Abstract
Objective:
To determine whether weight loss could reverse excessive sleep in high-fat diet-induced obesity.
Design:
Three groups of mice participated in the study. A weight gain/loss group was fed with high-fat food for 6 weeks (weight gain), and regular food again for 4 weeks (weight loss). A control group and a weight gain only group were fed with regular food and high-fat food, respectively, for 10 weeks after the baseline.
Participants:
Adult male C57BL/6 mice.
Measurements:
The amounts of wake, rapid eye movement sleep (REMS) and non-REM sleep (NREMS) were determined at week 0 (baseline), week 6, and week 10.
Results:
The weight gain/loss group displayed a significant decrease in wakefulness and increases in NREMS and episodes of NREMS during 6 weeks of weight gain, which were reversed during subsequent 4 weeks of weight loss. The weight gain only group displayed significant decrease in wakefulness and increase of NREMS and REMS at both week 6 and week 10. The control group did not show significant sleep alterations during the experiment.
Conclusion:
These observations indicate that sleep alterations induced by weight gain are reversed by weight loss in obese animals. These data may shed light on the mechanisms underlying the well-established association between obesity and sleepiness in humans and may lead to new therapeutic strategies for these 2 increasingly prevalent problems in the modern societies.
Citation:
Guan Z; Vgontzas AN; Bixler EO; Fang J. Sleep is increased by weight gain and decreased by weight loss in mice. SLEEP 2008;31(5):627-633.
Keywords: sleep, obesity, high fat food, weight loss, mice
OBESITY IS A MAJOR HEALTH PROBLEM FOUND IN MODERN SOCIETIES AND IS STILL INCREASING IN MANY COUNTRIES. ABOUT 60% OF ADULTS ARE overweight or obese in the United States.1 Numerous studies indicate that obesity is closely associated with sleep disturbances, which are also a major health problem. Excessive daytime sleepiness (EDS) is one of the most common complaints of obese patients, and is also the most frequent complaint in a sleep clinic. Obesity is often associated with sleep disordered breathing, which might contribute to EDS. However, independent studies from different groups indicate that objective EDS occurs in obese patients regardless of the presence of sleep apnea.2–4 A study of the prevalence of EDS in a large general, randomized sample involving over 1,700 subjects also suggests that obesity is a significant risk factor for EDS independent of sleep disordered breathing and age.5 However, these studies are cross-sectional and do not provide any direction on the causality of the association.
We and others have recently observed that sleep is significantly increased in a diet-induced obesity (DIO) mouse model.6,7 In the DIO mice, the increase of sleep was significantly correlated with the increase of body weight, but not the increase of daily energy intake. Also, leptin-deficient ob/ob mice sleep more compared to non-obese controls.8 In both DIO and ob/ob mice, sleep was primarily or exclusively increased during the dark period, the active time period in mice, consistent with the increased sleepiness during the daytime in obese humans.
In order to understand further the direction of the association between obesity and excessive sleep, we examined sleep alterations in mice after weight gain and weight loss, induced by high-fat diet and regular lab chow (after the development of DIO), respectively. We hypothesized that the increases of sleep in obesity could be reversed by weight loss.
MATERIALS AND METHODS
Animals
Adult male C57BL/6 mice (6 months of age) were used in the experiment. All experimental protocols used in the experiment were approved by the Institutional Animal Care and Use Committee (IACUC). The animals were implanted with electroencephalogram (EEG) and electromyography (EMG) electrodes. The experiment started after 2 weeks of recovery from the surgery and additional 4 days of adaptation to the recording procedures. Animals were kept individually in the plastic cages at 23°C room temperature on a 12:12 h light-dark cycle with light onset at 06:00 during the recovery and adaptation, and throughout the experiment.
Experimental Procedures
The animals were fed with regular lab chows (Harlan Tekled, Madison, WC; Product No. 2018), in which fat, proteins, and carbohydrates provided 15.9%, 21.5%, and 63.1% of calories, respectively. Baseline EEG and EMG were continuously recorded for 24 h. After baseline EEG and EMG recording, the animals were divided into 3 groups: weight gain/loss group, control group and weight gain only group. The control animals (n = 6) were continuously fed with the regular lab chows during the subsequent 10 weeks. The weight gain/loss animals (n = 9) were fed with high-fat food (Bio-Serv, Frenchtown, NJ; Product No. F3282) for 6 weeks to induce obesity, and fed with regular food again for additional 4 weeks to induce weight loss. In the high-fat food, fat, protein and carbohydrate provided 59.3%, 16.2%, and 24.5% of calories, respectively. The weight gain only animals (n = 9) were fed with high-fat food for 10 weeks. Daily food intake was measured at weeks 0, 6, and 10. The regular lab chow and high-fat food provide 3.3 kcal/g and 5.29 kcal/g, respectively. The food intake data were converted into the total energy intake for statistical comparisons. The adjusted energy intake based on body mass was also calculated (adjusted energy intake = total energy intake [kcal]/body mass [gm]0.568; the value of 0.568 has been used to calculate the metabolism against body mass in small mammals.9 The EEG and EMG were further recorded at the end of the 6th and 10th weeks in all groups. The recording system consisted of a personal computer with an 12-bit analog-to-digital (AD) converter (Model PCI-6023E, National Instruments, Austin, TX), and a Grass Model 12 Neurodata amplifier system (Grass Instrument Division of Astro-Med, Inc., West Warwick, RI). The EEG and EMG signals were amplified with 12A5 amplifiers. The one-half cut-off frequencies in EEG recordings for low and high frequencies were at 0.5 and 35.0 Hz, respectively. The EMG signals were filtered with one-half cut-off for low and high frequencies at 100 and 10,000 Hz, respectively. The data collection was controlled by the computer and a computer program (SleepWave, developed in our lab). The J10 outputs from the 12A5 amplifiers were fed into the AD converter and displayed on the computer monitor. The EEG and EMG data were saved to the hard disk drive. The daily amounts of food intake were also measured during each recording period.
