Abstract
Study objective:
Short and long sleep duration have been linked to various risk factors for cardiovascular disease. In the present study, we evaluated the relationship between sleep duration and presence of the metabolic syndrome, which is a cluster of physiologically interrelated risk factors for cardiometabolic disease.
Design/Setting:
Cross-sectional community-based cohort study.
Participants:
One thousand two hundred fourteen participants from the Adult Health and Behavior Project registry (aged 30 to 54 years).
Measurements:
Participants were divided into 4 groups based upon their reported sleep duration. The metabolic syndrome was defined according to the American Heart Association/National Heart Lung and Blood Institute's criteria. Logistic regression was used to test the hypothesis that sleep duration is a significant correlate of the metabolic syndrome and its components.
Results:
The observed metabolic syndrome rate (22%) was similar to that of published health statistics for American adults. After covariate adjustment, the odds for having the metabolic syndrome increased by more than 45% in both short and long sleepers, compared with those sleeping 7 to 8 hours per night. Sleep duration was also associated individually with abdominal obesity, elevated fasting glucose, and hypertriglyceridemia. After further adjustment for use of antihypertensive medication, prevalence of the metabolic syndrome and its components remained elevated in short sleepers only.
Conclusion:
These data suggest that sleep duration is a significant correlate of the metabolic syndrome. Additional studies are needed to evaluate temporal relationships among these measures, the behavioral and physiologic mechanisms that link the two, and their impact on subsequent cardiometabolic disease.
Citation:
Hall MH; Muldoon MF; Jennings JR; Buysse DJ; Flory JD; Manuck SB. Self-reported sleep duration is associated with the metabolic syndrome in midlife adults. SLEEP 2008;31(5):635-643.
Keywords: Sleep, metabolic syndrome, obesity, cardiovascular disease, diabetes
MIDLIFE ADULTS WHO ARE SHORT SLEEPERS ARE AT INCREASED RISK FOR CARDIOVASCULAR DISEASE.1–4 BOTH CROSS-SECTIONAL AND PROSPECTIVE epidemiologic studies have linked sleep durations of 6 hours or less per night with prevalent and incident hypertension. For example, in an 8- to 10-year follow-up of the National Health and Nutrition Examination Survey-I cohort, Gangwisch and colleagues reported that short sleepers (≤ 5 hours per night) were at increased risk for hypertension, after adjusting for confounding variables including age, sex, education, health behaviors, depression, overweight/obesity, and diabetes.3 Significant relationships among short sleep duration and incident hypertension over a 5-year follow-up were observed among women only in the slightly older Whitehall II cohort.2 Strikingly, decreases in sleep duration over a 3- to 5-year period in the Whitehall II cohort were associated with a 110% excess risk of cardiovascular mortality after adjusting for prominent cardiovascular disease risk factors.4
Possible pathways linking short sleep duration to hypertension and cardiovascular disease events include increases in body weight and changes in glucose metabolism/diabetes. In a series of landmark studies, Spiegel and colleagues demonstrated that short-term experimental sleep restriction in healthy, lean, young adults profoundly altered key components of energy homeostasis, including glucose tolerance, food cravings, and hormones critical to appetite regulation.5 For example, experimental sleep restriction of 4 hours per night over two consecutive nights markedly increased the ghrelin-to-leptin ratio and reported cravings for calorie-dense and carbohydrate-rich foods.6 Observational data from the population-based Wisconsin Sleep Cohort study7 similarly demonstrated significant cross-sectional associations among short sleep duration and reduced leptin and elevated ghrelin, which regulate satiety and hunger, respectively. Given the metabolic and endocrine changes that favor increased appetite and hunger in association with sleep curtailment, it is not surprising that a number of prospective epidemiologic studies have reported significant relationships between short sleep duration and increased body mass index.8,9
With respect to diabetes, the experimental sleep-restriction studies of Spiegel and colleagues demonstrated that sleep curtailment for periods of less than one week leads to clinically significant decreases in glucose tolerance in lean young men.5 Although these experimental studies show that sleep restriction affects glucose metabolism, one has to look to the epidemiologic literature to evaluate the extent to which naturalistic sleep curtailment, as measured by short sleep duration, is associated with diabetes. To date, several epidemiologic studies have reported that diabetes risk is elevated in both short and long sleepers.10–12 For instance, after adjusting for diabetes risk factors, including waist girth, both the cross-sectional Sleep Heart Health Study and the prospective Massachusetts Male Aging Study reported U-shaped relationships between sleep duration and type 2 diabetes.11,12 Although similar univariate relationships were observed in 10-year follow-up data in the full Nurse's Health Study cohort, after adjusting for body mass index, diabetes risk remained elevated only in short and long sleepers with symptomatic disease (e.g., thirst, urinary frequency, weight loss).10 Increased body weight and glucose dysregulation, including diabetes, have pernicious and far-reaching consequences to cardiometabolic risk, including increased free fatty acid release, dyslipidemia, increased inflammation, macrovascular damage and remodeling, and endothelial dysfunction.13,14
If the U-shaped relationship between sleep duration and cardiometabolic risk is mediated, in part, by individual associations with obesity, glucose metabolism, and their downstream pathophysiologic consequences, it is logical to consider whether short and/or long sleep duration is related to the metabolic syndrome. The metabolic syndrome is a composite set of cardiometabolic risk factors, including central adiposity, glucose dysregulation, elevated blood pressure, and dyslipidemia, which covary within populations and aggregate in individuals.15 Despite the absence of international consensus regarding the definition of and scoring guidelines for the metabolic syndrome,16 it has been widely shown to predict cardiovascular disease risk,17–19 type 2 diabetes,20–22 and all-cause mortality.17,19 While there is also disagreement about the extent to which the individual components of the metabolic syndrome represent a unique physiologic substructure or are merely a cluster of health hazards, there is no dispute that individual components of the metabolic syndrome are physiologically interrelated.23 To this end, evaluation of relationships among sleep and the metabolic syndrome is critical to understanding the synergistic mechanisms by which sleep affects and is affected by health. Indeed, recent studies have reported increased prevalence of the metabolic syndrome in individuals who snore and/or have sleep apnea, as well as rotating shiftworkers.24–26 We have recently shown that subjective sleep quality, as measured by the Pittsburgh Sleep Quality Index, is a significant correlate of the metabolic syndrome in a subsample (n = 210) of the participants included in this report.27 In the present larger study, we hypothesized that both short and long sleep duration would be associated with increased prevalence the metabolic syndrome.
