Abstract
Study Objectives:
We examined the individual association between sleep duration and a high serum triglyceride, low HDL cholesterol, or high LDL cholesterol level.
Design and Setting:
The present study analyzed data from the National Health and Nutrition Survey that was conducted in November 2003 by the Japanese Ministry of Health, Labour and Welfare. This survey was conducted on residents in the districts selected randomly from all over Japan.
Participants:
The subjects included in the statistical analysis were 1,666 men and 2,329 women aged 20 years or older.
Intervention:
N/A
Measurements and Results:
Among women, both short and long sleep durations are associated with a high serum triglyceride level or a low HDL cholesterol level. Compared with women sleeping 6 to 7 h, the relative risk of a high triglyceride level among women sleeping <5 h was 1.51 (95% CI, 0.96–2.35), and among women sleeping ≥8 h was 1.45 (95% CI, 1.00–2.11); the relative risk of a low HDL cholesterol level among women sleeping <5 h was 5.85 (95% CI, 2.29–14.94), and among women sleeping ≥8 h was 4.27 (95% CI, 1.88–9.72). On the other hand, it was observed that the risk of a high LDL cholesterol level was lower among men sleeping ≥8 h. These analyses were adjusted for the following items: age, blood pressure, body mass index, plasma glucose level, smoking habit, alcohol consumption, dietary habits, psychological stress, and taking cholesterol-lowering medications.
Conclusions:
Usual sleep duration is closely associated with serum lipid and lipoprotein levels.
Citation:
Kaneita Y; Uchiyama M; Yoshiike N; Ohida T. Associations of usual sleep duration with serum lipid and lipoprotein levels. SLEEP 2008;31(5):645-652.
Keywords: Dyslipidemia, triglyceride, high density lipoprotein cholesterol, low density lipoprotein cholesterol, sleep duration
IN THE LATE 1960S, IT WAS REVEALED THAT SHORTER OR LONGER SLEEP DURATION RESULTED IN AN INCREASED RISK OF MORTALITY IN HUMANS, AND that there was a U-shaped association between sleep duration and mortality risk.1 Subsequently, several large-scale studies demonstrated similar findings.2–4 In recent years, U-shaped associations have been demonstrated between sleep duration and morbidity risk for diabetes mellitus,5–7 obesity,8 hypertension,9 coronary heart disease (CHD),10 and atherosclerosis.11 It has been increasingly recognized that sleep habits, along with other lifestyle habits, such as eating, exercising, smoking, and drinking, are potential risk factors for diabetes mellitus, obesity, hypertension, and cardiovascular disease (CVD).
Dyslipidemia, such as an increase in the level of triglyceride, a decrease in the level of high-density-lipoprotein (HDL) cholesterol, and an increase in the level of low-density-lipoprotein (LDL) cholesterol, increases the risk of CVD morbidity.12–14 In order to prevent CVD, it is important to identify and change lifestyles that are associated with serum triglyceride, HDL cholesterol or LDL cholesterol level. It is well known that these serum lipid and lipoprotein levels are strongly influenced by lifestyles. Smoking decreases the level of HDL cholesterol and increases the level of triglyceride in blood, whereas alcohol consumption increases the levels of both.15 Exercise increases the HDL cholesterol level and decreases the triglyceride level in blood.15 In addition, alcohol consumption is reported to decrease the level of LDL cholesterol.16,17
In 1999, Nakanishi et al. indicated that there was no significant association between sleep duration and serum lipid and lipoprotein levels among men.18 However, their sample was comprised of male office workers from a single company and these findings cannot reasonably be extrapolated to the general Japanese population. To clarify this issue, the present study examined the individual associations of sleep duration with the levels of serum triglyceride, HDL cholesterol and LDL cholesterol.
METHODS
Study Subjects and Data Collection
The present study was performed using data collected by the National Health and Nutrition Survey that was conducted in November 2003 by the Japanese Ministry of Health, Labour and Welfare. The National Health and Nutrition Survey is a cross-sectional survey that is conducted annually in order to obtain epidemiological data for national health promotion.19 The subjects of this national survey comprised approximately 15,000 residents aged 1 year or more in the 300 districts randomly selected from the national census unit districts.
The survey comprised three parts: (1) examination of physical status, (2) dietary intake survey, and (3) questionnaire on lifestyles. Actual data collection was performed by the staff of the local public health centers that exercised jurisdiction over the selected districts.
For the physical status examination, the subjects were invited to public facilities within the districts. The heights and weights of all the participants aged 1 year or older, abdominal circumference, and blood pressure of all participants aged 15 years or older were measured. In addition, participants aged 20 years or older were interviewed with regard to any medications taken on a regular basis, and blood samples were taken from each subject for subsequent analysis.
For dietary assessment, the staff of the public health centers visited the subjects' households, distributed the recording sheets, and explained how to complete them. The subjects were requested to record the name and amount of all food consumed by each household member aged 1 year or older on a selected weekday.
The lifestyle questionnaire, which was self-administered, was issued to all participants older than 15 years of age, with instructions on how to complete it. The lifestyle questionnaire included items related to diet, smoking, drinking, exercise, sleep, and dental hygiene. When the staff of the public health centers visited the subjects' households, the subjects were informed of the due consideration given to confidentiality of all personal data based on the Health Promotion Law.