Scoring of Sleep Data
Sleep-wake states were scored visually on a personal computer. The EEG and EMG records were scored as previously described.6 Sequential 10-s segments of EEG, EMG, and the results of fast Fourier transformation (FFT) of EEG signals were graphically displayed in both condensed form (12-min data on the bottom of the screen) and expanded form (30-s data on the top of the screen) to provide both a good overview of the data and the details of EEG and EMG patterns. Sleep data were scored in 10-s segments. The vigilance states were defined according to the following criteria: NREMS was identified by high-voltage delta waves in the EEG and decreased muscle tone; REMS by predominant theta activity (6–10 Hz) in the EEG and absence of muscle tone with occasional muscle twitches; and wakefulness by low-voltage EEG activity, and increased and varying levels of muscle activities. The average number and duration of the episodes for each behavioral state were computed according to the scoring results. The criterion used was that each episode should last at least 30 s.
EEG Power Spectrum Analyses
The EEG data were subjected to off-line FFT. However, the EEG power alterations could not be compared between baseline and subsequent recordings in 2 of 9 animals in the weight gain/loss group because EEG recorded at week 6 and week 10 was recorded from different EEG leads (due to the poor recording quality from the original EEG leads). Therefore, although the FFT signals were still useful by providing information in addition to EEG and EMG patterns for identifying sleep stages, these 2 animals were excluded from EEG power analyses. In the EEG power analyses, sequential 2 s of EEG data (256 samples) were treated with a Hanning window and the power spectrum data with 0.5 Hz resolution were obtained after FFT. The power spectrum data were averaged every 10 s to match the sleep scoring results. The data from NREM sleep were used to compute EEG slow wave activity (SWA). The total power from 0.5 to 4.0 Hz was calculated for each 10-s segment. The results were further averaged for each 3-h time block. Since power spectrum data are sensitive to subtle changes in EEG amplitude, the EEG SWA data were normalized using the average of EEG SWA across 24 h during the baseline (week 0) as 100 in each animal.
Statistics
Two-way analysis of variance (ANOVA) for repeated measures was used to determine effects of weight gain and weight loss on sleep and EEG SWA. In the sleep analyses, the first independent variable was the treatment condition (comparisons between the baseline, week 6 and week 10), and the second independent variable was the time of day (light vs. dark). If significant differences were observed in the treatment condition or in the interaction between treatment conditions and time of day, Tukey multiple comparison procedures were used to compare individual differences between different time points. One way ANOVA was used to compared the differences in the body weight and energy intake. When the assumption of equal variance was violated, Friedman repeated measures ANOVA on ranks were used. A probability of ≤0.05 was considered statistically significant in all conditions.
RESULTS
Change in Body Weight and Energy Intake during High-Fat Feeding
The alterations of body weight and energy intake across the 10-week experimental period are shown in Table 1. In the weight gain/loss group, high-fat food significantly increased the body weight by 32.57% compared to the baseline, whereas switching back to regular food reduced body weight to only 12.62% above the baseline (F2,16 = 64.763, P < 0.001; week 6 vs. week 0, q3,16 = 15.961, P < 0.001; week 6 vs. week 10, q3,16 = 9.778, P < 0.001; week 10 vs. week 0, q3,16 = 6.183, P = 0.001). In the weight gain only group, the body weight increased by 34.98% at week 6 and continued to increase to 54.18% above the baseline level at week 10 (F2,16 = 140.397, P < 0.001; week 6 vs. week 0, q3,16 = 15.088, P < 0.001; week 6 vs. week 10, q3,16 = 8.282, P < 0.001; week 10 vs. week 0, q3,16 = 23.370, P < 0.001). The control mice did not gain weight significantly at 6 weeks, but showed slight (4.49% above the baseline) but significant increases in their body weight at week 10 compared to the baseline and week 6 (F2,10 = 18.079, P < 0.001; week 6 vs. week 0, q3,10 = 1.123, not significant; week 6 vs. week 10, q3,10 = 6.738, P < 0.002; week 10 vs. week 0, q3,10 = 7.862, P < 0.001).
