal muscles within each limb. Phasic EMG activity was not analyzed during non-REM sleep.
Periodic leg movements in sleep (PLMS) were scored in both the right and left tibialis anterior muscles following standard criteria.27 In addition, we used the same criteria for scoring PLMS in the left extensor digitorum brevis muscle. The PLMS index was calculated as the number of periodic leg movements per hour of sleep. In order to distinguish PLMS from RBD-related phasic EMG activity in the tibialis anterior and the extensor digitorum brevis muscles, we first reviewed each REM sleep period in general and carefully identified PLMS based upon (1) their regular periodicity and EMG criteria27 and (2) their characteristic appearance by videography. Once PLMS were identified, they were excluded from the quantitative analysis of RBD-related phasic EMG activity.
Statistical Analysis
SPSS 12.0 for Windows (SPSS, Inc., Chicago, IL) was used for all statistical analyses. Descriptive values are given as means ± standard deviations or frequencies (percentages), as applicable. To test for normal distribution, Shapiro-Wilks test was used. The distribution of all measures of phasic EMG activity for different muscles followed a normal distribution across individuals; therefore Student t-test for comparison between different RBD etiology (classified in idiopathic RBD and RBD associated with PD) and Bonferroni adjusted analysis of variance for repeated measurements were used to analyze differences of phasic EMG activity in respect to individual body regions and individual muscles.
For all mini-epochs, distal and proximal limb phasic EMG activity was calculated for the upper limb and for the lower limb. To examine whether distal limb muscles had a different frequency of phasic EMG activity than proximal limb muscles, a paired Student t-test was performed for muscles of the upper limbs and the lower limbs. In addition, for all mini-epochs, simultaneous and nonsimultaneous phasic EMG activity in upper limb and lower limb muscles was counted. To examine whether upper and lower limb muscles were activated differently, we used the Student t-test. We also evaluated simultaneous and nonsimultaneous phasic EMG activity in distal and proximal limb muscles using the McNemar χ2 test with continuity correction.
For all the mini-epochs, bilaterally simultaneous and unilateral left and right tibialis anterior phasic EMG activity was calculated. A similar procedure was done for the left and right rectus femoris. To examine whether bilaterally simultaneous phasic EMG activity was more frequent than unilateral phasic EMG activity in each pair of muscles, a paired Student t-test was performed.
To determine the combination of muscles resulting in the highest rates of phasic EMG activity, all pairs of muscles were first analyzed. Once the pair of muscles showing the highest rates of phasic EMG activity was found, we sequentially added the remaining muscles to find the 3-muscle combination with the highest rate of phasic EMG activity. The same procedure was repeated increasing the number of muscles until reaching a 95% threshold in detecting phasic EMG activity in all mini-epochs. A Student t-test for paired samples was used to compare the rates of phasic EMG activity of the different combinations of muscles.
Differences in PLMS indices between the left and right tibialis anterior muscles and extensor digitorum brevis muscle were calculated using nonparametric Friedman test, since these indices were not normally distributed. Linear logistic regression analysis (Wald backward procedure) was performed to analyze potential relations between total phasic EMG activity and RBD duration, sex, age, and daily levodopa-equivalent dose.
RESULTS
We studied 17 right-handed patients with RBD, 13 men and 4 women, with a mean age of 60.8 ± 9.7 (range, 43–73) years and a mean RBD duration of 6.1 ± 4.8 (range, 1–20) years. Eight patients had idiopathic RBD (mean age, 68.0 ± 4.1 years), and 9 had RBD associated with PD (mean age, 54.3 ± 8.7 years). None of the patients with idiopathic RBD was taking central nervous system active drugs. Patients with PD were taking dopaminergic agents (n = 8), hypnotics (n = 3), antidepressants (n = 2), and neuroleptics (n = 2). Patients' demographic and clinical data and use of psychoactive medications are presented in Table 1. Polysomnographic data are shown in Table 2. A mean of 1459.6 ± 613.8 3-second mini-epochs were analyzed per patient.
Table 1.