Measures and Definitions
According to criteria determined by the Japan Society for the Study of Obesity, a BMI of 25 kg/m2 or higher was considered to indicate overweight.20 Before recording the blood pressure, activities that might potentially affect the blood pressure readings (blood sampling, exercise, eating, and smoking) were prohibited. Each participant was instructed to urinate prior to recording the blood pressure. After 5 min of rest, blood pressure was measured in the right upper arm with the participant seated on a chair. Blood pressure was measured twice with an interval of 1 minute between the 2 measurements, and the 2 measurements were used for statistical analyses.
In accordance with criteria determined by the World Health Organization, International Society of Hypertension, and Japanese Society of Hypertension, a mean systolic blood pressure of 140 mm Hg or higher or a mean diastolic blood pressure of 90 mm Hg or higher was considered to indicate hypertension.
Blood tests included the following items: white blood cell count, red blood cell count, platelet count, hemoglobin concentration, ferritin level, total protein level, albumin level, total cholesterol level, HDL cholesterol level, triglyceride level, glucose level, and hemoglobin A1c level. The serum triglyceride and HDL cholesterol levels were measured using the enzyme and direct methods, respectively. In the participants whose serum triglyceride level was lower than 400 mg/dL, the level of LDL cholesterol was calculated from total cholesterol, HDL cholesterol and triglyceride levels using Friedewald's formula.21 The serum triglyceride level was 400 mg/dL or more in 93 participants (2.3%), and we excluded cases showing this value from statistical analysis involving LDL cholesterol. In accordance with the criteria determined by the Japan Atherosclerosis Society, a serum triglyceride level ≥150 mg/dL was considered to be high, an HDL cholesterol level <40 mg/dL was defined as low, and an LDL cholesterol level ≥140 mg/dL was considered to be high. Furthermore, in accordance with the criteria determined by the Japan Diabetes Society, a fasting glucose level ≥126 mg/dL was considered to represent hyperglycemia.
The questionnaire on lifestyles included the question: “What was your daily average sleep duration during the past month?” The 6 options provided as responses to this question were: (a) less than 5 h, (b) 5 h or more but less than 6 h, (c) 6 h or more but less than 7 h, (d) 7 h or more but less than 8 h, (e) 8 h or more but less than 9 h, and (f) 9 h or more. Categories (e) and (f) were integrated for the purpose of statistical analysis. Subjects who answered “every day” and “sometimes” to the question “Do you smoke currently? (within one month)” were categorized as current smokers. Those who answered “3 days or more per week” to the question “How many days per week do you consume alcoholic beverages?” were categorized as habitual drinkers. Those who had exercised twice or more per week for ≥30 min over the past one year or more were categorized as habitual exercisers. Questions regarding the frequencies of skipping meals, eating between meals, and eating out were asked separately. If the frequency of any of the above eating patterns was once or more per week, it was considered to be a habit. The questionnaire also included a question on psychological stress levels. To the question “Have you felt stress caused by dissatisfaction, worries, or troubles during the past month?” the following 4 options were provided: definitely, occasionally, not much, and never. The participants who selected “definitely” as the answer to this question were regarded as those “Definitely feeling psychological stress.”
Statistical Analyses
After seeking permission from the Ministry of Health, Labour and Welfare, we performed statistical analysis of the anonymized dataset obtained from the National Health and Nutrition Survey.
A total of 11,630 individuals participated in at least one of the three parts of the National Health and Nutrition Survey, and the response rate was approximately 77.5%. Of these participants, the following were sequentially excluded from the dataset; those aged 20 years or younger (2,199); those who did not participate in a blood test (4,124); pregnant women or women who had given birth in the last 6 months (43); those from whom blood was collected within 4 h after a meal (1,234); those for whom the serum triglyceride and HDL cholesterol levels could not be measured due to technical errors such as an insufficient quantity of collected blood (8); and those who did not answer the question on sleep duration (27). The data for the remaining 3,995 cases (men: 1,666, women: 2,329) were used for statistical analyses.
All statistical analyses were conducted separately by gender. Unadjusted differences in continuous and categorical variables across sleep duration categories were assessed for significance using single-factor analysis of variance or contingency table analysis, as appropriate. Logistic regression analyses were conducted to assess the relation of usual sleep duration to a high triglyceride, low HDL cholesterol or high LDL cholesterol level, adjusting for relevant covariates. Covariates included in the model were age, blood pressure, BMI, fasting plasma glucose level, smoking habit, alcohol consumption, dietary habits, psychological stress, and taking cholesterol-lowering medications. Odds ratios were calculated from logistic regression analyses with 95% confidence intervals. Finally, participants who were taking cholesterol-lowering medications were excluded, and the same analyses as described above were performed. All analyses were performed using SPSS 12.0 for Windows.