Table 1.
Body Weight and Energy Intake in Different Groups
| Week 0 Mean ± SE | Week 6 Mean ± SE | Week 10 Mean ± SE | ||
|---|---|---|---|---|
| Body Weight (gm) | ||||
| Weight gain/loss group | * | 29.23 ± 0.19 | 38.76 ± 0.53 a, b | 32.92 ± 0.38 a |
| Control group | * | 28.58 ± 0.13 | 28.77 ± 0.15 b | 29.87 ± 0.14 a |
| Weight gain only group | * | 30.84 ± 0.14 | 41.63 ± 0.39 a, b | 47.56 ± 0.49 a |
| Total Energy Intake (kcal/day) | ||||
| Weight gain/loss group | * | 15.29 ± 0.13 | 18.85 ± 0.30 a, b | 14.34 ± 0.22 a |
| Control group | 15.29 ± 0.21 | 15.18 ± 0.11 | 15.18 ± 0.17 | |
| Weight gain only group | * | 15.00 ± 0.39 | 18.21 ± 0.41 b | 14.45 ± 0.30 |
| Adjusted Energy Intake [kcal/mass (gm)0.568/day] | ||||
| Weight gain/loss group | * | 2.25 ± 0.02 | 2.38 ± 0.04 b | 1.95 ± 0.02 |
| Control group | 2.28 ± 0.03 | 2.25 ± 0.02 | 2.20 ± 0.02 | |
| Weight gain only group | * | 2.14 ± 0.06 | 2.21 ± 0.05 b | 1.62 ± 0.04 a |
SE: standard error. *Significant differences between weeks. a: Significant difference from the baseline (week 0). b: Significant difference from week 10. See additional details in the text.
In the weight gain/loss group, the amount of energy intake was significantly higher at week 6 compared to the baseline and week 10 (F2,16 = 16.486, P < 0.001; week 6 vs. week 0, q3,16 = 7.703, P = 0.001; week 6 vs. week 10, q3,16 = 6.077, P = 0.002). The weight gain group showed similar pattern but the differences between week 6 and week 0 did not reach statistical significance (F2,16 = 4.178, P < 0.025; week 6 vs. week 0, q3,16 = 3.308, P = 0.079; week 6 vs. week 10, q3,16 = 6.738, P < 0.025). Despite the fact that these animals were continuously fed with high-fat diet, their energy intake levels were reduced to the baseline level. In the meanwhile their body weight further increased significantly from week 6 to week 10. The control animals did not show clear change in energy intake across the experimental period.
The adjusted energy intake based on the body mass (kcal/mass [gm]0.568/day) did not increase at week 6 compared to the baseline and was decreased in week 10 compared to week 6 in the weight gain/loss group (F2,16 = 7.170, P = 0.006; week 6 vs. week 10, q3,16 = 5.237, P = 0.005), and was significantly decreased at week 10 compared to both baseline and week 6 in the weight gain only group (χ2(2) = 10.667, P = 0.005; baseline vs. week 10, P < 0.05; week 6 vs. week 10, P < 0.05). The adjusted energy intake was not altered in the control group during the experimental period.
The Amounts of Wakefulness and Sleep
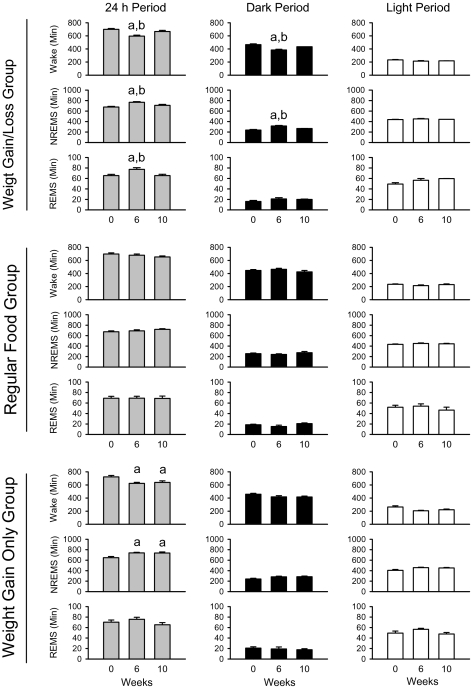
In the weight gain/loss group, the development of obesity and subsequent weight loss significantly altered the amounts of wakefulness, NREMS and REMS (Figure 1 and Figure 2). Wakefulness was significantly reduced from 698.72 ± 13.58 min/day at baseline to 596.56 ± 13.24 min/day after weight gain and returned to 665.72 ± 18.01 min/day after weight loss (F2,16 = 27.717, P < 0.001, main effect [treatment condition]): significant decrease at week 6 compared to the baseline (Tukey test: q3,16 = 10.515, P < 0.001), and compared to week 10 (weight loss) [q3,16 = 5.737, P = 0.003]. There was a significant treatment condition and time of day interaction (F2,16 = 9.973, P < 0.002). The amount of wakefulness was significantly less at week 6 than in week 0 (q3,16 = 11.772, P < 0.001) and week 10 (q3,16 = 7.069, P < 0.001) during the dark period. NREMS was also significantly different between treatment conditions (F2,16 = 24.758, P < 0.005), but not during the light period. REMS was significantly increased after weight gain at week 6 and returned to baseline levels after weight loss at week 10 (F2,16 = 12.971, P < 0.001; week 6 vs. week 0, q3,16 = 6.697, P < 0.001; week 6 vs. week 10, q3,16 = 5.646, P = 0.003; week 10 vs. week 0, q3,16 = 1.051, P = 0.742, not significant). There was no significant interaction between treatment condition and time of day for REMS.