Demographic, Clinical, and CNS-Active–Drug Characteristics in Patients with RBD
| Patients with idiopathic RBD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|
| Age, y | 73 | 72 | 72 | 67 | 67 | 62 | 68 | 63 | |
| Sex | m | m | m | m | f | m | m | m | |
| RBD duration, y | 4 | 4 | 7 | 12 | 7 | 20 | 10 | 3 | |
| Patients with secondary RBD due to PD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| Age, y | 63 | 43 | 45 | 57 | 63 | 67 | 47 | 53 | 51 |
| Sex | m | f | m | f | m | m | f | m | m |
| Hoehn – Yahr stage in “on” | 1.5 | 2.5 | 3 | 3 | 3 | 2 | 3 | 3 | 4 |
| Age at onset of parkinsonism, y | 60 | 39 | 33 | 50 | 58 | 57 | 43 | 44 | 30 |
| RBD duration, y | 3 | 1 | 2 | 7 | 2 | 10 | 3 | 5 | 3 |
| Daily levodopa equivalent dose, mg | 0 | 405 | 600 | 1060 | 1065 | 1000 | 535 | 1200 | 1420 |
| Antidepressants | Isocarboxazide | - | - | - | - | - | Amitriptyline | - | - |
| Atypical neuroleptics | - | - | - | - | Quetiapine | - | - | Quetiapine | - |
| Hypnotics | - | - | - | Bromazepam | - | - | - | Zolpidem | Zolpidem |
| Timing of RBD onset as related to treatment onset with CNS active drugs | After | After | After | After | After | Before | After | After | After |
| Clinical effect of different medications on RBD symptoms | No effecta | No effecta | No effecta | No effecta | No effecta | No effecta | No effecta | No effecta | No effecta |
m refers to male; f, female; PD: Parkinson disease
Rapid eye movement sleep behavior disorder (RBD) symptoms persisted after discontinuation of previous central nervous system (CNS) active medication.
Table 2.
Polysomnographic Variables of Patients with RBD
| Mean ± SD | |
|---|---|
| Time in bed, min | 466.6 ± 22.1 |
| Total sleep time, min | 370.1 ± 25.2 |
| Sleep efficiency, % | 79.5 ± 6.0 |
| Wake after sleep onset, min | 78.6 ± 28.0 |
| Sleep stage, % Total sleep time | |
| 1 | 18.1 ± 9.2 |
| 2 | 54 ± 10.7 |
| 3-4 | 7.6 ± 11.3 |
| REM | 20.0 ± 6.7 |
| Sleep-onset latency (S2), min | 14.2 ± 9.7 |
| REM sleep latency, min | 109.5 ± 75.9 |
| REM periods, no. | 3.4 ± 1.0 |
| Three-second REM sleep mini-epochs, no. | 1459.6 ± 613.8 |
RBD refers to rapid eye movement (REM) sleep behavior disorder.
Rates of Phasic EMG Activity in REM Sleep in the Different Muscles
Table 3 and Figure 1 show the mean percentage, standard deviation, and range of muscle activity in individual muscles for each patient and the mean for the whole group of patients.
Table 3.
Percentage of Phasic Electromyographic Activity in Individual Muscles for Each Patient According to RBD Etiology (Idiopathic or PD) and for the Whole Group of Patients
| Idiop | Ment | Ster | Delt | Bic | Flex | Abd | Pasp | Lfem | Rfem | Lta | Rta | Gast | Ext |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 14.3 | 5.2 | 4.2 | 3.6 | 28.7 | 13.0 | 10.7 | 2.0 | 1.6 | 1.0 | 0.0 | 8.8 | 11.7 |
| 2 | 59.8 | 30.9 | 33.3 | 37.0 | 42.1 | 25.9 | 6.7 | 9.7 | 5.7 | 6.5 | 4.6 | 7.1 | 17.0 |
| 3 | 70.7 | 41.8 | 35.0 | 26.6 | 39.2 | 45.8 | 17.2 | 5.2 | 7.7 | 8.1 | 8.8 | 13.9 | 13.1 |
| 4 | 38.2 | 28.9 | 17.5 | 16.8 | 27.2 | 28.6 | 8.2 | 3.8 | 3.2 | 3.5 | 3.7 | 9.5 | 10.9 |