METHODS
Data for the present study were derived from the University of Pittsburgh's Adult Health and Behavior (AHAB) registry, which is a compendium of behavioral and biologic measurements on midlife adults recruited via mass-mail solicitation from communities of Southwestern Pennsylvania (principally Allegheny County). Study participants in the AHAB registry were 1295 male and female AHAB participants, all between 30 and 54 years of age. Participants had no clinical history of atherosclerotic cardiovascular disease, chronic kidney or liver disease, cancer treatment within the preceding year, or major neurologic disorders, schizophrenia, or other psychotic illness. Other AHAB study exclusions included pregnancy and the use of insulin, glucocorticoid, antiarrhythmic, psychotropic or weight-loss medications. Data collection occurred over multiple laboratory sessions, and informed consent was obtained in accordance with approved guidelines of the University of Pittsburgh Institutional Review Board.
Sleep
Sleep duration was measured by trained interviewers as a component of the Stanford Five-City Physical Activity Interview.28 Participants were asked the number of hours per night they had slept over the past 7 days and were asked separately about sleep duration on weeknights (Sunday–Thursday) and weekend nights (Friday, Saturday). Consistent with previous studies that have evaluated relationships among sleep duration and indices of cardiometabolic risk, weekly average reported sleep duration was calculated as the weighted average of weeknight and weekend values [(5 × weekday sleep duration) + (2 × weekend sleep duration)/7].7,29 This definition makes no assumptions regarding work, leisure, or other constraints on weekday and weekend sleep hours. Participants were divided into 4 groups based upon their reported sleep duration: < 6 hours = “very short sleepers,” 6 to 6.99 hours = “short sleepers,” 7 to 8 hours = “reference group,” and > 8 hours = “long sleepers.” In exploratory analyses, we computed a measure of presumed sleep debt in order to explore the extent to which sleep-curtailment patterns were associated with the metabolic syndrome and its components.29 Sleep duration difference scores were calculated as weekend sleep duration — weeknight sleep duration. The majority of the sample had weekday versus weekend sleep duration discrepancies of less than 1 hour (77%); 16% slept over an hour longer on weekend compared with weekday nights, whereas 7% slept over an hour longer on weekday compared with weekend nights. Because we could not presume that participants “paid their sleep debt” on weekend nights, presumed sleep debt was dichotomized as a weekend versus weekday sleep duration difference of greater than 1 hour, regardless of whether the longer sleep bout occurred on a week night or weekend night.
Cardiometabolic Risk
Components of the metabolic syndrome were assessed in the morning following a 12-hour overnight fast. Blood pressure measurements were obtained by trained staff, using a mercury sphygmomanometer and cuff size appropriate to the participant's arm circumference. Blood pressure was calculated as the mean of two consecutive readings obtained on the right arm, in a seated position, and following 10 minutes' rest. Measurements were obtained of participants' height, weight, and waist circumference (at the umbilicus). Study staff also collected a venous blood sample, as well as medication usage and health status data at this time. Determination of fasting serum lipids, glucose, and insulin was performed by the Heinz Nutrition Laboratory, University of Pittsburgh Graduate School of Public Health, as described previously.30 The metabolic syndrome was defined according to the American Heart Association/National Heart Lung and Blood Institute's (AHA/NHLBI) criteria15 as the presence of three or more of the following: (1) waist circumference greater than 102 cm in men or greater than 88 cm in women; (2) fasting serum glucose of 100 mg/dL or greater or use of oral hypoglycemic medication; (3) blood pressure of 130 mm Hg systolic, 85 mm Hg diastolic or higher or use of antihypertensive medication; (4) serum triglycerides of 150 mg/dL or higher or medication for hypertriglyceridemia; and (5) high-density lipoprotein (HDL) cholesterol of less than 40 mg/dL in men or 50 mg/dL in women, or use of medication for low HDL cholesterol.
Covariates
Traditional and emerging biologic and sociodemographic factors, health behaviors, and indices of health were evaluated as potential confounds of relationships among sleep duration and cardiometabolic risk. Risk factors that have been robustly associated with sleep duration and/or the metabolic syndrome and its individual components included age, sex, race, socioeconomic status, smoking, alcohol use, physical activity, and cholesterol.31–33 The following were assessed by self report: age, sex, race (non-Hispanic Caucasian, African American), socioeconomic status as indexed by educational attainment (“college degree or greater” = 1 compared to “associate's degree or less” = 0), smoker status (dichotomized as “never,” “past smoker” = 0 versus “current smoker” = 1), alcohol use (average number of alcoholic beverages consumed per week), and physical activity operationalized as average weekly kilocalories.34 Fasting low-density lipoprotein (LDL)-cholesterol levels were determined according to the Friedewald equation.35 LDL-cholesterol levels were selected as a covariate in lieu of total cholesterol or the total cholesterol:HDL ratio given the high degree of collinearity between the latter two measures and indices of dyslipidemia, which constitute two of the five components of the metabolic syndrome.
Accumulating cross-sectional and longitudinal evidence also suggests that depression is a significant correlate of sleep duration33–36and cardiometabolic risk.37–39 For instance, two prospective studies reported that symptoms of depression, independent of age and health behaviors, were associated with increased risk of developing the metabolic syndrome.38,39 The majority of cross-sectional studies similarly support a strong association between symptoms of depression and the metabolic syndrome in mid- to late-life adults.37 In the present study, symptoms of depression were measured with the self-report Center for Epidemiological Studies Depression Scale,40 minus sleep items.