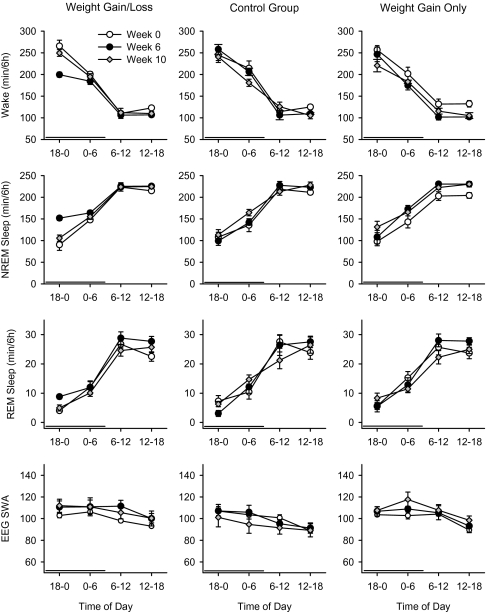
Figure 1.
Sleep patterns after weight gain and weight loss in mice. The 24-h patterns of wakefulness, NREMS, and REMS are displayed in 6-h blocks. In the weight gain/loss group, the weight gain (after 6 weeks feeding with high-fat food) decreased wakefulness and increased NREMS and REMS (left). These alterations returned to the baseline (week 0) at week 10 (after 4 weeks of refeeding with regular food). The control animals did not show significant alterations in the amounts of wakefulness, NREMS, and REMS (middle). In the weight gain only group (right), the amount of wakefulness was significantly decreased and the amount of NREM sleep was significantly increased at week 6 and week 10 compared to baseline. The time is expressed as minute/6 hours. The error bars indicate the standard error. The horizontal thick black bars above the abscissa indicate the dark period.
Figure 2.
Effects of weight gain and weight loss on sleep. In the weight gain/loss group, weight grain (after 6 weeks of high-fat feeding) significantly decreased wakefulness, and increased NREMS and REMS compared to baseline (week 0) and weight loss at week 10 (after 4 weeks of refeeding with regular food): a, significant difference compared to baseline; b, significant difference from the weight loss. The control animals fed with regular food did not display significant alterations in sleep during the same experimental period. See text for details in statistical analyses. In the weight gain only group, the amount of wakefulness was significantly decreased and the amount of NREM sleep was significantly increased at week 6 and week 10 compared to the baseline.
In contrast to the weight gain/loss group, the control mice fed with regular food did not show clear sleep alterations in the amounts of sleep and wakefulness (F2,10 = 1.699, P = 0.237), NREMS (F2,10 = 1.855, P = 0.206) and REMS (F2,10 = 0.0226, P = 0.978) during the same periods.
Similar to the weight gain/loss group, the weight gain only group also displayed significant reduction of wakefulness and significant increases of NREM sleep at week 6 and week 10 compared to the baseline (Wakefulness: F2,16 = 8.231, P < 0.003; week 6 vs. week 0, q3,16 = 5.288, P = 0.005; week 10 vs. week 0, q3,16 = 4.573, P < 0.02. NREMS: F2,16 = 9.273, P < 0.002; week 6 vs. week 0, q3,16 = 5.355, P < 0.005; week 10 vs. week 0, q3,16 = 5.189, P = 0.006). There was no significant treatment and time of day interactions. The amount of REMS was slightly greater at week 6 compared to week 0 and week 10, but this trend was not significant (F2,16 = 2.726, P = 0.096).
Sleep Structure: Number and Duration of Sleep Episodes
The number and duration of episodes for each behavioral state were further analyzed (Table 2).
Table 2.
