Abstract
Study Objectives:
To evaluate the impact of enhanced slow wave sleep (SWS) on behavioral, psychological, and physiological changes resulting from sleep restriction.
Design:
A double-blind, parallel group, placebo-controlled design was used to compare gaboxadol (GBX) 15 mg, a SWS-enhancing drug, to placebo during 4 nights of sleep restriction (5 h/night). Behavioral, psychological, and physiological measures of the impact of sleep restriction were assessed in both groups at baseline, during sleep restriction and following recovery sleep.
Setting:
Sleep research laboratory.
Participants:
Forty-one healthy adults; 9 males and 12 females (mean age: 32.0 ± 9.9 y) in the placebo group and 10 males and 10 females (mean age: 31.9 ± 10.2 y) in the GBX group.
Interventions:
Both experimental groups underwent 4 nights of sleep restriction. Each group received either GBX 15 mg or placebo on all sleep restriction nights, and both groups received placebo on baseline and recovery nights.
Measurements and Results:
Polysomnography documented a SWS-enhancing effect of GBX with no group difference in total sleep time during sleep restriction. The placebo group displayed the predicted deficits due to sleep restriction on the multiple sleep latency test (MSLT) and on introspective measures of sleepiness and fatigue. Compared to placebo, the GBX group showed significantly less physiological sleepiness on the MSLT and lower levels of introspective sleepiness and fatigue during sleep restriction. There were no differences between groups on the psychomotor vigilance task (PVT) and a cognitive test battery, but these measures were minimally affected by sleep restriction in this study. The correlation between change from baseline in MSLT on Day 6 and change from baseline in SWS on Night 6 was significant in the GBX group and in both groups combined.
Conclusions:
The results of this study are consistent with the hypothesis that enhanced SWS, in this study produced by GBX, reduces physiological sleep tendency and introspective sleepiness and fatigue which typically result from sleep restriction.
Citation:
Walsh JK; Snyder E; Hall J; Randazzo AC; Griffin K; Groeger J; Eisenstein R; Feren SD; Dickey P; Schweitzer PK. Slow Wave Sleep Enhancement with Gaboxadol Reduces Daytime Sleepiness During Sleep Restriction. SLEEP 2008;31(5):659–672.
Keywords: Slow wave sleep, slow wave activity, sleep restriction, sleepiness, gaboxadol
A NUMBER OF INVESTIGATORS HAVE PROPOSED THAT INCREASED SLOW WAVE SLEEP (SWS), AS MEASURED VISUALLY, OR ITS SPECTRAL POWER DENSITY counterpart, slow wave activity (SWA), represent ongoing cortical recovery from prior wakefulness. Moreover, NREM sleep periods with more SWS/SWA are hypothesized to be periods of relatively heightened neurophysiologic restoration or recuperation.1,2 The hypothesized role of SWS in sleep homeostatic regulation has been the result of a number of findings, including: (1) SWA increase in proportion to the duration of prior wakefulness,3 (2) reduced SWA during nocturnal sleep following afternoon/evening naps,4 (3) the decline in SWA across a night of sleep,5 and (4) increased SWS following fragmented sleep.6 The two-process model of sleep regulation views heightened SWS/SWA as reflecting Process S, the homeostatic component.7 Some authors have proposed that increased SWS/SWA, represents ongoing cortical recovery from prior wakefulness activities. That is, periods with more SWS/SWA have been widely hypothesized to be a time of relatively heightened neurophysiologic restoration or recuperation.8,9 Tononi and Cirelli10 hypothesize more specifically that SWS/SWA reflect synaptic changes necessary to conserve energy, save space for future synaptic growth, and to enhance signal-to-noise ratio.