Statistical Analysis
Twenty-eight participants were excluded from analysis due to missing data on one or more sentinel variables (viz., sleep record, central adiposity, fasting lipids, or glucose concentration). An additional 53 participants taking statins were not included since these agents are generally prescribed for elevated LDL-cholesterol but have concurrent effects on triglycerides and HDL cholesterol, which confound dyslipidemia classification. Consistent with AHA/NHLBI guidelines regarding the diagnosis of the metabolic syndrome, participants taking fibrates and nicotinic acid (n = 7) were retained in the analyses as they were presumed to have high triglycerides and low HDL cholesterol.15 Therefore, the present analyses were conducted on a total sample of 1214 participants.
Descriptive statistics were used to characterize the sample, evaluate distributions, and guide the formation of categorical or transformed variables. Multivariable logistic regression was used to test the hypothesis that sleep duration is a significant correlate of the metabolic syndrome and its components. Covariates were age, sex, race, educational attainment, smoker status, physical activity, fasting LDL-cholesterol level, and symptoms of depression. Alcohol use was not used as a covariate as it was unrelated to sleep duration and the metabolic syndrome in the present sample. Secondary analyses explored the extent to which presumed sleep debt was a significant correlate of the metabolic syndrome and its components. Analyses of presumed sleep debt were considered exploratory given that we did not have a measure of preferred sleep duration29 nor were we able to verify that participants worked during the week and slept longer hours (i.e., paid their sleep debt) on the weekend.
A series of sensitivity analyses were conducted to evaluate the possibility that several key variables significantly confounded study results. Given the strong association between the abdominal obesity and glucose components of the metabolic syndrome (χ2 = 52.64, P < 0.001), two additional models tested relationships between (a) sleep duration and the abdominal obesity criterion in participants who did not meet the glucose criterion (n = 864, 71% of the sample) and (b) sleep duration and the glucose criterion in participants who did not meet the abdominal obesity criterion (n = 802, 66% of the sample). These analyses allowed us to evaluate associations between sleep duration and abdominal obesity, not confounded by elevated glucose, as well as sleep duration and glucose, not confounded by abdominal obesity. Because β-blockers and diuretics may indirectly increase glucose concentrations, sensitivity analyses were also performed in the subsample of participants who were not taking these medications (n = 1173, 96.6% of the sample). These analyses allowed us to evaluate associations between sleep duration and cardiometabolic risk, not confounded by antihypertensive medication use.
RESULTS
Background characteristics of the sample are shown in Table 1. Sixteen percent of the sample (n = 199) was self-identified as African American, and, overall, 62% of the sample were overweight or obese, as defined by a body mass index (kg/m2) of 25 or greater. These statistics parallel those observed in US adults of similar average age and racial distribution.23 Presence of the metabolic syndrome in this sample (22%) was also similar to that of published prevalence statistics for US adults.15 An additional 46% (n = 560) of the sample met one or two criteria for the metabolic syndrome, whereas 32% (n = 386) met no criteria. Short sleep duration was common, with 20% of the sample reporting sleep durations of less than 6 hours per night. In contrast, only 8% of the sample reported sleep durations of longer than 8 hours per night.
Table 1.
Sample Characteristics
| Characteristic | Results |
|---|---|
| Age, y | 44.4 ± 6.8 |
| Men | 568 (46.6) |
| Non-Hispanic Caucasian | 1019 (83.7) |
| College graduate | 816 (67.2) |
| Current smoker | 231 (19.0) |
| Physical activity, weekly kcala | 2431.7 ± 1841.0 |
| Symptoms of depression, CES-Da | 7.4 ± 1.8 |
| Reported sleep duration, h | |
| < 6 (very short sleepers) | 187 (15.4) |
| 6.0–6.99 (short sleepers) | 402 (33.1) |
| 7.0–8.0 (referent group) | 525 (43.2) |
| > 8.0 (long sleepers) | 100 (8.2) |
| Presumed sleep debt | 281 (23.1) |
| Metabolic syndrome | 272 (22.3) |
| Central adiposity criterion | 412 (33.9) |
| Glucose criterion | 350 (28.8) |
| Blood pressure criterion | 317 (31.9) |
| Triglycerides criterion | 276 (22.7) |
| HDL criterion | 338 (27.8) |
| Medications | |
| Diabetic | 5 (0.4) |
| Antihypertensives | 79 (6.5) |
| Dyslipidemiab | 7 (0.6) |
| Fasting Glucose, mg/dL | 96.1 ± 17.5 |
| BMI | 27.4 ± 5.8 |
| Blood pressure | |
| Systolic | 116.3 ± 13.5 |
| Diastolic | 78.3 ± 9.5 |
| Triglycerides, mg/dL | 119.7 ± 81.4 |
| HDL, mg/dL | 53.6 ± 14.7 |
| LDL, mg/dL | 123.39 ± 32.15 |
Data are presented as mean ± SD or number (%). CES-D refers to Center for Epidemiological Studies Depression Scale; BMI, body mass index; LDL, low-density lipoprotein.
Symptoms of Depression (CES-D) and Physical Activity (kcal) were log transformed prior to hypothesis testing.
Use of fibrates or nicotinic acid for treatment of hypertriglyceridemia or low high-density lipoprotein (HDL) cholesterol.
Presumed sleep debt is defined as > 1 hour weekday – weekend difference.
Logistic regression model results are summarized in Table 2. With respect to background variables included in the models, older age was associated with the metabolic syndrome and each of its components (P values < 0.01) with the exception of the low HDL criterion. Although metabolic syndrome rates did not differ between Caucasians and African Americans (21.8% and 24.6%, respectively), a greater percentage of the African Americans ranked above the defined cutoffs for central adiposity and blood pressure and below the threshold for triglycerides, as compared with the Caucasians (all P values < 0.001). Metabolic syndrome rates were twice as high in men, as compared with women (30.3% versus 15.2%, respectively; P < 0.001). Increased prevalence of the metabolic syndrome in men was related to the following criteria: glucose, blood pressure, and triglycerides. Although rates of very short sleep duration (< 6 hours/night) were comparable in men and women (18.8% and 12.5%, respectively), very short sleep durations were more common among African Americans (23.6%) than among Caucasians (13.8%) (Pearson χ2 = 12.33, P < 0.001). The metabolic syndrome and elevated blood pressure were less common in participants who achieved greater educational attainment (19% and 27.6%, respectively), compared with those with an associate's degree or less (26.8% and 38%, respectively). Consistent with previous studies, the metabolic syndrome and each of its components was inversely associated with physical activity, as measured by weekly kilocalorie expenditure, and positively associated with LDL-cholesterol levels. In multivariable models, smoker status and symptoms of depression were unrelated to the metabolic syndrome or its individual components.