RESULTS
Among men, the percentages of subjects who slept <6 h and ≥8 h per night were 23.3% and 13.6%, respectively. Among women, the corresponding percentages were 31.2% and 8.2%, respectively. The number of subjects with shorter sleep duration was greater for women than for men, and the number of subjects with longer sleep duration was smaller for women than for men (P < 0.001). The mean (standard deviation [SD]) serum triglyceride level was 153.9 (112.2) mg/dL for men and 123.2 (86.5) mg/dL for women, and was thus significantly higher among men (P < 0.001). The mean (SD) serum HDL cholesterol level was 56.3 (15.0) mg/dL for men and 64.8 (15.6) mg/dL for women, and was thus significantly lower among men (P < 0.001). The mean (SD) serum LDL cholesterol level was 113.2 (31.5) mg/dL for men and 118.6 (32.0) mg/dL for women, and was thus significantly higher among women (P < 0.001). The prevalence of a high triglyceride level was 36.5% among men and 24.0% among women, and was thus significantly higher among men (P < 0.001). The prevalence of a low HDL cholesterol level was 12.1% among men and 3.4% among women, and was thus significantly higher among men (P < 0.001). The prevalence of a high LDL cholesterol level was 17.6% among men and 23.9% among women, and was thus significantly higher among women (P < 0.001).
Among men with shorter sleep duration, the number of men who answered that they skipped meals or ate out once or more per day was observed to be high (Table 1). Additionally, a high number of men in this group answered that they experienced high levels of psychological stress. A significant association was observed between serum LDL cholesterol levels and sleep duration among men. The mean serum LDL cholesterol level of those who slept ≥8 h was approximately 9.2 mg/dL lower than that of those who slept for 6 to 7 h. Among men, there were no evident significant associations between sleep duration and serum triglyceride or serum HDL cholesterol level.
Table 1.
Characteristics of the Male Participants According to Reported Usual Sleep Duration
| Characteristic | Reported Usual Sleep Duration, h/night |
P value | ||||
|---|---|---|---|---|---|---|
| <5 | 5 to <6 | 6 to <7 | 7 to <8 | ≥8 | ||
| No. of participants | 70 | 318 | 596 | 455 | 227 | |
| Age, y | 52.7 (19.9) | 52.4 (16.8) | 53.6 (15.6) | 56.3 (16.1) | 64.1 (15.2) | <0.001 |
| BMI | 22.9 (3.2) | 23.9 (3.5) | 23.6 (3.1) | 23.2 (3.2) | 23.1 (3.4) | 0.008 |
| Systolic blood pressure, mm Hg | 133.4 (17.1) | 134.0 (21.3) | 134.3 (19.6) | 136.4 (19.1) | 136.7 (18.1) | 0.181 |
| Diastolic blood pressure, mm Hg | 78.5 (10.4) | 81.3 (13.0) | 82.2 (11.5) | 82.7 (11.0) | 80.6 (11.2) | 0.015 |
| Drinking alcohol ≥3 days/week, % | 44.3 | 50.8 | 57.0 | 61.3 | 59.5 | 0.009 |
| Current smoking, % | 43.3 | 47.9 | 42.5 | 46.9 | 45.9 | 0.515 |
| Exercising at least twice per week, % | 40.0 | 28.6 | 29.8 | 29.3 | 29.2 | 0.433 |
| Skipping meal ≥1 time/day, % | 21.4 | 5.4 | 7.9 | 7.7 | 3.5 | <0.001 |
| Eating between meals ≥1 time/day, % | 28.6 | 31.6 | 27.7 | 28.1 | 34.8 | 0.273 |
| Eating out ≥1 time/day, % | 22.9 | 12.6 | 9.7 | 5.5 | 2.6 | <0.001 |
| Definitely feeling psychological stress, % | 37.1 | 18.6 | 9.2 | 7.0 | 4.0 | <0.001 |
| Fasting plasma glucose, mg/dL | 101.6 (26.6) | 104.8 (32.3) | 104.5 (29.5) | 108.8 (43.9) | 112.6 (44.8) | 0.021 |
| Hemoglobin A1c, % | 5.41 (0.97) | 5.33 (0.78) | 5.40 (0.86) | 5.47 (1.07) | 5.55 (1.03) | 0.069 |
| Triglyceride, mg/dL | 134.5 (70.9) | 159.0 (110.3) | 155.3 (120.6) | 157.7 (115.4) | 141.7 (94.1) | 0.198 |
| HDL cholesterol, mg/dL | 57.8 (16.2) | 56.1 (14.6) | 56.1 (14.4) | 56.5 (15.7) | 56.1 (15.6) | 0.915 |
| LDL cholesterol, mg/dL | 109.0 (29.0) | 113.1 (31.7) | 116.4 (32.3) | 112.7 (31.1) | 107.2 (29.6) | 0.004 |
| Total cholesterol, mg/dL | 193.7 (33.9) | 199.5 (34.2) | 202.1 (37.0) | 199.3 (33.4) | 191.8 (35.2) | 0.003 |
Data are presented as mean (SD) or percentages.
Significance tests for the unadjusted difference across categories of sleep duration are based on the contingency table analysis for categorical variables and single-factor analysis of variance for continuous variables.