On the other hand, most investigations of selective deprivation of SWS or stage 4 alone have failed to support the concept of enhanced recuperative “value” of SWS relative to other sleep stages. Neither performance nor alertness has been found to be impaired after reduction of SWS by approximately 25% to 90% relative to baseline.11–14 Significant methodological limitations probably contribute to the negative findings of these studies. For example, in one study12 more than a 50% reduction in SWS was observed in the “control” condition (i.e., designed to retain SWS), versus an 85% reduction in the “no-SWS” condition. With the additional influence of approximately 55–65 experimental arousals per night, finding differences in performance between two conditions with 50% and 85% reductions of SWS would be unlikely. Other studies by Lubin et al.13 and Johnson et al.14 deprived subjects of stage 4 only, not SWS, and therefore considerable SWS occurred. Compared to baseline, SWS was reduced by 78% and 63% in the 2 studies, respectively. Moreover, the sample size per condition in both studies was small (N = 4 and 7, respectively). The statistical power to detect differences in performance associated with sleep stage differences when sleep is highly disrupted by the experimental procedures is likely to be exceedingly low. Similar concerns exist for the other SWS deprivation studies.11
Drugs with varying mechanisms of action have been found to increase SWS and/or SWA, including several antagonists of 5HT2A receptors,15,16 gabapentin and pregabalin, which are alpha-2-delta calcium channel modulators,17,18 tiagabine,19–21 a selective GABA reuptake inhibitor, and gaboxadol (GBX), a selective (for alpha4 delta receptors) extrasynaptic GABAA agonist.22 Whether the increases in SWS/SWA with one or more of these drugs reflect the same or similar neural processes to those which characterize natural SWS/SWA is an important scientific question.
One experimental approach to testing whether pharmacologically enhanced SWS has a functional correlate involves production of increased SWS simultaneously with sleep restriction. If enhanced SWS increases the restorative capacity of NREM sleep, the predictable consequences of sleep restriction, such as those documented by Dinges and colleagues,23,24 should be reduced or prevented. In a prior investigation using that experimental approach, tiagabine 8 mg was found to enhance SWS and to markedly attenuate the deficit in sustained attention seen with sleep restriction, although physiologic sleep tendency was not altered by tiagabine.25
In the present study we investigated the impact of enhanced SWS/SWA with GBX 15 mg on behavioral, psychological, and physiological changes resulting from sleep restriction. GBX is a direct GABAA agonist which is selective for the extrasynaptically located alpha4-delta receptor subtype.26 When activated, alpha4-delta receptors produce a tonic inhibitory conductance which is thought to result in a more stable inhibitory pattern, as compared to phasic synaptic inhibition.27 GBX has consistently increased SWS/SWA, in a dose-related manner, in adult and elderly healthy subjects and in primary insomnia patients.22,28–30
METHODS
Study Design and General Methods
A randomized, double-blind, placebo-controlled, parallel groups design was used to compare GBX 15 mg and placebo. Each participant's activities consisted of: (1) a screening office visit, (2) 8 consecutive nights/days of sleep laboratory procedures: 2 screening/baseline nights and days; 4 sleep restriction nights and 2 days; 2 recovery nights and 1 day; and (3) end-of-study procedures. Subjects received single-blind placebo (PBO) on screening /baseline nights as well as on both recovery nights. Subjects received PBO or GBX 15 mg in randomized double-blind fashion on the four sleep restriction nights. Study drug was administered 30 min prior to scheduled bedtime. For each subject the study period was a minimum of 11 days and a maximum of 28 days from initial screening to follow-up. The protocol was approved by the institutional review board of St. Luke's Hospital. All subjects signed an informed consent and were compensated for participation. The study was performed in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines.
The study design and methods were jointly developed by authors (JKW, JG, PKS) and sponsor representatives. Full disclosure of the data was provided by the sponsor to the authors. Data analyses were performed by Dr. Snyder, a Merck employee, and key analyses were confirmed by Dr. Schweitzer. The writing of the manuscript and interpretation of study findings was the sole responsibility of the authors.