| 5 | 60.6 | 45.1 | 22.9 | 33.1 | 13.6 | 36.0 | 11.6 | 9.8 | 11.0 | 4.2 | 11.5 | 17.7 | 13.6 |
| 6 | 26.7 | 10.9 | 10.1 | 6.8 | 32.0 | 26.9 | 1.8 | 8.1 | 6.4 | 22.6 | 31.0 | 25.0 | 36.9 |
| 7 | 51.4 | 21.5 | 19.4 | 16.9 | 31.1 | 23.8 | 5.4 | 7.5 | 7.7 | 20.1 | 17.7 | 26.7 | 28.5 |
| 8 | 29.4 | 15.2 | 17.7 | 17.1 | 25.9 | 29.4 | 2.7 | 15.6 | 4.3 | 18.3 | 14.3 | 12.4 | 24.2 |
| PD-related RBD | |||||||||||||
| 1 | 40.8 | 22.1 | 29.0 | 26.1 | 48.5 | 31.4 | 11.2 | 26.0 | 23.6 | 28.7 | 37.2 | 31.2 | 40.3 |
| 2 | 25.9 | 10.7 | 16.8 | 21.9 | 33.4 | 23.6 | 4.6 | 10.5 | 10.6 | 14.5 | 12.5 | 10.8 | 32.7 |
| 3 | 22.6 | 15.2 | 4.1 | 4.3 | 15.2 | 19.3 | 1.5 | 12.9 | 9.0 | 18.1 | 19.1 | 12.5 | 8.7 |
| 4 | 61.3 | 33.6 | 24.9 | 22.2 | 32.8 | 24.3 | 1.6 | 17.5 | 2.4 | 31.1 | 30.3 | 26.4 | 42.1 |
| 5 | 14.7 | 7.2 | 3.7 | 2.9 | 7.6 | 12.9 | 0.7 | 5.4 | 3.9 | 8.4 | 7.0 | 8.0 | 12.6 |
| 6 | 65.0 | 27.0 | 32.5 | 29.4 | 45.3 | 3.4 | 4.5 | 16.6 | 13.3 | 23.1 | 24.5 | 21.4 | 28.4 |
| 7 | 53.4 | 26.6 | 18.6 | 20.7 | 35.0 | 4.8 | 8.5 | 12.7 | 13.2 | 30.8 | 28.9 | 30.0 | 41.9 |
| 8 | 19.1 | 6.3 | 1.7 | 1.5 | 5.0 | 5.0 | 0.4 | 3.8 | 3.8 | 9.6 | 5.4 | 8.9 | 13.7 |
| 9 | 51.2 | 25.7 | 29.5 | 33.0 | 33.0 | 18.0 | 9.4 | 21.2 | 23.0 | 23.4 | 40.0 | 31.3 | 10.1 |
| Whole | |||||||||||||
| Mean | 41.5 | 22.0 | 18.9 | 18.8 | 29.1 | 21.9 | 6.3 | 11.1 | 8.8 | 16.0 | 17.4 | 17.7 | 22.7 |
| SD | 18.4 | 11.7 | 10.8 | 11.2 | 12.1 | 11.2 | 4.6 | 6.4 | 6.3 | 9.6 | 12.1 | 8.7 | 11.9 |
| Min | 14.3 | 5.2 | 1.7 | 1.5 | 5.0 | 3.4 | 0.4 | 2.0 | 1.6 | 1.0 | 0.0 | 7.1 | 8.7 |
| Max | 70.7 | 45.1 | 35.0 | 37.0 | 48.5 | 45.8 | 17.2 | 26.0 | 23.6 | 31.1 | 40.0 | 31.3 | 42.1 |
RBD refers to rapid eye movement sleep behavior disorder; idiop, idiopathic; PD, Parkinson disease; Ment, mentalis muscle; Ster, sternocleidomastoid muscle; Delt, deltoid muscle; Bic, biceps brachii muscle; Flex, flexor digitorum superficialis muscle; Abd, abductor pollicis brevis muscle; Pasp, thoraco-lumbar paraspinal muscles; Lfem, left rectus femoris muscle; Rfem, right rectus femoris muscle; Lta, left tibialis anterior muscle; Rta, right tibialis anterior muscle; Gast, gastrocnemius muscle; Ext, extensor digitorum brevis muscle.
Figure 1.
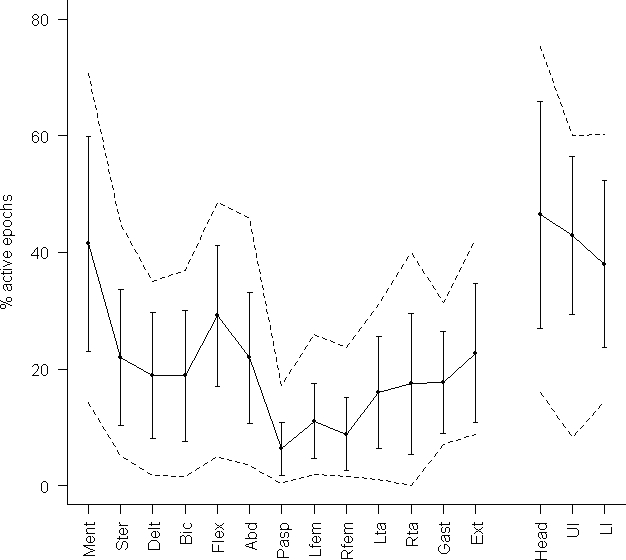
Mean phasic electromyographic activity (continuous line), standard deviation (vertical bars) and range (discontinuous lines) over individual muscles and (right side of the figure) over 3 different regions of the body (head, upper limb, lower limb). Ment refers to mentalis muscle; Ster, sternocleidomastoid muscle; Delt, deltoid muscle; Bic, biceps brachii muscle; Flex, flexor digitorum superficialis muscle; Abd, abductor pollicis brevis muscle; Pasp, thoraco-lumbar paraspinal muscles; Lfem, left rectus femoris muscle; Rfem, right rectus femoris muscle; Lta, left tibialis anterior muscle; Rta, right tibialis anterior muscle; Gast, gastrocnemius muscle; Ext, extensor digitorum brevis muscle. Head, Mentalis plus sternocleidomastoid muscles; Ul, upper limb muscles; Ll, lower limb muscles.