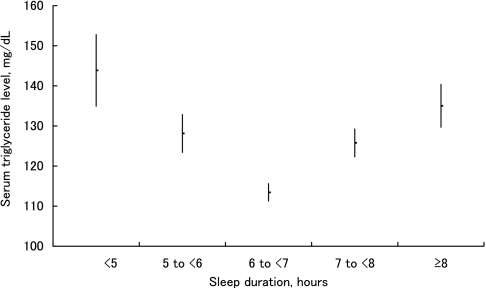
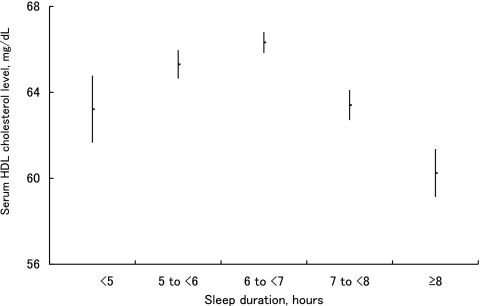
Similarly, among women with shorter sleep duration, the number of subjects who skipped meals, ate out, or experienced heavy psychological stress was large (Table 2). The mean serum triglyceride level was lowest in women who slept 6 to 7 h and became higher as the sleep duration became shorter than 6 h or longer than 7 h. Compared with those who slept for 6 to 7 h, the mean serum triglyceride level in those who slept <5 h, and in those who slept ≥8 h, was approximately 30.5 mg/dL and 21.6 mg/dL higher, respectively. Thus, a U-shaped association was observed between sleep duration and serum triglyceride level (Figure 1). In contrast, the mean serum HDL cholesterol level was highest for sleep durations of 6 to 7 h, and became lower as the sleep duration became shorter than 6 h or longer than 7 h. Compared with those who slept for 6 to 7 h, the mean serum HDL cholesterol level in those who slept <5 h, and in those who slept ≥8 h was approximately 3.1 mg/dL and 6.1 mg/dL lower, respectively. Thus, an inverted U-shaped association was observed (Figure 2). Furthermore, U-shaped associations were observed between sleep duration and systolic blood pressure, and sleep duration and fasting plasma glucose. Among women, there was no significant association between sleep duration and serum LDL cholesterol level.
Table 2.
Characteristics of the Female Participants According to Reported Usual Sleep Duration
| Characteristic | Reported Usual Sleep Duration, h/night |
P value | ||||
|---|---|---|---|---|---|---|
| <5 | 5 to <6 | 6 to <7 | 7 to <8 | ≥8 | ||
| No. of participants | 125 | 601 | 919 | 493 | 191 | |
| Age, y | 56.6 (16.7) | 53.2 (15.1) | 53.2 (15.3) | 57.1 (17.2) | 65.0 (16.9) | <0.001 |
| BMI | 23.3 (4.2) | 22.8 (3.6) | 22.7 (3.4) | 22.8 (3.3) | 22.6 (3.6) | 0.457 |
| Systolic blood pressure, mm Hg | 133.9 (21.7) | 128.4 (20.7) | 128.6 (20.6) | 131.0 (22.0) | 134.9 (19.3) | <0.001 |
| Diastolic blood pressure, mm Hg | 77.9 (11.6) | 76.8 (11.4) | 77.5 (11.2) | 77.2 (12.1) | 77.9 (12.0) | 0.691 |
| Drinking alcohol ≥3 days/week, % | 8.0 | 15.5 | 15.3 | 12.4 | 13.1 | 0.127 |
| Current smoking, % | 12.8 | 12.5 | 10.2 | 6.9 | 7.9 | 0.023 |
| Exercising at least twice per week, % | 21.8 | 26.1 | 25.5 | 25.3 | 20.6 | 0.535 |
| Skipping meal ≥1 time/day, % | 11.2 | 3.7 | 4.0 | 4.3 | 6.8 | 0.002 |
| Eating between meals ≥1 time/day, % | 40.8 | 52.8 | 49.0 | 47.6 | 52.9 | 0.086 |
| Eating out ≥1 time/day, % | 5.6 | 4.3 | 2.1 | 1.6 | 0.5 | 0.001 |
| Definitely feeling psychological stress, % | 29.6 | 18.1 | 11.6 | 7.3 | 4.7 | <0.001 |
| Fasting plasma glucose, mg/dL | 105.0 (35.0) | 103.3 (25.3) | 102.2 (26.3) | 104.7 (25.7) | 110.9 (36.9) | 0.002 |
| Hemoglobin A1c, % | 5.39 (0.72) | 5.34 (0.77) | 5.30 (0.74) | 5.34 (0.68) | 5.39 (0.83) | 0.427 |
| Triglyceride, mg/dL | 143.9 (100.0) | 128.1 (115.7) | 113.4 (66.0) | 125.8 (77.2) | 135.0 (74.1) | <0.001 |
| HDL cholesterol, mg/dL | 63.2 (17.4) | 65.3 (16.2) | 66.3 (14.8) | 63.4 (15.5) | 60.2 (15.4) | <0.001 |
| LDL cholesterol, mg/dL | 117.4 (31.4) | 116.7 (31.8) | 118.1 (32.3) | 120.7 (31.4) | 122.6 (32.9) | 0.114 |
| Total cholesterol, mg/dL | 209.6 (38.4) | 207.0 (37.1) | 207.1 (35.9) | 208.8 (34.8) | 209.5 (34.5) | 0.770 |
Data are presented as mean (SD) or percentages.
Significance tests for the unadjusted difference across categories of sleep duration are based on the contingency table analysis for categorical variables and single-factor analysis of variance for continuous variables.
Figure 1.
The relationship between serum triglyceride level and sleep duration. The mean value (point) and standard error (bar) of the serum triglyceride level for different sleep duration groups are shown. A U-shaped association is observed between serum triglyceride level and sleep duration.