Table 2.
Relationships Among the Metabolic Syndrome, Components of the Metabolic Syndrome, Sleep Duration and Covariates
| Sleep Duration and Covariates | Individual components of the metabolic syndrome |
|||||
|---|---|---|---|---|---|---|
| Metabolic syndrome OR (95% CI) | Central adiposity criterion OR (95% CI) | Glucose criterion OR (95% CI) | Blood pressure criterion OR (95% CI) | Triglycerides criterion OR (95% CI) | HDL criterion OR (95% CI) | |
| Reported sleep duration, hd | ||||||
| < 6 | 1.83 (1.19–2.80) | 1.73 (1.21–2.57) | 1.74 (1.18–2.55) | 1.10 (0.75–1.63) | 1.32 (0.85–2.05) | 1.34 (0.91–1.99) |
| 6-< 7 | 1.41 (1.05–2.10) | 1.64 (1.22–2.20) | 1.11 (0.82–1.52) | 0.97 (0.72–1.32) | 1.53 (1.10–2.14) | 1.24 (0.91–1.69) |
| 7– 8 | Ref | Ref | Ref | Ref | Ref | Ref |
| > 8 | 1.81 (1.04–3.15) | 1.47 (0.91–2.40) | 1.70 (1.04–2.80) | 1.00 (0.59–1.69) | 0.93 (0.50–1.73) | 1.54 (0.95–2.50) |
| Biologic and sociodemographicsa | ||||||
| Age | 1.05 (1.02–1.07) | 1.02 (1.00–1.04) | 1.04 (1.02–1.06) | 1.07 (1.05–1.10) | 1.03 (1.01–1.05) | 0.98 (0.96–1.00) |
| Sex | 2.60 (1.90–3.55) | 1.01 (0.78–1.32) | 2.44 (1.85–3.21) | 3.09 (2.33–4.08) | 3.20 (2.34–4.38) | 1.16 (0.88–1.52) |
| Race | 0.99 (0.65–1.49) | 1.79 (1.26–2.52) | 1.17 (0.81–1.70) | 2.10 (1.46–3.02) | 0.31 (0.19–0.53) | 0.79 (0.54–1.14) |
| Educational attainment | 0.68 (0.50–0.93) | 0.78 (0.60–1.02) | 0.94 (0.71–1.24) | 0.66 (0.50–0.87) | 0.74 (0.54–1.01) | 0.59 (0.44–0.77) |
| Health behaviorsb | ||||||
| Smoker | 1.03 (0.71–1.50) | 0.78 (0.56–1.09) | 1.27 (0.91–1.77) | 0.86 (0.61–1.22) | 0.98 (0.67–1.43) | 1.15 (0.82–1.61) |
| Physical activity | 0.61 (0.51–0.73) | 0.68 (0.58–0.80) | 0.82 (0.69–0.96) | 0.79 (0.67–0.93) | 0.65 (0.54–0.78) | 0.67 (0.57–0.79) |
| Indices of healthc | ||||||
| LDL cholesterol | 1.01 (1.00–1.01) | 1.01 (1.01–1.01) | 1.01 (1.00–1.01) | 1.00 (1.00–1.01) | 1.01 (1.00–1.01) | 1.00 (1.00–1.00) |
| Symptoms of depression | 1.03 (0.88–1.21) | 1.08 (0.94–1.23) | 0.96 (0.83–1.10) | 0.93 (0.81–1.07) | 1.05 (0.90–1.23) | 0.93 (0.81–1.07) |
HDL refers to high-density lipoprotein; OR, odds ratio; CI, confidence interval.
Age = 1-year increase; Sex = males compared to females; Race = African American compared to Caucasian; Educational attainment = bachelor's degree or higher compared to associate's degree or lower.
Smoker = “current smoker” vs “never” or “past”; Physical activity = 1 SD increase.
Low-density lipoprotein (LDL)-cholesterol = 1 SD increase; Symptoms of depression = 1 SD increase.
Sleep duration = 7– 8 hours as referent.
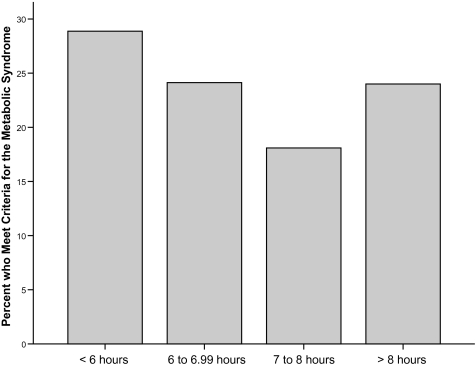
The proportion of participants in each sleep duration category with the metabolic syndrome is shown in Figure 1. As detailed in Table 2, after adjusting for age, sex, race, educational attainment, smoker status, physical activity, LDL-cholesterol, and symptoms of depression, the odds for having the metabolic syndrome increased between 48% and 83% in both groups of short sleepers and the long sleeper group, compared with those sleeping 7 to 8 hours per night. Relationships between reported sleep duration and individual components of the metabolic syndrome were similarly evaluated, and significant relationships were noted for three of the five metabolic syndrome criteria. Very short and short sleepers were at least 1.6 times more likely to meet criteria for abdominal obesity (waist circumference > 102 cm for men or 88 cm for women) compared with individuals who slept 7 to 8 hours per night. Sensitivity analyses confirmed that significant relationships among very short (odds ratio [OR] = 2.05, 95% confidence interval [CI]= 1.27–3.31) and short sleepers (OR = 1.62, 95% CI = 1.12–2.34) and the abdominal-obesity criterion remained significant in the subsample of participants who did not meet the glucose criterion (n = 864, 71% of the sample).