The highest rates of phasic EMG activity were found in the mentalis, flexor digitorum superficialis, and extensor digitorum brevis. In individual muscles, the mentalis showed a statistically significant higher rate of phasic EMG activity than the remaining muscles except the flexor digitorum superficialis (P = 0.390) and the extensor digitorum brevis (P = 0.156). The greatest amount of phasic EMG activity was detected in cranial nerve-innervated muscles (46.5% ± 19.5%), followed by muscles of the upper (42.9%% ± 13.6%), and lower limbs (38.0% ± 14.3%), although these differences were not statistically significant (p > 0.12). Phasic EMG activity was less prominent in the axial paraspinal muscles (6.3% ± 4.6%, P < 0.0001).
The rates of phasic EMG activity in several combinations of muscles for each patient are shown in Table 4. Simultaneous recording of the mentalis, flexor digitorum superficialis, and extensor digitorum brevis detected phasic EMG activity in 82% (mean, 954 mini-epochs) of all the mini-epochs containing phasic EMG activity (mean, 1033 mini-epochs; 95% confidence interval [CI], 770–1295). This was similar in patients with idiopathic RBD and in those with RBD related to PD. The combination of mentalis, flexor digitorum superficialis, and extensor digitorum brevis provided higher rates of phasic EMG activity than the standard combination used in routine polysomnographic studies (mentalis plus left and right tibialis anterior) and than any other 3-muscle combination, including those currently used in sleep centers for RBD evaluation, such as mentalis, flexor digitorum superficialis, and tibialis anterior or mentalis, biceps brachii, and tibialis anterior (Table 5). The muscles showing the lowest rates of phasic EMG activity not simultaneous with other muscles were the rectus femoris, deltoid, biceps brachii, and paraspinal. A combination of at least 7 muscles was necessary to reach a 95% threshold in detecting phasic EMG activity in all mini-epochs.
Table 4.
Rates of Phasic Electromyographic Activity in Several Combinations of Muscles for Each Patient
| RBD patient | A ment | B ment+flex | C ment+flex+ext | D ment+flex+ext+abd | E ment+flex+ext+ abd+ster | F ment+flex+ext+ abd+ster+lta | G ment+flex+ext+abd+ster+lta+gast |
|---|---|---|---|---|---|---|---|
| Idiopathic RBD | |||||||
| 1 | 23.8 | 63.8 | 74 | 82.2 | 83.8 | 83.8 | 89.2 |
| 2 | 75.4 | 90.7 | 92.3 | 95 | 96.1 | 96.5 | 96.6 |
| 3 | 81.4 | 88.7 | 90.3 | 93.8 | 97.6 | 97.8 | 98.3 |
| 4 | 55.6 | 76.6 | 79.8 | 89.1 | 93.2 | 93.4 | 94.6 |
| 5 | 72.1 | 75.6 | 78.9 | 88.7 | 96 | 96.1 | 96.8 |
| 6 | 33.7 | 62.3 | 80.7 | 86.6 | 88.5 | 91.9 | 96.1 |
| 7 | 63.6 | 78.2 | 86.3 | 90.1 | 92.2 | 94.4 | 97.2 |
| 8 | 42.2 | 65.5 | 76.8 | 85.2 | 87.2 | 92.3 | 94.3 |
| PD-related RBD | |||||||
| 1 | 49.2 | 79.7 | 87.3 | 89 | 89.9 | 92.9 | 94.2 |
| 2 | 37.5 | 69.1 | 85.4 | 89.4 | 91 | 93.6 | 94.1 |
| 3 | 36.6 | 53.5 | 60.7 | 72.1 | 80.9 | 89.3 | 92.1 |
| 4 | 70.7 | 80.5 | 90 | 92.9 | 96 | 97.4 | 98.2 |
| 5 | 32.9 | 45.8 | 65.1 | 79.2 | 83.9 | 89.8 | 93.9 |
| 6 | 79 | 92.8 | 95.2 | 95.3 | 96.5 | 98.2 | 98.5 |
| 7 | 63.5 | 80.4 | 90.7 | 91.3 | 93.1 | 95.1 | 96.6 |
| 8 | 51.5 | 60.5 | 83.4 | 85.7 | 86.8 | 93.2 | 96.2 |
| 9 | 61 | 75.5 | 78.1 | 80.2 | 84.4 | 87.6 | 92.1 |
| Mean | 54.69 | 72.89 | 82.06 | 87.40 | 90.42 | 93.14 | 95.24 |
| SD | 17.99 | 13.01 | 9.39 | 6.19 | 5.26 | 3.85 | 2.53 |
| t Student | A vs B | B vs C | C vs D | D vs E | E vs F | F vs G | |
| P value after Bonferroni correction | < 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.001 | 0.0002 | |
RBD refers to rapid eye movement sleep behavior disorder; PD, Parkinson disease; ment, mentalis muscle; flex, flexor digitorum superficialis muscle; ext, extensor digitorum brevis muscle; abd, abductor pollicis brevis muscle; ster, sternocleidomastoid muscle; lta, left tibialis anterior muscle; gast, gastrocnemius muscle.