Figure 2.
The relationship between serum HDL cholesterol level and sleep duration. The mean value (point) and standard error (bar) of serum HDL cholesterol level for different sleep duration groups are shown. An inverted U-shaped association is observed between serum HDL cholesterol level and sleep duration.
Among women, both univariate and multivariate logistic models showed statistically significant associations between sleep duration and a high triglyceride level and sleep duration and a low HDL cholesterol level (Table 3). Both the associations were U-shaped. The ORs for a high triglyceride and a low HDL cholesterol levels were the lowest for sleep durations of 6 to 7 h and became higher as the sleep duration became shorter or longer than 6 to 7 h. Among men, no statistically significant association was observed between sleep duration and a high triglyceride level, or sleep duration and a low HDL cholesterol level.
Table 3.
Odds Ratios for Dyslipidemia by Reported Sleep Duration
| Reported Usual Sleep Duration, h/night | High triglyceride Crude odds ratio | 95% CI | P value | Adjusted odds ratio* | 95% CI | P value |
|---|---|---|---|---|---|---|
| Male | 0.364 | 0.229 | ||||
| <5 | 1.02 | 0.61–1.71 | 1.52 | 0.85–2.70 | ||
| 5 to <6 | 1.25 | 0.95–1.66 | 1.36 | 1.00–1.84 | ||
| 6 to <7 | 1.00 | referent | 1.00 | referent | ||
| 7 to <8 | 1.10 | 0.85–1.41 | 1.24 | 0.94–1.63 | ||
| ≥8 | 0.89 | 0.64–1.23 | 1.09 | 0.76–1.56 | ||
| Female | <0.001 | 0.048 | ||||
| <5 | 1.82 | 1.21–2.75 | 1.51 | 0.96–2.35 | ||
| 5 to <6 | 1.41 | 1.10–1.80 | 1.42 | 1.09–1.84 | ||
| 6 to <7 | 1.00 | referent | 1.00 | referent | ||
| 7 to <8 | 1.28 | 0.98–1.66 | 1.15 | 0.87–1.53 | ||
| ≥8 | 1.98 | 1.41–2.79 | 1.45 | 1.00–2.11 | ||
| Reported Usual Sleep Duration, h/night | Low HDL cholesterol | |||||
| Male | 0.951 | 0.694 | ||||
| <5 | 1.15 | 0.54–2.41 | 1.35 | 0.60–3.03 | ||
| 5 to <6 | 1.02 | 0.67–1.57 | 0.95 | 0.60–1.50 | ||
| 6 to <7 | 1.00 | referent | 1.00 | referent | ||
| 7 to <8 | 1.16 | 0.80–1.68 | 1.19 | 0.80–1.77 | ||
| ≥8 | 1.09 | 0.68–1.75 | 0.87 | 0.51–1.46 | ||
| Female | <0.001 | 0.001 | ||||
| <5 | 6.40 | 2.60–15.78 | 5.85 | 2.29–14.94 | ||
| 5 to <6 | 3.14 | 1.51–6.52 | 3.25 | 1.55–6.85 | ||
| 6 to <7 | 1.00 | referent | 1.00 | referent | ||
| 7 to <8 | 3.86 | 1.85–8.02 | 3.15 | 1.49–6.65 | ||
| ≥8 | 7.04 | 3.18–15.57 | 4.27 | 1.88–9.72 | ||
| Reported Usual Sleep Duration, h/night | High LDL cholesterol | |||||
| Male | 0.005 | 0.048 | ||||
| <5 | 0.49 | 0.23–1.06 | 0.66 | 0.29–1.48 | ||
| 5 to <6 | 0.90 | 0.64–1.28 | 0.91 | 0.62–1.31 | ||
| 6 to <7 | 1.00 | referent | 1.00 | referent | ||
| 7 to <8 | 0.80 | 0.59–1.11 | 0.80 | 0.57–1.13 | ||
| ≥8 | 0.42 | 0.26–0.68 | 0.45 | 0.27–0.76 | ||
| Female | 0.333 | 0.515 | ||||
| <5 | 1.28 | 0.84–1.96 | 1.24 | 0.78–1.97 | ||
| 5 to <6 | 0.87 | 0.68–1.12 | 0.88 | 0.67–1.14 | ||
| 6 to <7 | 1.00 | referent | 1.00 | referent | ||
| 7 to <8 | 1.10 | 0.85–1.42 | 1.10 | 0.84–1.45 | ||
| ≥8 | 1.11 | 0.78–1.59 | 0.98 | 0.66–1.45 |
Multivariate logistic regression analyses were conducted with adjustment for age, blood pressure, body mass index, fasting plasma glucose level, smoking habit, alcohol consumption, dining habits, psychological stress, and using anti-cholesterol medicine.
A significant association was observed among men with regard to LDL cholesterol level and sleep duration. Specifically, the OR with regard to a high LDL cholesterol level among those who slept ≥8 h was significantly lower than for those who slept for 6 to 7 h. On the other hand, there was no significant association between sleep duration and LDL cholesterol level among women.
Finally, 370 participants (9.3%) who took cholesterol-lowering medications were excluded, and the same analyses as those described above were performed. There were no substantial differences between the results of those analyses and the above-mentioned analyses.