The Number and Duration of the Episodes for Each Behavioral State
| Week 0 Mean ± SE | Week 6 Mean ± SE | Week 10 Mean ± SE | ||
|---|---|---|---|---|
| Number of Episodes | ||||
| Weight gain/loss group | Wake * | 57.00 ± 3.58 | 75.89 ± 3.81 a | 68.33 ± 4.14 |
| NREMS * | 100.56 ± 4.61 | 124.22 ± 8.50 a,b | 106.11 ± 6.12 | |
| REMS | 48.67 ±2.37 | 57.56 ± 4.58 | 46.33 ± 1.96 | |
| Control group | Wake | 60.17 ± 5.61 | 70.50 ± 6.83 | 66.83 ± 5.81 |
| NREMS | 98.50 ± 6.81 | 109.50 ± 6.16 | 104.50 ± 8.78 | |
| REMS | 49.33 ± 2.84 | 47.67 ± 3.03 | 48.17 ± 3.37 | |
| Weight gain only group | Wake * | 69.22 ± 3.76 | 72.33 ± 7.33 | 81.78 ± 10.47 a |
| NREMS * | 106.44 ± 4.63 | 116.44 ± 7.14 | 119.89 ± 8.16 a | |
| REMS | 48.11 ± 2.91 | 53.11 ± 2.99 | 47.78 ± 3.00 | |
| Duration of Episodes (minutes) | ||||
| Weight gain/loss group | Wake * | 12.80 ± 1.09 | 8.03 ± 0.49 a | 10.02 ± 0.61 |
| NREMS | 6.84 ± 0.33 | 6.37 ± 0.37 | 6.81 ± 0.34 | |
| REMS | 1.35 ± 0.04 | 1.38 ± 0.06 | 1.41 ± 0.02 | |
| Control group | Wake | 12.06 ± 1.05 | 9.92 ± 0.61 | 10.20 ± 1.09 |
| NREMS | 7.03 ± 0.60 | 6.44 ± 0.50 | 7.11 ± 0.55 | |
| REMS | 1.40 ±0.03 | 1.46 ± 0.05 | 1.43 ±0.03 | |
| Weight gain only group | Wake * | 10.79 ± 0.85 | 9.31 ± 0.87 a | 8.48 ± 0.79 a |
| NREMS | 6.13 ± 0.21 | 6.51 ± 0.34 | 6.37 ± 0.43 | |
| REMS | 1.47 ±0.05 | 1.44 ± 0.05 | 1.37 ±0.04 | |
The number and duration of episodes for each state are calculated for 24-hour period. SE: standard error. *: Significant differences between weeks. a: Significant difference from the baseline (week 0). b: Significant difference from weight loss (week 10). See additional details in the text.
In the weight gain/loss group, the number of waking episodes was significantly increased after weight gain (F2,16 = 12.081, P < 0.001, main effect [treatment condition]) compared to the baseline (q2,16 = 6.703, P < 0.001) and to week 10 (q2,16 = 4.947, P < 0.01). By week 10, the number of waking episodes was not significantly different from the baseline. The duration of waking episodes was significantly altered (F2,16 = 11.509, P = 0.001, main effect [weeks]): significant decrease in week 6 compared to the baseline (q2,16 = 6.783, P < 0.001 for 24-h period); and no significant change at week 10 compared to the baseline or week 6. The number of NREM sleep episodes were significantly increased after weight gain (F2,16 = 6.582, P < 0.01; week 6 vs. week 0, q2,16 = 4.921, P < 0.01; week 6 vs. week 10, q2,10 = 1.201, P < 0.05; week 10 vs. week 0, q2,10 = 3.720, P < 0.05), whereas the duration of NREMS episodes were not significantly altered (F2,16 = 2.552, P = 0.109, main effect [treatment condition]). The number of REMS was significantly altered (F2,16 = 2.211, P = 0.160), but post hoc comparisons did not reveal significant differences between specific group comparisons. The duration of REMS was not significantly altered during the experiment (F2,16 = 0.0716, P = 0.931).
The control group did not display a significant change in the number and duration of waking (number, F2,10 = 1.628, P = 0.244; duration, F2,10 = 2.033, P = 0.182), NREMS (number, F2,10 = 1.667, P = 0.237; duration, F2,10 = 2.693, P = 0.116) and REMS (number, F2,10 = 0.234, P = 0.795; duration, F2,10 = 1.050, P = 0.385) episodes during the same experimental period.
In the weight gain only group, the number waking episodes was slightly increased at week 10 compared to week 0 (F2,16 = 3.898, P < 0.05, main effect; q2,16 = 3.801, P < 0.05). The duration of waking episodes was significantly decreased after weight gain (F2,16 = 10.016, P = 0.002; week 6 vs. week 0, q2,16 = 4.733, P < 0.02; week 10 vs. week 0, q2,16 = 6.006, P = 0.002). The number of NREMS episodes was significantly increased at week 10 compared to week 0 (F2,16 = 3.898, P < 0.05, main effect; q2,16 = 3.801, P < 0.05), whereas the duration of NREMS episodes was not significantly altered across the 10-week period (F2,16 = 0.472, P = 0.632). The number and duration of REMS episodes were not significantly altered.