Figure 1.
Proportion of participants in each sleep duration category who meet criteria for the metabolic syndrome.
In the full sample, a U-shaped curvilinear relationship was observed between sleep duration and the glucose criterion, defined as a fasting glucose level of 100 mg/dL or greater or use of oral hypoglycemic medication (Table 2). Compared with the reference group, the odds of meeting the glucose criterion were at least 1.7 times greater in the very short (< 6 hours) and long (> 8 hours) sleeper groups. Sensitivity analyses in the subsample of participants who did not meet the abdominal-obesity criterion (n = 802, 66% of the sample) revealed that the odds for meeting the glucose criterion remained elevated in short sleepers (OR = 2.08, 95% CI = 1.27–3.43) but not significantly so in long sleepers (OR = 1.72, 95% CI = 0.90–3.29).
In the full sample, the odds for meeting the elevated triglyceride criterion (triglycerides ≥ 150 mg/dL or use of dyslipidemic medication) were increased by 53% in short sleepers, compared with the reference group of men and women who slept 7 to 8 hours per night. The odds of meeting the triglyceride criterion did not differ in the very short or long sleepers, compared with the reference group. Sleep duration was not associated with the blood pressure criterion or the low HDL-cholesterol criterion.
Sensitivity analyses indicated that use of potentially confounding antihypertensive medications had relatively minor effects on observed relationships between sleep duration and cardiometabolic risk. As detailed in Table 3, among participants not taking β-blockers and/or diuretics (n = 1173, 96.6% of the sample), the odds for meeting metabolic syndrome criteria remained elevated in the very short and short sleepers but declined somewhat in the long sleepers and was no longer statistically significant. Similarly, the odds for meeting the glucose criterion remained elevated in the very short sleepers but not in the long sleepers. Significant relationships among sleep duration and both the abdominal obesity criterion and the triglyceride criterion remained unchanged in the subsample of participants not taking antihypertensive medications. Long sleep duration, which was weakly related to the HDL criterion in the full sample (P = 0.08), was associated with a significant 77% increase in this cardiometabolic risk factor in the subsample of participants not taking β-blockers or diuretics.
Table 3.
Sensitivity Analysis Results for Relationships Between Sleep Duration and Cardiometabolic Risk in the Subsample of Participants not Taking Antihypertensive Medication (n = 1173, 96.6% of the Sample)
| Reported sleep duration, hours | Individual components of the metabolic syndrome |
|||||
|---|---|---|---|---|---|---|
| Metabolic syndrome OR (95% CI) | Central adiposity criterion OR (95% CI) | Glucose criterion OR (95% CI) | Blood pressure criterion OR (95% CI) | Triglycerides criterion OR (95% CI) | HDL criterion OR (95% CI) | |
| < 6 | 1.76 (1.13–2.74) | 1.75 (1.19–2.56) | 1.76 (1.19–2.60) | 1.13 (0.76–1.68) | 1.29 (0.82–2.03) | 1.31 (0.87–1.95) |
| 6 – < 7 | 1.47 (1.03–2.11) | 1.64 (1.22–2.22) | 1.06 (0.77–1.45) | 0.96 (0.70–1.31) | 1.51 (1.07–2.13) | 1.26 (0.92–1.73) |
| 7– 8 | Ref | Ref | Ref | Ref | Ref | Ref |
| > 8 | 1.65 (0.91–3.00) | 1.41 (0.85–2.34) | 1.56 (0.93–2.63) | 0.87 (0.49–1.54) | 0.91 (0.48–1.74) | 1.77 (1.08–2.89) |
Sleep duration = 7–8 hours as referent; covariates included age, sex, race, educational attainment, smoker, physical activity, low-density lipoprotein (LDL) cholesterol and symptoms of depression (odds ratio [OR] and 95% confidence interval [CI] not shown). HDL refers to high-density lipoprotein.
Results of exploratory analyses revealed that presumed sleep debt, as indexed by a weekend versus weeknight sleep-duration discrepancy of greater than 8 hour, was not a significant correlate of the metabolic syndrome or any of its components (data not shown).
DISCUSSION
In recent years, there has been a proliferation of studies focused on sleep duration and indices of cardiometabolic risk. Short and long sleep duration have been linked to various risk factors for cardiovascular disease, including increased body mass index, diabetes, metabolic dysregulation, elevated blood pressure, dyslipidemia, and others.7,33,41–44 Each of these large epidemiologic studies has generally focused on a single cardiometabolic risk factor and adjusted for other risk factors to the extent possible. In contrast, the epidemiologic and clinical concept of the metabolic syndrome presumes that abdominal obesity, glucose dysregulation, elevated blood pressure, and dyslipidemia are a cluster of risk factors that act synergistically to influence subsequent disease processes.15 Although some find this approach unsatisfying because evaluation of a syndrome makes it difficult to identify individual physiologic pathways to disease, the central notion of the metabolic syndrome is that these individual risk factors have, in part, shared etiologies. Growing appreciation of the behavioral and physiologic interconnectedness of cardiometabolic risk factors has led some to evaluate relationships between sleep and the metabolic syndrome. Published studies have examined relationships between the metabolic syndrome and sleep disordered breathing, snoring, shift work, and subjective sleep-quality complaints.24–27 To our knowledge, the current investigation is the first to evaluate relationships between sleep duration and the metabolic syndrome.