Subject Recruitment and Screening
Subjects were recruited via media advertisements. A general description of the study was provided and preliminary screening was conducted by telephone. Interested and qualified persons were scheduled for a clinical screening visit during which a thorough explanation of the study was provided and subjects gave written informed consent. Clinical screening procedures included a sleep, psychiatric, and medical history; physical examination; ECG; clinical laboratory testing (hematology, chemistry, urinalysis); and urine screen for drugs of abuse. These procedures insured that subjects were free of chronic sleep disturbance, DSM-IV psychiatric diagnoses including substance abuse in the past 2 years, and current or recent medical illness. Females could not be pregnant or lactating and had to confirm the use of adequate contraceptive procedures throughout the study.
During the prior 2 months the subjects must have maintained a bedtime between 22:00 and 24:00 at least 5 nights per week, and usual nightly sleep duration between 6.5 and 9 h. A body mass index < 34 kg/m2 was also required.
Subjects could not work night or rotating shifts or have crossed more than 3 time zones in the prior 2 weeks. Subjects were also excluded if they used any psychotropic medication or sedating or alerting over-the-counter drugs during the prior 2 weeks or 5 half-lives (whichever was longer), usually consumed more than 500 mg caffeine per day, or were regular users of nicotine. Participation in a clinical research trial or weight loss program within 30 days, prior exposure to GBX, history of a positive test for human immunodeficiency virus, hepatitis B surface antigen, and/or hepatitis C virus were additional exclusion criteria.
The screening visit also included training on performance tests used in the study, and completion of the Horne-Ostberg Morningness-Eveningness Questionnaire31 and the Sleep Timing Questionnaire.32 Polysomnographic (PSG) screening was performed during the first 2 nights in the sleep laboratory, and a multiple sleep latency test (MSLT) followed each screening PSG. On Night 1, respiratory recordings and leg electromyography were included. Participation was discontinued if on PSG Night 1 the apnea-hypopnea index was >10/h or the periodic leg movement arousal index was >10/h. On Night 2, total sleep time (TST) was required to be ≤ 510 min (time in bed was 540 min), and the mean latency on the Day 2 MSLT was required to be ≥ 7 min.
Seventy-one individuals signed informed consent. Six persons withdrew consent prior to randomization. Twenty-three individuals failed screening (13 MSLT, 1 TST, 8 medical, 1 positive drug screen) and one person was discontinued prior to randomization because enrollment was met. Forty-one healthy male and female subjects aged 18-55 inclusive, were randomized to the 2 study groups, 21 to PBO and 20 to GBX. Randomization was based on preliminary TST and MSLT scoring and confirmed subsequently by final scoring. Thirty-nine subjects completed all study procedures; 2 subjects terminated participation early for personal reasons, 1 following Night 6 and 1 following Night 7. The 2 study groups were similar in age, sex distribution, body mass index, Horne-Ostberg Morningness-Eveningness, and usual rise time and bedtime from the Sleep Timing Questionnaire (Table 1).
Table 1.
Demographic and Baseline Characteristics of the Study Groups
| Placebo (N = 21) | Gaboxadol (N = 20) | |
|---|---|---|
| Mean (SD) Age in years | 32.0 (9.9) | 31.9 (10.2) |
| Sex | 9 male, | 10 male, |
| 12 female | 10 female | |
| Race | 1 Asian, 3 black, | 4 black, |
| 17 white | 16 white | |
| Mean (SD) BMI, kg/m2 | 25.8 (2.8) | 26.6 (3.9) |
| Morningness-Eveningnessa: | ||
| Moderate Morning, N | 7 | 8 |
| Neither, N | 12 | 11 |
| Moderate Evening, N | 2 | 1 |
| Sleep Timing:b | ||
| Mean Bedtime (SD) | 23:15 (00:36) | 23:08 (00:49) |
| Mean Rise time (SD) | 07:47 (00:50) | 07:40 (01:02) |
| Mean (SD) Day 2 MSLT, min. | 13.0 (4.1) | 12.4 (4.4) |
| Mean (SD) Night 2 TST, min. | 475.7 (37.8) | 479.2 (26.6) |
From the Horne and Ostberg Questionnaire at baseline
From the Sleep Timing Questionnaire at baseline
General Study Procedures
Participation involved 8 consecutive nights and the intervening 7 days from laboratory screening to study completion for each subject. PSG Nights 1 and 2 were screening/baseline nights. The MSLT on Day 2 served as a screening procedure. Baseline MSLT values were calculated as the mean of MSLTs on Days 1 and 2. Data from Days 1 and 2 served as baseline for other daytime measures. PSG recording time was 10 h on Night 1 (22:00 to 08:00) and 9 h on Night 2 (23:00 to 08:00). The durations of the recordings on Nights 1 and 2 were selected to minimize the impact of prior sleep history. Single-blind placebo was administered on both nights to all subjects, 30 min prior to bedtime.