EEG SWA
EEG SWA was not significantly altered in any experimental group across the 10-week experimental period (Figure 1).
DISCUSSION
This is the first study to demonstrate that diet-induced weight loss in the obese animals is associated with normalization of sleep patterns. Previously, our group and others have observed that sleep is increased in DIO mice6,7 and ob/ob mice.8 In the present study, we confirmed previous observations and further showed that DIO-induced sleep alterations are reversible by weight loss. The increased sleep along with the shorter and more frequent waking episodes in DIO mice are consistent with the observations in humans that obesity is associated with EDS,3–5,10,11 whereas weight loss leads to improvement of sleep and sleepiness.12–17 Furthermore, the increase of sleep with weight gain and the subsequent decrease of sleep with weight loss cannot be explained by the recovery from surgery, the adaptation to the environment, or aging process, since the control animals fed with regular food did not display such sleep alterations during the same experimental period.
The increase of NREMS and the decrease of wakefulness reached significance by week 6 and remained stable throughout week 10 in DIO mice, even though the levels of total energy intake had returned to the baseline level and the adjusted energy intake (based on the body mass) had dropped below the baseline level by week 10. This observation suggests that the NREMS alterations associated with weight gain and loss cannot be explained by the acute effect of food intake, which is consistent with our previous results that sleep alterations are associated with weight gain but not daily amounts of energy intake. That REMS in both weight gain/loss group and weight gain only group showed an increase at week 6 and a return to baseline line level at week 10 suggests that the increase of REMS is either transiently associated with DIO at a certain stage or is simply an acute effect of food intake.
Although sleep was consistently increased in DIO animals, both in the weight gain/weight loss group and the weight gain only group, some other aspects of sleep alterations were less consistent. For example, the increase of sleep and decrease of wakefulness were found only in the dark period in the weight gain/loss group, but distributed across a 24-h period in the weight gain only group in the present study and in our previous study. This difference could be due to individual differences and/or other unknown factors. Additional studies are needed to clarify what factors may influence the distribution of increased sleep across the light-dark cycle in DIO mice.
The increased number but not duration of NREMS episodes indicates lack of sleep fragmentation in the DIO mice, which is different from the ob/ob mice.8 The reasons for this difference are unclear. A minor difference in methodology might contribute to this difference. The previous study on ob/ob mice used the minimal 20-s episode length in the calculation of duration of NREMS, whereas we used the minimal 30-s requirement in our studies as has been used in numerous other studies. Another possibility is that the development of sleep fragmentation may depend on the length and severity of DIO. Finally, it is possible that hypothalamic-pituitary-adrenal axis-mediated arousal18 may be heightened in ob/ob mice since corticosterone levels are elevated in ob/ob mice19 but not in DIO mice.20 Regardless of the interpretation, the increase of sleep in the absence of evidence for sleep fragmentation in the DIO mice suggests that the increase of NREMS was not the result of poor sleep and strengthens the human data that obesity is a significant risk factor for EDS independent of night sleep disturbances.3,5 Our data also show that the EEG SWA was not altered during the weight gain period, indicating that the depth or intensity of sleep was not reduced in DIO mice. These observations further support that the increase of NREMS in DIO mice was not a compensatory response secondary to poor/shallow sleep.
The underlying mechanisms for the reversible sleep alterations are not known. Reduced levels of orexin, a wake-promoting peptide,21 have been associated with obesity and EDS in narcolepsy.22 Decreases of orexin in obese ob/ob mice23 and db/db mice24 were observed in 2 studies, but not in another study.25 However, high-fat diet increases orexin in the hypothalamus in both rats and mice,26,27 suggesting that the increase of NREMS reported here was not due to reduced orexin activities.
Obesity, both in humans and animals, is considered a state of low grade inflammation associated with peripheral increases of several sleep-promoting inflammatory cytokines such as interleukin-1beta (IL-1β), tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6).2, 28–32 IL-1β and TNF-α33–35 are involved in physiologic sleep regulation. Exogenous IL-6 also induces sleep alterations in humans and rats,36,37 whereas endogenous IL-6 levels are altered by sleep-wake/light-dark cycles and sleep deprivation.38,39 Further, blood levels of TNF-α and IL-6 are elevated in disorders of EDS whereas neutralization of TNF-α is associated with decrease of sleepiness in obese humans.2,28,40 The above reported studies suggest that these inflammatory cytokines may mediate the excessive sleep observed in DIO animals.
In summary, we have observed that sleep is increased by weight gain and decreased by subsequent weight loss in mice. EDS is a frequent complaint among obese patients and its reversal by weight loss may also improve daytime functioning and quality of life in obese patients.