Table 5.
Rates of Phasic Electromyographic Activity in REM Sleep in Several 3-Muscle Combinations
| RBD patient | A ment+lta+rta | B ment+bic+lta | C ment+flex+lta | D ment+flex+ext |
|---|---|---|---|---|
| Idiopathic RBD | ||||
| 1 | 24.9 | 29.7 | 64.9 | 74 |
| 2 | 77.8 | 85.7 | 91.1 | 92.3 |
| 3 | 84.1 | 86.1 | 89.4 | 90.3 |
| 4 | 58.7 | 68.3 | 77.3 | 79.8 |
| 5 | 76 | 83.4 | 76.6 | 78.9 |
| 6 | 67.9 | 56.3 | 72.1 | 80.7 |
| 7 | 76.9 | 77.5 | 83.9 | 86.3 |
| 8 | 63.1 | 67.3 | 74.9 | 76.8 |
| PD-related RBD | ||||
| 1 | 77.5 | 74.4 | 85.4 | 87.3 |
| 2 | 55.2 | 64.9 | 75.9 | 85.4 |
| 3 | 67.2 | 58.9 | 69.9 | 60.7 |
| 4 | 82.8 | 82.4 | 85.6 | 90 |
| 5 | 54.5 | 51.1 | 58 | 65.1 |
| 6 | 87.6 | 90 | 95.7 | 95.2 |
| 7 | 83 | 82.1 | 88.2 | 90.7 |
| 8 | 75.2 | 69.2 | 74.8 | 83.4 |
| 9 | 83.7 | 79.7 | 81.3 | 78.1 |
| Mean rates | 70.36 | 71.00 | 79.12 | 82.06 |
| SD | 15.68 | 15.55 | 9.86 | 9.39 |
| t Student | A vs. B | B vs. C | C vs. D | |
| P value after Bonferroni correction | 1.0000 | 0.0018 | 0.0344 | |
PD refers to Parkinson disease; Ment, mentalis muscle; Bic, biceps brachii muscle; Flex, flexor digitorum superficialis muscle; Lta, left tibialis anterior muscle; Rta, right tibialis anterior muscle; Ext, extensor digitorum brevis muscle.
There was no correlation between mean phasic EMG activity and RBD duration, age, sex, and daily levodopa equivalent dose.
Comparison of Proximal Versus Distal Limb Emg Activity
Phasic EMG activity was detected more frequently in distal muscles than in proximal muscles. In the upper limb, 62.8% ± 10.9% of phasic EMG activity occurred in distal muscles, whereas proximal muscles only displayed 37.2% ± 11 % (P = 0.0001). In the lower limb, 72.3% ± 7.8% of phasic EMG activity occurred in distal muscles versus 27.7% ± 7.7% in the proximal muscles (P <0.001). Phasic EMG activity in proximal limb muscles tended to appear simultaneously with distal limb EMG activity, whereas phasic EMG activity in distal limb muscles tended to appear independently from proximal limb muscles, in both upper (χ2 1661.22; P < 0.0001) and lower limbs (χ2 4859.68; P < 0.0001).
Bilateral Distribution of Phasic EMG Activity
Phasic EMG activity was more frequently detected unilaterally than bilaterally both in the tibialis anterior (67.0% ± 14.1% versus 32.6% ± 14.1%; P > 0.001) and in the rectus femoris (67.9% ± 13% versus 32.2% ± 12.7%; P > 0.001).
Periodic Leg Movements in Sleep
The PLMS index did not differ between both tibialis anterior muscles (right: 49.7 ± 30.0; left: 38.5 ± 26.3) and the left extensor digitorum brevis muscle (50.1 ± 30.6) (P = 0.779).
DISCUSSION
To the best of our knowledge, this is the first study to examine multiple muscle recordings in patients with RBD. Our results show that simultaneous recording of the mentalis, flexor digitorum superficialis, and extensor digitorum brevis provided the highest rates of phasic EMG activity seen in subjects with RBD. In addition, we found that excessive phasic EMG activity was more prominent in distal than in proximal muscles.