DISCUSSION
In the present study, we found U-shaped associations between sleep duration and a high triglyceride or a low HDL cholesterol level among women. Using the data of a cohort study conducted on 71,617 women in the USA, Ayas et al. examined the associations between sleep duration and CHD.10 They reported that the relative risk of CHD was significantly higher among those with shorter or longer sleep durations, and that the association was U-shaped.10 Recently, in a study comprising 2,437 participants from the general population in Germany, Wolff et al. reported that the carotid intima-media thickness was greater among those with short and long sleep durations.11 From these study results, it is suggested that both short and long sleep durations can be regarded as individual risk factors of CVDs such as CHD and atherosclerosis. Since an increase in the triglyceride level or a decrease in the HDL cholesterol level in blood are risk factors for the onset of CVD,12–14 the present results are important for explaining the association between sleep duration and CVD. It is logical to consider that the incidence or prevalence of a high triglyceride or a low HDL cholesterol level are high among individuals with short and long sleep durations, predisposing them to a higher relative risk of CVD. There are associations between two of the three elements (sleep duration, dyslipidemia, and CVD) and each element can produce a confounding effect on the association between the other two elements. These associations must be examined individually in the future using a study design that can account for the confounding effects of all the above elements.
While we were preparing the present report, a study on the associations between sleep duration and dyslipidemia was published by another group.22 Williams et al. examined the associations between sleep duration and biomarkers that could be risk factors for CVDs in 935 women with type 2 diabetes. They indicated that among the subjects whose blood pressure was within the normal range, the serum HDL cholesterol level was low among those with both short and long sleep durations. They stated that the result partially explained how sleep habit could become a risk factor for CVDs. A simple comparison between their study and ours is not warranted because in their study the subjects were limited to women with type 2 diabetes. However, the data are helpful for clarifying the associations between dyslipidemia and sleep duration, i.e., an inverted U-shaped association was observed between serum HDL cholesterol levels and sleep duration in both studies.
Recently, it has become increasingly clear that sleep has a strong influence on the metabolic hormones that regulate energy balance. Sleep restriction lowers the blood concentration of leptin, which acts to suppress appetite, and increases the blood concentration of ghrelin, which promotes appetite.8,23–25 In addition, it is known that administration of leptin decreases serum triglyceride level.26,27 In addition, it was recently reported that short sleep duration was associated with a reduced leptin level and being overweight.28 Mechanisms such as a decrease in the blood concentration of leptin or an increase in the blood concentration of ghrelin due to sleep restriction may be involved in the biological mechanisms responsible for the associations between short sleep duration and dyslipidemia: associations that were observed among women.
Meanwhile, it is not easy to explain the biological mechanism responsible for the association between long sleep duration and a high triglyceride or a low HDL cholesterol level. Existing knowledge of metabolic hormones and sleep duration cannot explain this association. Certain metabolic endocrinological changes caused by long sleep duration may result in increased triglyceride level and decreased HDL cholesterol level. However, because it is difficult to experimentally induce individuals to sleep for long periods, data related to this field are sparse. Meanwhile, there is a possibility that a specific factor may be associated separately with long sleep duration and a high triglyceride or a low HDL cholesterol level, and that through this unidentified confounding factor, an apparent association between long sleep duration and these dyslipidemia becomes evident. In this study, as age, overweight, hypertension, and glucose intolerance could have been potential confounding elements, various covariates, including the above factors, were fed into multivariate logistic models to study the association between long sleep duration and serum lipid and lipoprotein levels. However, the associations were independent of these factors, and could not be justified using them. Several previous studies have reported that various pathologic features such as obesity, hypertension, and glucose intolerance are associated with long sleep duration.5–9 However, in those studies, biological mechanisms responsible for such associations were not completely elucidated. Therefore, studies on the physiological characteristics of long sleep must be conducted in the future.
Previous studies have reported that the relative risk of death or CHD was lowest among those who slept for 7 to 8 h.1–4,10,29 Meanwhile, in the present study, the relative risk of a high triglyceride level or a low HDL cholesterol level was lowest among women who slept for 6 to 7 h. Thus, the optimal sleep duration suggested in the present study was not in accord with those indicated by previous studies. However, the results of the present and the previous studies are similar in that the relative risks were lowest among the categories of sleep duration to which the largest numbers of participants belonged. In an attempt to interpret the optimal sleep durations for disease prevention based on epidemiological data, it is inferred that the optimal sleep durations vary with the target population. In addition, when considering optimal sleep duration, bidirectional causal relationships must be taken into consideration from a biological viewpoint. In other words, sleep duration may affect physical status, but conversely, physical status may also affect sleep duration. It must be recognized that according to the type of disease being examined, the optimal sleep duration may differ.
With regard to the associations between sleep duration and mortality among Japanese, 3 cohort studies have been reported so far, but their results were discordant.4,29,30 Kojima et al. reported that a U-shaped association was observed among male subjects,29 whereas Tamakoshi et al. reported that a U-shaped association was observed among women.4 Conversely, Amagai et al. reported that a U-shaped association was not observed among either men or women.30 The reason for these gender-based differences in the associations between sleep duration and mortality among the studies is unclear. In the present study, a U-shaped association between sleep duration and dyslipidemia was recognized among women. Our data support the results of Tamakoshi et al. Further studies will be necessary to clarify the associations of sleep duration with dyslipidemia and mortality among Japanese.