After all screening and baseline procedures were completed subjects were randomized to receive GBX 15 mg or matching placebo on all 4 sleep restriction nights (Nights 3–6). Group assignment was stratified to ensure approximate balance in age and sex distribution. Double-blind study drug was administered at 00:30 on Nights 3–6, and PSG start time was 01:00. Preliminary sleep scoring was performed in real time so that total sleep time would be as close as possible to 5 h on each sleep restriction night. Night 3–6 PSGs were terminated at variable times when sleep duration was judged to be 5 h. Actual PSG termination time ranged from 06:05 to 06:30. Following the first 2 sleep restriction nights (Nights 3 and 4), subjects were allowed to go about their normal routines from about 08:00 until approximately 22:30. They were monitored with actigraphy for compliance with the instruction not to sleep when out of the laboratory. They were cautioned about the effects of sleep loss on driving and other potentially dangerous activities. Following the last 2 sleep restriction nights (Nights 5 and 6), subjects remained in the laboratory to complete the MSLT, subjective scales, neurocognitive tests, and mood measures, and collection of saliva, urine, and electrocardiographic samples (Days 5 and 6).
All subjects received single-blind placebo on Nights 7 and 8 at 21:30. PSGs were recorded from 22:00 to 10:00. Twelve hours were allotted to observe the potential differential effects of sleep restriction with and without GBX upon recovery sleep. On Day 7, between Nights 7 and 8, MSLT, subjective scales, neurocognitive tests, and mood measures were completed. Urine and saliva samples and electrocardiographic data were collected.
Alcohol was prohibited beginning 24 hours prior to laboratory Night 1 for the duration of participation. Caffeine consumption on study Days 3 and 4 was limited to a single drink within 1 h of morning awakening. No caffeine was allowed on the remaining study days. Vigorous exercise was prohibited on study Days 1, 2, 5, 6, and 7.
Polysomnography
Digital PSG recordings were made on all 8 study nights. The recording montage for all nights included the following: right and left electrooculogram, submental electromyogram (EMG), electrocardiogram (V5), and 10 EEG derivations (C3-A2, C4-A1, O1-A2, O2-A1, FP1-A2, FP2-A1, F3-A2, F4-A1, F7-A2, F8-A1). On Night 1, the recording also included nasal thermocouple, oximetry, respiratory movement, and right and left anterior tibialis EMG. Sampling rate for all EEG signals was 200 Hz. All PSGs were scored according to standard methods33 using the C3-A2 derivation. Each subject's PSGs were scored by a single scorer. Spectral analysis of the EEG reported here are from the C3-A2 recording.
Daytime Testing
Multiple Sleep Latency Test
The MSLT evaluates sleep propensity by electrophysiologically measuring the latency to fall asleep at multiple times throughout the day. MSLT subtests were conducted at 10:00, 12:00, 14:00, 16:00, and 18:00 on Days 1, 2, 5, 6 and 7. The 10:00 subtest was omitted on Day 7 because of the extended time in bed for recovery sleep PSGs. MSLTs were conducted using standard procedures34 and all MSLTs for the study were scored by a single scorer.