Rates of Phasic EMG Activity in REM Sleep in the Different Muscles
Correct diagnosis of RBD is important because patients with the idiopathic form have an increased risk for developing a neurodegenerative disease.19,20 Therefore, polysomnographic detection of increased EMG activity during REM sleep is crucial to confirm the diagnosis of RBD. However, it was previously unknown in which muscle or muscle group this excessive EMG activity is more prominent.
In a standard polysomnographic study, EMG of the mentalis or submentalis muscles is used as a criterion for scoring REM sleep.25 In our study, the mentalis showed the highest rate of phasic EMG activity. Isolated recording of the mentalis, however, only detected 55% of all the mini-epochs containing phasic EMG activity in REM sleep. Confirming the diagnosis of RBD using only the mentalis may be limited by (1) relatively low rates (55%) of phasic EMG activity, (2) high tendency of this muscle to be superimposed by artifacts such as snoring, (3) difficulties in distinguishing phasic from tonic EMG activity, and the fact that (4) most abnormal sleep behaviors seen in RBD correspond to movements of the limbs.1,28 Thus, other muscles, including those in the limbs, should be added to the mentalis in the polysomnographic montage. Our study showed that detection of 95% of the mini-epochs containing phasic EMG activity was only obtained when at least 7 muscles from the head and upper and lower limbs were recorded simultaneously. Evaluation of 7 muscles is not feasible in most sleep centers. We found that a more simple 3-muscle combination of the mentalis, flexor digitorum superficialis, and extensor digitorum brevis provided high rates of increased phasic EMG activity of RBD. With this 3-muscle combination, 82% of overall phasic EMG activity could be detected in patients with RBD. An additional advantage of this 3-muscle combination is that it examines the common abnormal EMG activity of RBD occurring in the cranial and upper and lower limb muscles.1,18 Our findings were not influenced by RBD duration, age, sex, etiology (idiopathic RBD versus RBD linked to PD), or levodopa-dose intake.
Comparison of Proximal Versus Distal Limb EMG Activity
In healthy people, occasional and brief bursts of phasic EMG activity can be recorded during REM sleep, particularly in distal muscles of the limbs.26,29 Cats have a similar type of activity, which is more frequently detected in distal than in proximal limb muscles.30 Our study showed that in patients with RBD, phasic EMG activity during REM sleep was more common in distal than in proximal limb muscles. Thus, it can be speculated that the excessive EMG expression of RBD in the distal limb muscles represents an abnormal increase of a physiologic tendency occurring in REM sleep. The reason why distal muscles are more active or less inhibited in normal REM sleep as well as in RBD is unknown, but several explanations can be offered. First, to protect an individual from dangerous motion during sleep, atonia may be more necessary in proximal limb muscles than in distal limb muscles. Second, distal limb muscles may be more active in RBD because, as has been recently suggested,22 movements in REM sleep in RBD might be generated in the cortex where the distal limb has a larger cortical somatotopic representation than does the proximal limb. We can not exclude that a similar somatotopic representation exists in those brainstem nuclei that mediate muscle atonia during REM sleep (e.g., subceruleus nucleus, pedunculopontine nucleus, magnocellularis nucleus) that are thought to be impaired in RBD.1 To the best of our knowledge, the motor somatotopic distribution within these brainstem nuclei has not been studied.
Bilateral Distribution of Phasic EMG Activity
The findings that phasic EMG activity was often not simultaneous over both sides of the body and that it is common that movements in REM sleep are limited to 1 extremity in normal subjects,26 indicate that it would be useful that the polysomnographic montage contained EMG recording of the 4 limbs.
Periodic Leg Movements in Sleep
When compared with the tibialis anterior, the extensor digitorum brevis provided higher rates of phasic EMG events and similar rates of PLMs in both REM and non-REM sleep. Recording from the extensor digitorum brevis instead of the tibialis anterior does not decrease detection of PLMS in subjects with suspected RBD.
This study has some potential limitations. First, we did not include a control group. This prevented us from assessing normative EMG data in the muscles investigated. Our study, however, was aimed at determining, in patients with RBD, which muscles provide the highest rates of phasic EMG activity rather than determining the normal quantity of this activity. Therefore, the lack of a control group does not diminish the conclusions of our study. Second, patients included were affected by different forms of RBD (idiopathic RBD and RBD associated with PD) and were using different medications. A heterogeneous sample of patients was included in our study because we aimed to evaluate the habitual subject presenting to a sleep center to confirm the diagnosis of RBD. The results of our study are in line with previous observations showing that the global amount of phasic EMG activity (1) is not different in patients with idiopathic RBD than in those with RBD associated with PD and (2) is not related to daily levodopa-equivalent dose.18 Patients taking clonazepam, however, were excluded because this medication reduces phasic EMG activity in RBD.4 Third, since patients were evaluated only 1 night, our results might be affected by the night-to-night variability of this parasomnia.29 In our study, the internight variability might have been minimized by measuring more than 1000 mini-epochs per patient. Fourth, tonic EMG activity was not assessed. Our study focused on quantification of phasic EMG events because this activity is always increased in idiopathic RBD and sporadic PD, reflects the occurrence of abnormal sleep behaviors,18 and is easy to be recognized and scored. In contrast, tonic EMG activity may not be elevated in some patients with RBD.28 When tonic EMG activity is increased, this activity is normally not associated with vocalizations or body movements and is sometimes difficult to distinguish from a superimposed phasic component.28 We think therefore that the comprehensiveness of the investigation corroborates the validity of our findings.