In this study, unlike the situation in women, no significant associations were observed between sleep duration and serum triglyceride or HDL cholesterol level among men. However, the risk of a high LDL cholesterol level was lower among men who slept ≥8 h. From the viewpoint of CVD prevention, it was suggested that long sleep duration was not favorable for women, whereas it was favorable for men. Many previous studies have already reported that there is a gender-specific difference in the prevalence of dyslipidemia because sex hormones (estrogen, in particular) strongly affect lipoprotein metabolism.31–33 It has also been reported that certain gender-specific differences in sleep habits are influenced by differences in social or household roles,34 or in sex hormones.35 Since there are gender-based differences in the onset of dyslipidemia and sleep habits, it is not unusual to observe a gender-specific difference in the association between them. In any event, until the biological mechanisms associated with the relationship between sleep duration and dyslipidemia are elucidated, the reasons for the gender-specific difference in these associations will remain unclear. This issue should be addressed in future epidemiological and physiological investigations.
Several studies have reported U-shaped associations between sleep duration and various diseases. On the other hand, several studies have reported that the associations were negative linear, and not U-shaped (i.e., the risk was higher only among those with short sleep durations).36–39 In the present study, adjusted analyses failed to detect any significant associations of BMI, blood pressure, and fasting plasma glucose level with sleep duration; this was despite the fact that significant associations were recognized during unadjusted analyses (data not shown). Thus, the results of the present study did not always agree with those of previous ones. It is inferred that these differences were due to firstly, differences in the sampling of subjects, and secondly, differences in adjustment factors. It is important that future epidemiological studies regarding the associations between sleep duration and diseases are carefully designed to minimize the selection bias and are adjusted for confounding factors. Subsequently, the results from such studies should be integrated through a meta-analysis, and a consensus should be reached. Future development of studies along these lines is expected.
The present study had several limitations. First, as this was a cross-sectional study, causal relationships could not be determined, even for items between which an association was indicated. When examining a causal relationship, a longitudinal study such as a cohort study is required, and such a study will be required in the future. Second, there may have been a non-response bias. Since the subjects were asked to come to public facilities in each district on a particular day during the survey period for examination of physical status, many of them may not have been able to participate because they had to go to work. The percentage of subjects who did not participate in the survey of physical status is estimated to have been approximately 37%. Among the cases analyzed, the number of subjects in the 20 to 49-yr age group and that of male participants were relatively small. Third, objective data could not be used for the present evaluation of sleep habits. Lauderdale et al. showed that the self-reported sleep duration was systematically biased along gender and race line when compared to measured sleep duration.40 Therefore, the bias due to the use of self-reported data on sleep duration in this study remains to be resolved. Hereafter, the advantages of using measured data, such as those obtained with an actigraph, should be examined in a future study.
In conclusion, the results of this study indicate that both short and long sleep durations are associated with a high serum triglyceride level or a low HDL cholesterol level among women. Conversely, it was observed that the risk of a high LDL cholesterol level was lower among men who slept ≥8 h. Usual sleep duration is closely associated with serum lipid and lipoprotein levels.
ACKNOWLEDGMENTS
We wish to express our thanks to Eise Yokoyama M.D., Satoru Harano M.D., and Tetsuo Tamaki M.D. (Department of Public Health, School of Medicine, Nihon University) for their help in this study. This study was supported by a Health Science Research Grant from the Ministry of Health, Labour and Welfare of the Japanese Government.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Hammond EC. Some preliminary findings on physical complaints from a prospective study of 1,064,004 men and women. Am J Public Health Nations Health. 1964;54:11–23. doi: 10.2105/ajph.54.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kripke DF, Simons RN, Garfinkel L, et al. Short and long sleep and sleeping pills. Is increased mortality associated? Arch Gen Psychiatry. 1979;36:103–16. doi: 10.1001/archpsyc.1979.01780010109014. [DOI] [PubMed] [Google Scholar]
- 3.Kripke DF, Garfinkel L, Wingard DL, et al. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 4.Tamakoshi A, Ohno Y, JACC Study Group Self-reported sleep duration as a predictor of all-cause mortality: results from the JACC study, Japan. Sleep. 2004;27:51–4. [PubMed] [Google Scholar]