ACKNOWLEDGMENT
Research supported by NIH research grant HL-64415.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002;288:1723–7. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 2.Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82:1313–6. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 3.Vgontzas AN, Bixler EO, Tan TL, Kantner D, Martin LF, Kales A. Obesity without sleep apnea is associated with daytime sleepiness. Arch Intern Med. 1998;158:1333–7. doi: 10.1001/archinte.158.12.1333. [DOI] [PubMed] [Google Scholar]
- 4.Resta O, Foschino Barbaro MP, Bonfitto P, et al. Low sleep quality and daytime sleepiness in obese patients without obstructive sleep apnoea syndrome. J Intern Med. 2003;253:536–43. doi: 10.1046/j.1365-2796.2003.01133.x. [DOI] [PubMed] [Google Scholar]
- 5.Bixler EO, Vgontzas AN, Lin HM, Calhoun SL, Vela-Bueno A, Kales A. Excessive daytime sleepiness in a general population sample: The role of sleep apnea, obesity, diabetes and depression. J Clin Endocrinol Metab. 2005;90:4510–15. doi: 10.1210/jc.2005-0035. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins JB, Omori T, Guan Z, Vgontzas AN, Bixler EO, Fang J. Sleep is increased in mice with obesity induced by high-fat food. Physiol Behav. 2006;87:255–62. doi: 10.1016/j.physbeh.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Laposky AD, Arbruzova J, Shelton J, Bass JT, Terek FW. High fat diet induces changes in sleep-wake patterns in mice. Sleep. 2006;29(Abstract Supplement):A38. [Google Scholar]
- 8.Laposky AD, Shelton J, Bass J, Dugovic C, Perrino N, Turek FW. Altered sleep regulation in leptin-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2006;290:R894–903. doi: 10.1152/ajpregu.00304.2005. [DOI] [PubMed] [Google Scholar]
- 9.Austad SN, Kristan DM. Are mice calorically restricted in nature? Aging Cell. 2003;2:201–7. doi: 10.1046/j.1474-9728.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- 10.Resnick H, Carter E, Aloia M, Phillips B. Cross-sectional relationship of reported fatigue to obesity, diet, and physical activity: results from the Third National Health and Nutrition Examination Survey. J Clin Sleep Med. 2006;2:163–9. [PubMed] [Google Scholar]
- 11.Theorell-Haglöw J, Lindberg E, Janson C. What are the important risk factor for daytime sleepiness and fatigue in woman? Sleep. 2006;29:751–7. doi: 10.1093/sleep/29.6.751. [DOI] [PubMed] [Google Scholar]
- 12.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PloS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vorona RD, Winn MP, Babineau TW, Eng BP, Feldman HR, Ware JC. Overweight and obese patients in a primary care population report less sleep than patients with a normal body mass index. Arch Intern Med. 2005;165:25–30. doi: 10.1001/archinte.165.1.25. [DOI] [PubMed] [Google Scholar]
- 14.Willi SM, Oexmann MJ, Wright NM, Collop NA, Key LL., Jr The effects of a high-protein, low-fat, ketogenic diet on adolescents with morbid obesity: body composition, blood chemistries, and sleep abnormalities. Pediatrics. 1998;101:61–7. doi: 10.1542/peds.101.1.61. [DOI] [PubMed] [Google Scholar]
- 15.Dixon JB, Schachter LM, O'Brien PE. Sleep disturbance and obesity: changes following surgically induced weight loss. Arch Intern Med. 2001;161:102–6. doi: 10.1001/archinte.161.1.102. [DOI] [PubMed] [Google Scholar]
- 16.Dixon JB, Schachter LM, O'Brien PE. Polysomnography before and after weight loss in obese patients with severe sleep apnea. Int J Obes (Lond) 2005;29:1048–54. doi: 10.1038/sj.ijo.0802960. [DOI] [PubMed] [Google Scholar]
- 17.Valencia-Flores M, Orea A, Herrera M, et al. Effect of bariatric surgery on obstructive sleep apnea and hypopnea syndrome, electrocardiogram, and pulmonary arterial pressure. Obes Surg. 2004;14:755–62. doi: 10.1381/0960892041590773. [DOI] [PubMed] [Google Scholar]
- 18.Chang FC, Opp MR. A corticotropin-releasing hormone antisense oligodeoxynucleotide reduces spontaneous waking in the rat. Regul Pept. 2004;117:43–52. doi: 10.1016/j.regpep.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Saito M, Bray GA. Diurnal rhythm for corticosterone in obese (ob/ob) diabetes (db/db) and gold-thioglucose-induced obesity in mice. Endocrinology. 1983;113:2181–5. doi: 10.1210/endo-113-6-2181. [DOI] [PubMed] [Google Scholar]
- 20.Bai H, Castonguay TW. Effects of adrenalectomy and hormone replacement on B6C3F1 mice fed a high-fat diet. Physiol Behav. 2001;72:493–8. doi: 10.1016/s0031-9384(00)00443-1. [DOI] [PubMed] [Google Scholar]