In conclusion, this is the first study to investigate which polysomnographic EMG montage is the most appropriate for the detection of the characteristic abnormal phasic EMG activity seen in RBD. According to our findings, a montage including the mentalis, flexor digitorum superficialis, and extensor digitorum brevis muscles provides the highest rates of phasic EMG activity during REM sleep in RBD. A further study is needed to establish the cut-off values of phasic EMG activity in different muscles in REM sleep in a healthy population.
ACKNOWLEDGMENTS
The authors wish to express their gratitude to Wolfgang Löscher, PhD (Innsbruck, Austria), for helpful discussion of the design of the EMG protocol and Hanno Ulmer, PhD (Innsbruck, Austria), for his expert statistical advices.
This work was partially supported by a public grant from CIBERNED, SPAIN.
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Frauscher has participated in a speaking engagement for Boehringer Ingelheim. Dr. Högl has participated in speaking engagements for GlaxoSmithKline, Boehringer Ingelheim, Silmar Pharma, and Novartis and has consulted for GlaxoSmithKline, Boehringer Ingelheim, Silmar Pharma, and UCB. Dr. Gschliesser has participated in a speaking engagement for Boehringer Ingelheim. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Schenck CH, Mahowald MW. REM sleep behavior disorder: clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep. 2002;25:120–138. doi: 10.1093/sleep/25.2.120. [DOI] [PubMed] [Google Scholar]
- 2.The International Classification of Sleep Disorders. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. REM sleep behavior disorder; pp. 148–152. [Google Scholar]
- 3.Iranzo A, Santamaria J. Severe obstructive sleep apnea/hypopnea mimicking REM sleep behavior disorder. Sleep. 2005;28:203–206. doi: 10.1093/sleep/28.2.203. [DOI] [PubMed] [Google Scholar]
- 4.Lapierre O, Montplaisir J. Polysomnographic features of REM sleep behavior disorder: development of a scoring method. Neurology. 1992;42:1371–1374. doi: 10.1212/wnl.42.7.1371. [DOI] [PubMed] [Google Scholar]
- 5.Tachibana N, Kimura K, Kitajima K, Nagamine T, Kimura J, Shibasaki H. REM sleep without atonia at early stage of sporadic olivopontocerebellar atrophy. J Neurol Sci. 1995;132:28–34. doi: 10.1016/0022-510x(95)00119-m. [DOI] [PubMed] [Google Scholar]
- 6.Wetter TC, Trenkwalder C, Gershanik O, Högl B. Polysomnographic measures in Parkinson's disease: a comparison between patients with and without REM sleep disturbances. Wien Klin Wochenschr. 2001;113:249–253. [PubMed] [Google Scholar]
- 7.Gagnon JF, Bedard MA, Fantini ML, et al. REM sleep behavior disorder and REM sleep without atonia in Parkinson's disease. Neurology. 2002;59:585–589. doi: 10.1212/wnl.59.4.585. [DOI] [PubMed] [Google Scholar]
- 8.Fantini ML, Gagnon JF, Filipini D, Montplaisir J. The effects of pramipexole in REM sleep behavior disorder. Neurology. 2003;61:1418–1420. doi: 10.1212/wnl.61.10.1418. [DOI] [PubMed] [Google Scholar]
- 9.Consens FB, Chervin RD, Koeppe RA, et al. Validation of a polysomnographic score for REM sleep behavior disorder. Sleep. 2005;28:993–997. doi: 10.1093/sleep/28.8.993. [DOI] [PubMed] [Google Scholar]
- 10.Stiasny-Kolster K, Doerr Y, Möller JC, et al. Combination of “idiopathic” REM sleep behaviour disorder and olfactory dysfunction as possible indicator for alpha-synucleinopathy demonstrated by dopamine transporter FP-CIT-SPECT. Brain. 2005;128:126–137. doi: 10.1093/brain/awh322. [DOI] [PubMed] [Google Scholar]
- 11.Boesch SM, Frauscher B, Brandauer E, Wenning GK, Högl B, Poewe W. Disturbance of rapid eye movement sleep in spinocerebellar ataxia type 2. Mov Disord. 2006;21:1751–1754. doi: 10.1002/mds.21036. [DOI] [PubMed] [Google Scholar]
- 12.Gagnon JF, Petit D, Fantini ML, et al. REM sleep behavior disorder and REM sleep without atonia in probable Alzheimer disease. Sleep. 2006;29:1321–1325. doi: 10.1093/sleep/29.10.1321. [DOI] [PubMed] [Google Scholar]