- 5.Ayas NT, White DP, Al-Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–4. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 6.Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165:863–7. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 7.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29:657–61. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 8.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29:1009–14. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 10.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 11.Wolff B, Volzke H, Schwahn C, Robinson D, Kessler C, John U. Relation of self-reported sleep duration with carotid intima-media thickness in a general population sample. Atherosclerosis. doi: 10.1016/j.atherosclerosis.2006.12.023. in press. [DOI] [PubMed] [Google Scholar]
- 12.Jeppesen J, Hein HO, Suadicani P, Gyntelberg F. Relation of high TG-low HDL cholesterol and LDL cholesterol to the incidence of ischemic heart disease. An 8-year follow-up in the Copenhagen Male Study. Arterioscler Thromb Vasc Biol. 1997;17:1114–20. doi: 10.1161/01.atv.17.6.1114. [DOI] [PubMed] [Google Scholar]
- 13.Gaziano JM, Hennekens CH, O'Donnell CJ, Breslow JL, Buring JE. Fasting triglycerides, high-density lipoprotein, and risk of myocardial infarction. Circulation. 1997;96:2520–5. doi: 10.1161/01.cir.96.8.2520. [DOI] [PubMed] [Google Scholar]
- 14.Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 15.Hata Y, Nakajima K. Life-style and serum lipids and lipoproteins. J Atheroscler Thromb. 2000;7:177–97. doi: 10.5551/jat1994.7.177. [DOI] [PubMed] [Google Scholar]
- 16.Choudhury SR, Ueshima H, Kita Y, et al. Alcohol intake and serum lipids in a Japanese population. Int J Epidemiol. 1994;23:940–7. doi: 10.1093/ije/23.5.940. [DOI] [PubMed] [Google Scholar]
- 17.Nakanishi N, Tatara K, Nakamura K, Suzuki K. Association of lifestyle with serum lipid levels: a study of middle-aged Japanese men. J Epidemiol. 2000;10:216–25. doi: 10.2188/jea.10.216. [DOI] [PubMed] [Google Scholar]
- 18.Nakanishi N, Nakamura K, Ichikawa S, Suzuki K, Tatara K. Relationship between lifestyle and serum lipid and lipoprotein levels in middle-aged Japanese men. Eur J Epidemiol. 1999;15:341–8. doi: 10.1023/a:1007527111946. [DOI] [PubMed] [Google Scholar]
- 19.Ministry of Health, Labour and Welfare. Report of National health and nutrition survey 2003. www.mhlw.go.jp/bunya/kenkou/eiyou-chosa2-01/index.html.
- 20.Examination Committee of Criteria for ‘Obesity Disease’ in Japan; Japan Society for the Study of Obesity. New criteria for ‘obesity disease’ in Japan [in Japanese] Journal of Japan Society for the Study of Obesity. 2000;6:18–28. [Google Scholar]
- 21.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 22.Williams CJ, Hu FB, Patel SR, Mantzoros CS. Sleep duration and snoring in relation to biomarkers of cardiovascular disease risk among women with type 2 diabetes. Diabetes Care. 2007;30:1233–40. doi: 10.2337/dc06-2107. [DOI] [PubMed] [Google Scholar]
- 23.Guilleminault C, Powell NB, Martinez S, et al. Preliminary observations on the effects of sleep time in a sleep restriction paradigm. Sleep Med. 2003;4:177–84. doi: 10.1016/s1389-9457(03)00061-3. [DOI] [PubMed] [Google Scholar]
- 24.Spiegel K, Leproult R, L'hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–71. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 25.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 26.Oral EA, Simha V, Ruiz E, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–8. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 27.Petersen KF, Oral EA, Dufour S, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109:1345–50. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaput JP, Despres JP, Bouchard C, Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: Results from the Quebec family study. Obesity (Silver Spring) 2007;15:253–61. doi: 10.1038/oby.2007.512. [DOI] [PubMed] [Google Scholar]
- 29.Kojima M, Wakai K, Kawamura T, et al. Sleep patterns and total mortality: a 12-year follow-up study in Japan. J Epidemiol. 2000;10:87–93. doi: 10.2188/jea.10.87. [DOI] [PubMed] [Google Scholar]
- 30.Amagai Y, Ishikawa S, Gotoh T, et al. Sleep duration and mortality in Japan: the Jichi Medical School Cohort Study. J Epidemiol. 2004;14:124–8. doi: 10.2188/jea.14.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Godsland IF, Wynn V, Crook D, Miller NE. Sex, plasma lipoproteins, and atherosclerosis: prevailing assumptions and outstanding questions. Am Heart J. 1987;114:1467–503. doi: 10.1016/0002-8703(87)90552-7. [DOI] [PubMed] [Google Scholar]
- 32.Sacks FM, Gerhard M, Walsh BW. Sex hormones, lipoproteins, and vascular reactivity. Curr Opin Lipidol. 1995;6:161–6. doi: 10.1097/00041433-199506000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Sattler AM, Soufi M, Maisch B, Schaefer JR. Lipids and lipoproteins in women. Herz. 2005;30:368–74. doi: 10.1007/s00059-005-2708-3. [DOI] [PubMed] [Google Scholar]
- 34.Park YM, Matsumoto K, Shinkoda H, Nagashima H, Kang MJ, Seo YJ. Age and gender difference in habitual sleep-wake rhythm. Psychiatry Clin Neurosci. 2001;55:201–2. doi: 10.1046/j.1440-1819.2001.00825.x. [DOI] [PubMed] [Google Scholar]
- 35.Antonijevic IA, Murck H, Frieboes R, Holsboer F, Steiger A. On the gender differences in sleep-endocrine regulation in young normal humans. Neuroendocrinology. 1999;70:280–7. doi: 10.1159/000054487. [DOI] [PubMed] [Google Scholar]
- 36.Hasler G, Buysse DJ, Klaghofer R, et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27:661–6. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 37.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28:1289–96. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 38.Mallon L, Broman JE, Hetta J. High incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes Care. 2005;28:2762–7. doi: 10.2337/diacare.28.11.2762. [DOI] [PubMed] [Google Scholar]
- 39.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 40.Lauderdale DS, Knutson KL, Yan LL, et al. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol. 2006;164:5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]