- 21.Sakurai T. Roles of orexin/hypocretin in regulation of sleep/wakefulness and energy homeostasis. Sleep Med Rev. 2005;9:231–41. doi: 10.1016/j.smrv.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Kok SW, Overeem S, Visscher TL, et al. Hypocretin deficiency in narcoleptic humans is associated with abdominal obesity. Obes Res. 2003;11:1147–54. doi: 10.1038/oby.2003.156. [DOI] [PubMed] [Google Scholar]
- 23.Stricker-Krongrad A, Richy S, Beck B. Orexins/hypocretins in the ob/ob mouse: hypothalamic gene expression, peptide content and metabolic effects. Regul Pept. 2002;104:11–20. doi: 10.1016/s0167-0115(01)00344-5. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto Y, Ueta Y, Date Y, et al. Down regulation of the prepro-orexin gene expression in genetically obese mice. Mol Brain Res. 1999;65:14–22. doi: 10.1016/s0169-328x(98)00320-9. [DOI] [PubMed] [Google Scholar]
- 25.Lin L, Wisor J, Shiba T, et al. Measurement of hypocretin/orexin content in the mouse brain using an enzyme immunoassay: the effect of circadian time, age and genetic background. Peptides. 2002;23:2203–11. doi: 10.1016/s0196-9781(02)00251-6. [DOI] [PubMed] [Google Scholar]
- 26.Wortley KE, Chang GQ, Davydova Z, Leibowitz SF. Peptides that regulate food intake: orexin gene expression is increased during states of hypertriglyceridemia. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1454–65. doi: 10.1152/ajpregu.00286.2002. [DOI] [PubMed] [Google Scholar]
- 27.Park ES, Yi SJ, Kim JS, et al. Changes in orexin-A and neuropeptide Y expression in the hypothalamus of the fasted and high-fat diet fed rats. J Vet Sci. 2004;5:295–302. [PubMed] [Google Scholar]
- 28.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151–8. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 29.Halle M, Korsten-Reck U, Wolfarth B, Berg A. Low-grade systemic inflammation in overweight children: impact of physical fitness. Exerc Immunol Rev. 2004;10:66–74. [PubMed] [Google Scholar]
- 30.Pitombo C, Araujo EP, De Souza CT, Pareja JC, Geloneze B, Velloso LA. Amelioration of diet-induced diabetes mellitus by removal of visceral fat. J Endocrinol. 2006;191:699–706. doi: 10.1677/joe.1.07069. [DOI] [PubMed] [Google Scholar]
- 31.De Souza CT, Araujo EP, Bordin S, et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–9. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 32.Silha JV, Weiler HA, Murphy LJ. Plasma adipokines and body composition in response to modest dietary manipulations in the mouse. Obesity (Silver Spring) 2006;14:1320–9. doi: 10.1038/oby.2006.150. [DOI] [PubMed] [Google Scholar]
- 33.Fang J, Wang Y, Krueger JM. Mice lacking the TNF 55 kDa receptor fail to sleep more after TNFalpha treatment. J Neurosci. 1997;17:5949–55. doi: 10.1523/JNEUROSCI.17-15-05949.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang J, Wang Y, Krueger JM. Effects of interleukin-1 beta on sleep are mediated by the type I receptor. Am J Physiol. 1998;274:R655–60. doi: 10.1152/ajpregu.1998.274.3.R655. [DOI] [PubMed] [Google Scholar]
- 35.Opp MR, Krueger JM. Interleukin-1 is involved in responses to sleep deprivation in the rabbit. Brain Res. 1994;639:57–65. doi: 10.1016/0006-8993(94)91764-7. [DOI] [PubMed] [Google Scholar]
- 36.Hogan D, Morrow JD, Smith EM, Opp MR. Interleukin-6 alters sleep of rats. J Neuroimmunol. 2003;137:59–66. doi: 10.1016/s0165-5728(03)00038-9. [DOI] [PubMed] [Google Scholar]
- 37.Späth-Schwalbe E, Hansen K, Schmidt F, et al. Acute effects of recombinant human interleukin-6 on endocrine and central nervous sleep functions in healthy men. J Clin Endocrinol Metab. 1998;83:1573–9. doi: 10.1210/jcem.83.5.4795. [DOI] [PubMed] [Google Scholar]
- 38.Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–26. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 39.Guan Z, Vgontzas AN, Omori T, Bixler EO, Fang J. Interleukin-6 levels fluctuate with the light-dark cycle in the brain and peripheral tissues in rats. Brain Behav Immunity. 2005;19:526–9. doi: 10.1016/j.bbi.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Vgontzas AN, Zoumakis E, Lin HM, Bixler EO, Trakada G, Chrrousos GP. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-alpha antagonist. J Clin Endocrinol Metab. 2004;89:4409–13. doi: 10.1210/jc.2003-031929. [DOI] [PubMed] [Google Scholar]