Psychomotor Vigilance Test
The PVT is a simple reaction time test which measures sustained attention and psychomotor function.35 The PVT was performed for 15 minutes at 08:25, 10:25, 12:25, 14:25, and 16:25 on Days 1, 2, 5, 6, and 7; the 08:25 test was omitted on Day 7 because subjects were still in bed. Dependent variables include: mean reaction time (RT), 1/RT, number of lapses (reaction time > 500 msec), square root transformed lapses √x + √(x+1)], mean of the slowest 10% reaction times, and mean of the fastest 10% reaction times. Ratings of sleepiness were collected on a visual analog scale immediately before and after each PVT session.
Profile of Mood States
The POMS is a self-administered questionnaire that measures 6 dimensions of affect or mood. Subjects rate how they feel “now” with respect to 65 adjectives on a 5-point scale (0 = “not at all,” 4 = “extremely”).36 The POMS was completed at 13:50 on Days 2, 6, and 7.
Karolinska Sleepiness Scale
The KSS is a 9-point rating scale which provides a subjective measurement of sleepiness (1 = very alert, 9 = very sleepy).37 The KSS was completed approximately 2 min prior to each MSLT subtest.
Morning and Evening Questionnaires
These were administered each evening and each morning and included visual analog scales for subjective ratings of daytime feelings including relaxation, energy, tiredness, and overall daytime function as well as ratings for sleep quality and the refreshing nature of sleep. Subjects also estimated sleep latency, sleep duration, and number of awakenings during sleep.
Salivary Cortisol Samples
Salivary cortisol samples were collected hourly from 14:20 to 21:20 on Days 2, 6, and 7. No food or drink was allowed for 30 min before each sample collection. Each subject inserted a cotton salivette (Sarstedt AG & Co., Numbrecht, Germany) into his or her mouth. The salivette was chewed until the subject could no longer prevent swallowing excess saliva produced. The salivette was then placed in a tube, weighed to assure adequate saturation, and then stored at −20°C until shipped to Esoterix, Inc. (Calabrasas Hills, CA) for analysis by radioimmunoassay.
Urine Catecholamine Determinations
These determinations were made from 2 contiguous 12-hour aliquots (22:00 to 10:00 and 10:00 to 22:00) on Night2/Day2, Night 6/Day 6, and Night7/Day 7. Subjects collected all urine for each aliquot in a single container which contained 6N hydrochloric acid (Fisher Scientific, Pittsburgh, PA) to preserve the specimen. At the end of each aliquot, approximately 50 milliliters of the well-mixed specimen was poured into a sealed container and stored at −20°C until sent to Esoterix for analysis by high performance liquid chromatography.
Procedural Memory Test
A Procedural Memory Test was administered at baseline and at the end of sleep restriction. The task required subjects to press a series of numeric keys in a predetermined sequence. The number sequence was continuously displayed throughout training and testing. Twelve 30-sec training trials occurred at 09:10 on Days 1 and 5. Each training session used a single unique number sequence. Three 30-sec testing trials were conducted approximately 24 hours later on Days 2 and 6, using the number sequence from the prior training day. The number and accuracy of sequences were recorded.
Cognitive Testing Battery
A battery consisting of 14 individual tests (see appendix) was administered at 10:40, 14:40, and 16:40 on Days 1, 2, 5, 6, and 7. Memory, attention, executive function, and other cognitive domains were assessed. Total testing time was 40 min.