- 13.Iranzo A, Munoz E, Santamaria J, Vilaseca I, Mila M, Tolosa E. REM sleep behavior disorder and vocal cord paralysis in Machado-Joseph disease. Mov Disord. 2003;18:1179–1183. doi: 10.1002/mds.10509. [DOI] [PubMed] [Google Scholar]
- 14.Arnulf I, Merino-Andreu M, Bloch F, et al. REM sleep behavior disorder and REM sleep without atonia in patients with progressive supranuclear palsy. Sleep. 2005;28:349–354. [PubMed] [Google Scholar]
- 15.Eisensehr I, Linke R, Tatsch K, et al. Increased muscle activity during rapid eye movement sleep correlates with decrease of striatal presynaptic dopamine transporters. IPT and IBZM SPECT imaging in subclinical and clinically manifest idiopathic REM sleep behavior disorder, Parkinson's disease, and controls. Sleep. 2003;26:507–512. doi: 10.1093/sleep/26.5.507. [DOI] [PubMed] [Google Scholar]
- 16.Bliwise DL, He L, Ansari FP, Rye DB. Quantification of electromyographic activity during sleep: a phasic electromyographic metric. J Clin Neurophysiol. 2006;23:59–67. doi: 10.1097/01.wnp.0000192303.14946.fc. [DOI] [PubMed] [Google Scholar]
- 17.Kumru H, Santamaria J, Tolosa E, et al. Rapid eye movement sleep behavior disorder in parkinsonism with parkin mutations. Ann Neurol. 2004;56:599–603. doi: 10.1002/ana.20272. [DOI] [PubMed] [Google Scholar]
- 18.Iranzo A, Santamaria J, Rye DB, et al. Characteristics of idiopathic REM sleep behavior disorder and that associated with MSA and PD. Neurology. 2005;65:247–252. doi: 10.1212/01.wnl.0000168864.97813.e0. [DOI] [PubMed] [Google Scholar]
- 19.Iranzo A, Molinuevo JL, Santamaria J, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disease: a descriptive study. Lancet Neurol. 2006;5:572–577. doi: 10.1016/S1474-4422(06)70476-8. [DOI] [PubMed] [Google Scholar]
- 20.Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38 % of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: Neurology. 1996;46:388–393. doi: 10.1212/wnl.46.2.388. [DOI] [PubMed] [Google Scholar]
- 21.García-Borreguero D, Caminero AB, de la Llave Y, et al. Decreased phasic EMG activity during rapid eye movement sleep in treatment-naïve Parkinson's disease: effects of treatment with levodopa and progression of illness. Mov Disord. 2002;17:934–941. doi: 10.1002/mds.10233. [DOI] [PubMed] [Google Scholar]
- 22.De Cock VC, Vidailhet M, Leu S, et al. Restoration of normal muscle control in Parkinson's disease during REM sleep. Brain. 2007;130:450–456. doi: 10.1093/brain/awl363. [DOI] [PubMed] [Google Scholar]
- 23.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Möller JC, Körner Y, Dodel RC, et al. Pharmacotherapy of Parkinson's disease in Germany. J Neurol. 2005;252:926–935. doi: 10.1007/s00415-005-0784-1. [DOI] [PubMed] [Google Scholar]
- 25.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 26.Gardner R, Jr, Grossman WI. Normal patterns in sleep in man. Adv Sleep Res. 1975;2:67–107. [Google Scholar]
- 27.Bonnet MH, Carley D, Carskadon MA, et al. Recording and scoring leg movements: The Atlas Task Force. Sleep. 1993;16:748–759. [PubMed] [Google Scholar]
- 28.Schenck C. Clinical and research implications of a validated polysomnographic scoring method for REM sleep behavior disorder. Sleep. 2005;28:917–919. doi: 10.1093/sleep/28.8.917. [DOI] [PubMed] [Google Scholar]
- 29.Rye DB. Modulation of normal and pathologic motoneuron activity during sleep. In: Chokroverty S, Hening WA, Walters AS, editors. Sleep and Movement Disorders. Philadelphia: Butterworth Heinemann; 2003. pp. 94–119. [Google Scholar]
- 30.Gassel MM, Marchiafava PL, Pompeiano O. Phasic changes in muscular activity during desynchronized sleep in unrestrained cats. An analysis of the pattern and organization of myoclonic twitches. Arch Ital Biol. 1964;102:449–470. [PubMed] [Google Scholar]