Results indicate that both short (< 7 hours per night) and long sleep duration (> 8 hours per night) are significant correlates of the metabolic syndrome in a community sample of midlife adults. Relationships between sleep duration and the metabolic syndrome were observed despite statistical adjustment for traditional Framingham risk factor components (age, sex, race, smoker status, and LDL cholesterol), educational attainment, physical activity, and symptoms of depression. After exclusion of individuals taking potentially confounding antihypertensive medications, the relationship between long sleep duration and the metabolic syndrome was no longer statistically significant. Sleep duration was independently associated with 3 components of the metabolic syndrome: abdominal obesity, elevated glucose, and elevated triglycerides. Men and women who slept less than 7 hours per night were nearly twice as likely to meet criteria for abdominal obesity, compared with those who reported sleeping 7 to 8 hours per night. Very short sleepers (< 6 hours per night) who were not obese and did not take antihypertensive medications were more likely to meet the elevated-glucose criterion, compared with men and women who reported sleeping 7 to 8 hours per night. Meeting the elevated-triglyceride criterion was more common in short (between 6 and 7 hours per night) but not in very short or long sleepers. Our cross-sectional results for sleep duration and the abdominal-obesity and glucose components of the metabolic syndrome are consistent with the results of several prospective epidemiologic studies that have linked sleep duration to subsequent weight gain and diabetes risk.1,9,12,33 Although others have reported univariate associations between sleep duration and lipids, relationships have not remained significant after adjustment for other cardiometabolic risk factors.41,41,44 Thus, our findings related to short sleep duration and elevated triglyceride levels must be considered preliminary.
Although several epidemiologic studies have reported significant associations between sleep duration and prevalent or incident hypertension,2,3,45 sleep duration was unrelated to the blood pressure component of the metabolic syndrome in our midlife sample of men and women. The significant National Health and Nutrition Examination Survey-I, Sleep Heart Health Study, and Whitehall II results were observed in samples of more than 4000 and results were shown to be age and sex specific, which might suggest that our study may have been underpowered to evaluate relationships between sleep duration and the blood-pressure component of the metabolic syndrome. Certainly, laboratory studies have demonstrated that sleep curtailment or deprivation acutely increases blood pressure in normotensive adults.46–48 Finally, presumed sleep debt, which was operationalized as a weekend-to-weekday difference in sleep duration of longer than 1 hour, regardless of whether the sleep debt was “paid” on the weekend or weekday night, was unrelated to the metabolic syndrome and its components. This was somewhat surprising given robust associations among short sleep duration and indices of cardiometabolic risk in this and other studies. These results suggest that short sleep duration cannot be presumed to reflect sleep debt and/or sleep debt must be more systematically measured.
Abdominal obesity and elevated glucose were among the individual metabolic syndrome components most strongly associated with short sleep duration in the present study. Although our cross-sectional data cannot address questions of causality, experimental studies suggest that sleep restriction may promote weight gain and alter glucose metabolism.49 One study6 demonstrated that experimental sleep restriction altered the dynamic balance between leptin, which promotes satiety, and ghrelin, which stimulates appetite. The increased ghrelin-to-leptin ratio was strongly associated with increased reports of hunger and appetite for calorie-rich and carbohydrate-dense foods.6 Experimental sleep restriction has similarly been shown to elicit clinically significant decreases in glucose tolerance in lean young men.50 Because the present sample of midlife men and women was older, less healthy, and more overweight than were the participants in the experimental sleep-restriction studies, we conducted sensitivity analyses to evaluate relationships between sleep duration and abdominal obesity, not confounded by elevated glucose, and vice-versa. In the present sample, the cross-sectional relationship observed between short sleep duration and abdominal obesity was independent of elevated glucose and use of antihypertensive medication. Relationships between short sleep duration and the glucose component of the metabolic syndrome were independent of abdominal obesity and use of antihypertensive medication. Consistent with those of the experimental sleep-restriction studies, our results support independent links between short sleep duration and both abdominal obesity and alterations in glucose metabolism. That these risk factors act synergistically to promote cardiometabolic disease suggests that sleep duration, if causally linked to abdominal obesity and alterations in glucose metabolism, may play an important mechanistic role in the development of cardiometabolic disease.
Other less-studied behavioral and physiologic pathways linking short sleep duration to components of the metabolic syndrome include increased opportunity for eating, increased physiologic arousal, and increased inflammation.5,49,51 Although intriguing, the behavioral hypothesis that short sleepers gain weight and develop type 2 diabetes because they have more opportunities to eat has not been fully explicated. Although short sleepers may indeed have more time to eat, they also have more opportunities to engage in a host of health behaviors known to increase or decrease cardiometabolic risk, including smoking, alcohol and caffeine use, and increased physical activity, and more opportunities to engage in social support. Although our analyses adjusted for smoking and physical activity, relationships among short sleep duration, health behaviors, and cardiometabolic risk are undoubtedly complex and warrant further empirical evaluation. Other physiologic pathways that may play a role in the link between short sleep duration and cardiometabolic risk include autonomic nervous system dysregulation, increased activity of the hypothalamic-pituitary-adrenal axis, and increased inflammation.5,9,52
The evidence linking long sleep duration to cardiometabolic risk is mixed. Aside from the few studies that have reported increased incidence of type 2 diabetes in long sleepers,10,2 others have reported increased major depression and lower socioeconomic status in long sleepers.31,33,53 More recently, Williams and colleagues reported that C-reactive protein was a significant correlate of long sleep duration in women with type 2 diabetes and no documented history of heart disease or stroke.44 They hypothesized that proinflammatory cytokines actively increase sleep duration as an adaptive mechanism to promote recovery. We found little support for a link between long sleep duration and cardiometabolic risk. Multivariate models revealed only two significant relationships between long sleep duration, defined as 8 hours or more of sleep, and the metabolic syndrome: compared to men and women who slept 7 to 8 hours per night, long sleepers were more likely to meet criteria for the metabolic syndrome and the elevated-glucose criterion. These results were no longer significant with adjustment for use of antihypertensive medication, which has been shown to impact fasting blood glucose levels. Similar to Williams et al,44 we found that long sleepers who were not taking antihypertensive medication were more likely to meet the criteria for low HDL cholesterol. Others have hypothesized that long sleep duration is a proxy for sleep disordered breathing and that sleep apnea drives the relationship between long sleep duration and health outcomes.54–55 Furthermore, sleep apnea is itself associated with heightened risk for the metabolic syndrome.25 Measures of sleep apnea, including snoring, were not available in the AHAB cohort, so we were unable to evaluate the extent to which observed relationships between sleep duration and cardiometabolic risk were robust to adjustment for sleep disordered breathing. However, recent reports from the Sleep Heart Health Study and Wisconsin Sleep Cohort indicate that individuals with long sleep durations do not have significantly elevated symptoms of sleep disordered breathing, as compared with individuals with short sleep durations.45,56 Although the present sample was generally healthy, unrecognized morbidity, including sleep apnea, or adverse health behaviors may have contributed to observed relationships between long sleep duration and the metabolic syndrome.