Statistical Analyses
The prespecified criteria for the full analyses set included all subjects who had a baseline value (which was used as a covariate in all models), took the randomized study medication on at least one sleep restriction night, and had a sleep restriction value. All 41 subjects (20 GBX and 21 placebo) took study medication on all study nights and all were included in the full analysis set. MSLT and SWS were also analyzed using the per-protocol set (N = 33), which excluded data for subjects with predefined protocol violations. Prior to unblinding, the following protocol violations were noted: 4 subjects had TST > 510 min on Night 2 (range 513.5–525 min with only one > 516 min) and one subject had mean MSLT on Day 2 of < 7 min (6.7 min). These violations occurred because randomization was based on preliminary scoring. In addition, actigraphy indicated napping during the daytime likely occurred on the first and/or second sleep-restriction day in three individuals. Analyses excluding the 8 protocol violators produced the same group differences, at times with increased significance levels, as compared to the full analysis set comparisons. Analysis of safety endpoints included all subjects as treated; in this study all 41 subjects received the correct treatment (i.e., that to which the subject was randomized).
For endpoints measured multiple times per day (MSLT, KSS, PVT and cognitive battery endpoints), a mixed model with an unstructured covariance matrix to account for the correlation between observations on the same subject over time was used to evaluate treatment group differences while controlling for age, sex, and baseline value. A general linear model controlling for age, sex, and baseline value was used for endpoints measured once per day (PSG variables, morning and evening diary endpoints, POMS endpoints and overnight differences in the Procedural Memory Test). The mean over the last 2 sleep restriction days (at each time point for MSLT, KSS, PVT and cognitive battery endpoints) was used for the treatment value and the mean over the 2 baseline days (at each time point for MSLT, KSS, PVT and cognitive battery endpoints) was used for the baseline value. The PSG results were similar when the mean of all four sleep restriction nights was used as the treatment value and PSG Night 2 alone was used as baseline. If the value for a time point was missing on one of the last 2 sleep restriction days then the value for the non-missing day was used rather than the mean. No imputation was made for missing data (at a time point) after the measures were averaged over the last 2 sleep restriction days. For daytime measures, all data were missing for Day 6 for one subject. That same subject was missing the 18:00 data point on the MSLT on Day 5. The other time points on Day 5 for this subject were included from the analysis which included a factor for time of day, since the model appropriately handles the missing data. This subject was also included in the ANCOVA performed using means for the entire day; Day 5 values were used as the mean of Days 5 and 6. A second subject, missing an 18:00 data point on Day 6, was also included in the analysis which included a factor for time of day, as well as in the ANCOVA. We included in the ANCOVA (for the MSLT, PVT, KSS, etc) all subjects having values for at least 4 time points on a given day. Age, sex, and baseline values were used as covariates in all analyses.
Because the hypotheses of interest were directional in nature (e.g., GBX group would show higher MSLT scores and higher SWS values), one-sided significance tests were used with α equal to 0.05. Power calculations were conducted with the assumption of one-sided statistical comparisons.
When statistical comparisons are being described the values reported in the text are least square means generated by the models (unless noted otherwise). Error estimates indicated by ± refers to the standard error for least square means and for difference scores, and standard deviations for unadjusted means. In Tables 2–5 and Figures 1 and 3 unadjusted (observed) means and standard deviations are presented for the readers' convenience.
Table 2.
Observed Mean (SD) Polysomnography Variables for Gaboxadol (GBX) and Placebo (PBO) Groups on Nights 1–8
| Baseline |
Sleep Restriction |
Recovery |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Night 1 |
Night 2 |
Night 3 |
Night 4 |
Night 5 |
Night 6 |
Night 7 |
Night 8 |
||||||||
| PBO N=21 | GBX N=20 | PBO N=21 | GBX N=20 | PBO N=21 | GBX N=20 | PBO N=21 | GBX N=20 | PBO N=21 | GBX N=20 | PBO N=21 | GBX N=20 | PBO N=21 | GBX N=20 | PBO N=21 | GBX N=20 |
| Total Sleep Time (min) | |||||||||||||||
| 503.9 | 506.5 | 475.7 | 479.2 | 299.7 | 301.5 | 299.1 | 302.1 | 297.9 | 299.9 | 301.0 | 300.0 | 616.8 | 606.3 | 482.1 | 500.0 |
| (75.4) | (39.5) | (37.8) | (26.7) | (5.2) | (6.6) | (9.1) | (3.8) | (9.6) | (5.9) | (3.5) | (3.4) | (69.5) | (60.8) | (61.6) | (75.0) |
| Latency to Persistent Sleep (min) | |||||||||||||||
| 31.0 | 26.9 | 18.0 | 17.0 | 15.5 | 12.0 | 10.0 | 6.5 | 6.6 | 5.6 | 8.5 | 6.9 | 21.9 | 26.3 | 60.7 | 69.6 |
| (18.2) | (17.5) | (12.3) | (11.0) | (14.0) | (9.9) | (12.2) | (5.5) | (11.5) | (7.0) | (17.3) | (7.3) | (18.1) | (22.3) | (32.6) | (50.0) |
| Stage 1 (min) | |||||||||||||||
| 84.5 | 79.4 | 72.5 | 66.5 | 39.2 | 26.0 | 33.5 | 20.9 | 28.7 | 22.1 | 28.5 | 19.2 | 82.0 | 73.8 | 74.6 | 76.5 |
| (24.1) | (31.9) | (29.5) | (27.7) | (17.9) | (14.5) | (13.7) | (10.6) | (13.6) | (11.2) | (13.7) | (8.5) | (33.4) | (23.9) | (23.5) | (27.8) |
| Stage 2 (min) | |||||||||||||||
| 254.2 | 256.9 | 239.3 | 232.2 | 138.5 | 123.9 | 137.0 | 120.5 | 127.4 | 121.8 | 126.5 | 112.3 | 307.2 | 295.9 | 243.0 | 249.6 |
| (52.6) | (40.2) | (34.0) | (32.8) | (24.0) | (34.3) | (25.3) | (36.5) | (28.3) | (34.0) | (23.2) | (38.9) | (46.0) | (32.2) | (38.8) | (40.2) |
| Stage 3 (min) | |||||||||||||||
| 39.4 | 38.1 | 43.7 | 42.0 | 38.2 | 41.1 | 37.9 | 40.6 | 40.5 | 39.3 | 40.8 | 45.7 | 45.9 | 44.2 | 39.7 | 36.2 |
| (17.4) | (15.1) | (19.0) | (17.3) | (19.5) | (19.7) | (18.9) | (16.0) | (17.9) | (16.0) | (16.6) | (18.8) | (22.5) | (17.6) | (19.2) | (17.7) |
| Stage 4 (min) | |||||||||||||||
| 23.7 | 31.0 | 23.2 | 35.8 | 25.6 | 48.6 | 23.7 | 58.6 | 26.5 | 53.2 | 29.5 | 52.8 | 27.7 | 37.9 | 19.8 | 30.9 |
| (21.9) | (25.0) | (21.5) | (26.7) | (24.0) | (32.9) | (26.3) | (35.0) | (26.2) | (32.6) | (27.5) | (37.3) | (27.3) | (31.6) | (21.4) | (26.5) |
| SWS (min) | |||||||||||||||
| 63.0 | 69.0 | 66.9 | 77.8 | 63.9 | 89.7 | 61.6 | 99.1 | 67.1 | 92.6 | 70.3 | 98.5 | 73.6 | 82.1 | 59.4 | 67.1 |
| (31.9) | (31.9) | (33.1) | (34.7) | (32.5) | (36.7) | (32.8) | (34.2) | (31.1) | (35.5) | (32.8) | (39.1) | (40.6) | (40.6) | (32.9) | (33.1) |