The limitations of the present study and the published literature suggest several directions for future research regarding the relationship between sleep and the metabolic syndrome. As already noted, the cross-sectional nature of these data precludes causal inferences regarding the relationship between sleep duration and the metabolic syndrome. Additional experimental and prospective observational studies are needed to evaluate the extent to which sleep duration affects, or is affected by, the metabolic syndrome, abdominal obesity, glucose and lipid metabolism, and blood pressure. Although the present study evaluated a community sample of healthy adults, relationships between sleep and the metabolic syndrome might differ in important ways in other populations. For instance, the present results cannot be generalized to adults with medical or psychiatric morbidities not included in the AHAB sample (e.g., patients with bipolar disorder or schizophrenia, who are at increased risk for the metabolic syndrome and cardiometabolic disease). Additionally, these results cannot be generalized to other races and ethnic groups with different cardiometabolic risk profiles or different sleep patterns. In terms of future research, evaluation of relationships between sleep duration and early markers of cardiometabolic risk in adolescents and young adults would be especially helpful for teasing out confounds related to morbidity and lifestyle factors. In future studies it will be important to evaluate other dimensions of sleep (e.g., sleep continuity, sleep architecture, sleep debt) beyond sleep disordered breathing, sleep quality, and sleep duration that may be related to the metabolic syndrome and its component risk factors. Finally, identification of the proximal behavioral and biologic pathways by which sleep affects components of the metabolic syndrome is essential to developing treatment strategies to augment behavioral and pharmacologic interventions for cardiometabolic disease.
ACKNOWLEDGMENTS
The authors appreciate and acknowledge the statistical input of Patricia Houck, M.S. The corresponding author and Dr. Stephen Manuck (PI) had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. This work was supported by National Institutes of Health Grants HL040962, HL065137, HL076852, and RR024153.
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Muldoon has consulted for Pro Consulting. Dr. Buysse has consulted for Actelion, Arena, Cephalon, Eli Lilly, GlaxoSmithKline, Merck, Neurocrine, Neurogen, Pfizer, Respironics, Sanofi-Aventis, Sepracor, Servier, Stress Eraser, and Takeda. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 2.Cappuccio FP, Stranges S, Kandala NB, et al. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50:693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–839. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 4.Ferrie JE, Shipley MJ, Cappuccio FP, et al. A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort. Sleep. 2007;30:1659–1666. doi: 10.1093/sleep/30.12.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spiegel K, Knutson K, Leproult R, et al. Sleep loss: a novel risk factor for insulin resistance and type 2 diabetes. J Appl Physiol. 2005;99:2008–2019. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 6.Spiegel K, Tasali E, Penev P, et al. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 7.Taheri S, Lin L, Austin D, et al. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gangwisch JE, Malaspina D, Boden-Albala B, et al. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28:1289–1296. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 9.Patel SR, Malhotra A, White DP, et al. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006;164:947–954. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayas NT, White DP, Al Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–384. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 11.Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165:863–867. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 12.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29:657–661. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 13.Hansen E, Hajri T, Abumrad NN. Is all fat the same? The role of fat in the pathogenesis of the metabolic syndrome and type 2 diabetes mellitus. Surgery. 2006;139:711–716. doi: 10.1016/j.surg.2005.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ritchie SA, Connell JM. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis. 2007;17:319–326. doi: 10.1016/j.numecd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 16.Stern N, Izkhakov Y. The metabolic syndrome revisited: “cardiometabolic risk” emerges as common ground between differing views of the ADA and AHA. J Cardiometab Syndr. 2006;1:362–363. doi: 10.1111/j.1524-6175.2006.05999.x. [DOI] [PubMed] [Google Scholar]
- 17.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 18.Hunt KJ, Resendez RG, Williams K, et al. National Cholesterol Education Program versus World Health Organization metabolic syndrome in relation to all-cause and cardiovascular mortality in the San Antonio Heart Study. Circulation. 2004;110:1251–1257. doi: 10.1161/01.CIR.0000140762.04598.F9. [DOI] [PubMed] [Google Scholar]
- 19.Hillier TA, Rizzo JH, Pedula KL, et al. Increased mortality associated with the metabolic syndrome in older women with diabetes. Diabetes Care. 2005;28:2258–2260. doi: 10.2337/diacare.28.9.2258. [DOI] [PubMed] [Google Scholar]
- 20.Hanson RL, Imperatore G, Bennett PH, et al. Components of the “metabolic syndrome” and incidence of type 2 diabetes. Diabetes. 2002;51:3120–3127. doi: 10.2337/diabetes.51.10.3120. [DOI] [PubMed] [Google Scholar]
- 21.Laaksonen DE, Lakka HM, Niskanen LK, et al. Metabolic syndrome and development of diabetes mellitus: application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. Am J Epidemiol. 2002;156:1070–1077. doi: 10.1093/aje/kwf145. [DOI] [PubMed] [Google Scholar]
- 22.Wannamethee SG, Shaper AG, Lennon L, et al. Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med. 2005;165:2644–2650. doi: 10.1001/archinte.165.22.2644. [DOI] [PubMed] [Google Scholar]
- 23.Johnson LW, Weinstock RS. The metabolic syndrome: concepts and controversy. Mayo Clin Proc. 2006;81:1615–1620. doi: 10.4065/81.12.1615. [DOI] [PubMed] [Google Scholar]
- 24.Leineweber C, Kecklund G, Akerstedt T, et al. Snoring and the metabolic syndrome in women. Sleep Med. 2003;4:531–536. doi: 10.1016/s1389-9457(03)00160-6. [DOI] [PubMed] [Google Scholar]
- 25.Coughlin SR, Mawdsley L, Mugarza JA, et al. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735–741. doi: 10.1016/j.ehj.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 26.Sookoian S, Gemma C, Fernandez Gianotti T, et al. Effects of rotating shift work on biomarkers of metabolic syndrome and inflammation. J Intern Med. 2007;261:285–292. doi: 10.1111/j.1365-2796.2007.01766.x. [DOI] [PubMed] [Google Scholar]
- 27.Jennings JR, Muldoon MF, Hall M, et al. Self-reported sleep quality is associated with the metabolic syndrome. Sleep. 2007;30:219–223. doi: 10.1093/sleep/30.2.219. [DOI] [PubMed] [Google Scholar]
- 28.Sallis JF, Haskell WL, Wood PD, et al. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121:91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 29.Knutson KL, Ryden AM, Mander BA, et al. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med. 2006;166:1768–1774. doi: 10.1001/archinte.166.16.1768. [DOI] [PubMed] [Google Scholar]
- 30.Muldoon MF, Nazzaro P, Sutton-Tyrrell K, et al. White-coat hypertension and carotid artery atherosclerosis: a matching study. Arch Intern Med. 2000;160:1507–1512. doi: 10.1001/archinte.160.10.1507. [DOI] [PubMed] [Google Scholar]
- 31.Prescott E, Godtfredsen N, Osler M, et al. Social gradient in the metabolic syndrome not explained by psychosocial and behavioural factors: evidence from the Copenhagen City Heart Study. Eur J Cardiovasc Prev Rehabil. 2007;14:405–412. doi: 10.1097/HJR.0b013e32800ff169. [DOI] [PubMed] [Google Scholar]
- 32.Skilton MR, Moulin P, Serusclat A, et al. A comparison of the NCEP-ATPIII, IDF and AHA/NHLBI metabolic syndrome definitions with relation to early carotid atherosclerosis in subjects with hypercholesterolemia or at risk of CVD: evidence for sex-specific differences. Atherosclerosis. 2007;190:416–422. doi: 10.1016/j.atherosclerosis.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 33.Patel SR, Malhotra A, Gottlieb DJ, et al. Correlates of long sleep duration. Sleep. 2006;29:881–889. doi: 10.1093/sleep/29.7.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1971;101:161–175. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 35.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 36.Perlman CA, Johnson SL, Mellman TA. The prospective impact of sleep duration on depression and mania. Bipolar Disord. 2006;8:271–274. doi: 10.1111/j.1399-5618.2006.00330.x. [DOI] [PubMed] [Google Scholar]
- 37.Gans RO. The metabolic syndrome, depression, and cardiovascular disease: interrelated conditions that share pathophysiologic mechanisms. Med Clin North Am. 2006;90:573–591. doi: 10.1016/j.mcna.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Raikkonen K, Matthews KA, Kuller LH. The relationship between psychological risk attributes and the metabolic syndrome in healthy women: antecedent or consequence? Metabolism. 2002;51:1573–1577. doi: 10.1053/meta.2002.36301. [DOI] [PubMed] [Google Scholar]
- 39.Raikkonen K, Matthews KA, Kuller LH. Depressive symptoms and stressful life events predict metabolic syndrome among middle-aged women: a comparison of World Health Organization, Adult Treatment Panel III, and International Diabetes Foundation definitions. Diabetes Care. 2007;30:872–877. doi: 10.2337/dc06-1857. [DOI] [PubMed] [Google Scholar]
- 40.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 41.Bjorvatn B, Sagen IM, Oyane N, et al. The association between sleep duration, body mass index and metabolic measures in the Hordaland Health Study. J Sleep Res. 2007;16:66–76. doi: 10.1111/j.1365-2869.2007.00569.x. [DOI] [PubMed] [Google Scholar]
- 42.Patel SR. Social and demographic factors related to sleep duration. Sleep. 2007;30:1077. doi: 10.1093/sleep/30.9.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel SR, Ayas NT, Malhotra MR, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27:440–444. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 44.Williams CJ, Hu FB, Patel SR, et al. Sleep duration and snoring in relation to biomarkers of cardiovascular disease risk among women with type 2 diabetes. Diabetes Care. 2007;30:1233–1240. doi: 10.2337/dc06-2107. [DOI] [PubMed] [Google Scholar]
- 45.Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29:1009–1014. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 46.Lusardi P, Mugellini A, Preti P, et al. Effects of a restricted sleep regimen on ambulatory blood pressure monitoring in normotensive subjects. Am J Hypertens. 1996;9:503–505. doi: 10.1016/0895-7061(95)00389-4. [DOI] [PubMed] [Google Scholar]
- 47.Ogawa Y, Kanbayashi T, Saito Y, et al. Total sleep deprivation elevates blood pressure through arterial baroreflex resetting:a study with microneurographic technique. Sleep. 2003;26:986–989. doi: 10.1093/sleep/26.8.986. [DOI] [PubMed] [Google Scholar]
- 48.Tochikubo O, Ikeda A, Miyajima E, et al. Effects of insufficient sleep on blood pressure monitored by a new multibiomedical recorder. Hypertension. 1996;27:1318–1324. doi: 10.1161/01.hyp.27.6.1318. [DOI] [PubMed] [Google Scholar]
- 49.Knutson KL, Spiegel K, Penev P, et al. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 51.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 52.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 53.Ohayon MM, Vecchierini MF. Normative sleep data, cognitive function and daily living activities in older adults in the community. Sleep. 2005;21:981–989. [PubMed] [Google Scholar]
- 54.Alvarez GG, Ayas NT. The impact of daily sleep duration on health: a review of the literature. Prog Cardiovasc Nurs. 2004;19:56–59. doi: 10.1111/j.0889-7204.2004.02422.x. [DOI] [PubMed] [Google Scholar]
- 55.Kripke DF, Ancoli-Israel S, Mason WJ, et al. Sleep apnea: association with deviant sleep durations and increased mortality. In: Guilleminault C, Partinen M, editors. Sleep Apnea Syndrome: Clinical Research and Treatment. New York: Raven Press; 1990. pp. 9–14. [Google Scholar]
- 56.Reichmuth KJ, Austin D, Skatrud JB, et al. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172:1590–1595. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]