| REM (min) | |||||||||||||||
| 102.1 | 101.3 | 97.1 | 102.6 | 58.2 | 62.0 | 67.0 | 61.6 | 74.7 | 63.4 | 75.8 | 69.9 | 154.0 | 154.6 | 105.1 | 106.8 |
| (31.0) | (21.3) | (25.0) | (25.0) | (11.6) | (15.3) | (13.7) | (15.2) | (16.8) | (17.2) | (16.2) | (15.8) | (31.0) | (30.9) | (25.5) | (30.4) |
| Wake After Sleep Onset (min) | |||||||||||||||
| 71.0 | 72.6 | 49.2 | 47.6 | 13.1 | 11.0 | 13.8 | 6.6 | 10.5 | 7.9 | 10.2 | 10.6 | 83.3 | 92.2 | 181.7 | 155.0 |
| (70.1) | (38.1) | (37.9) | (28.0) | (8.5) | (8.6) | (19.3) | (4.5) | (8.8) | (5.5) | (7.2) | (14.1) | (66.5) | (62.6) | (65.6) | (94.7) |
| Number of Shifts to Wake or S1 | |||||||||||||||
| 46.4 | 50.1 | 50.8 | 50.3 | 31.9 | 28.4 | 31.0 | 23.3 | 26.3 | 23.6 | 28.0 | 21.8 | 42.6 | 40.4 | 43.3 | 40.7 |
| (14.5) | (12.8) | (11.7) | (13.9) | (11.0) | (12.7) | (9.5) | (10.6) | (11.1) | (9.6) | (10.2) | (8.4) | (14.7) | (8.2) | (13.4) | (10.3) |
| Number of Awakenings | |||||||||||||||
| 14.8 | 13.7 | 10.3 | 9.2 | 4.6 | 2.7 | 3.4 | 2.0 | 3.3 | 1.8 | 3.5 | 2.0 | 13.0 | 10.7 | 13.5 | 13.9 |
| (6.6) | (6.7) | (6.2) | (4.3) | (3.3) | (2.5) | (3.5) | (2.2) | (3.3) | (1.7) | (2.7) | (2.4) | (9.3) | (6.6) | (8.1) | (7.7) |
| Latency to Slow Wave Sleep (min) | |||||||||||||||
| 29.9 | 28.7 | 20.6 | 16.6 | 19.7 | 16.2 | 15.4 | 13.6 | 15.8 | 16.7 | 15.7 | 16.9 | 33.4 | 24.0 | 25.3 | 26.9 |
| (22.0) | (21.7) | (13.5) | (5.4) | (15.9) | (7.2) | (7.9) | (4.6) | (12.5) | (13.6) | (11.0) | (12.0) | (56.1) | (14.7) | (19.2) | (18.9) |
| REM Latency (min) | |||||||||||||||
| 93.2 | 100.7 | 63.3 | 66.6 | 57.8 | 66.6 | 57.8 | 66.7 | 47.5 | 70.6 | 48.8 | 53.5 | 67.5 | 56.7 | 85.0 | 63.2 |
| (41.4) | (37.1) | (16.1) | (22.9) | (21.7) | (24.1) | (31.8) | (31.6) | (28.3) | (30.2) | (22.6) | (8.5) | (60.9) | (9.3) | (67.0) | (20.8) |
Table 3.
Observed Mean (SD) Self-Reported Sleep Variables for Gaboxadol (GBX) and Placebo (PBO) Groups on Nights 1–8
| Screening / Baseline |
Sleep Restriction |
Recovery |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Night 1 |
Night 2 |
Night 3 |
Night 4 |
Night 5 |
Night 6 |
Night 7 |
Night 8 |
||||||||
| PBO | GBX | PBO | GBX | PBO | GBX | PBO | GBX | PBO | GBX | PBO | GBX | PBO | GBX | PBO | GBX |
| Total Sleep Time (min) | |||||||||||||||
| N=21 | N=19 | N=21 | N=19 | N=20 | N=19 | N=21 | N=20 | N=21 | N=20 | N=21 | N=20 | N=20 | N=20 | N=20 | N=18 |
| 517.0 | 539.8 | 500.7 | 500.8 | 317.6 | 333.9 | 311.0 | 318.7 | 325.5 | 314.7 | 304.8 | 306.1 | 635.7 | 652.5 | 556.0 | 582.1 |
| (91.2) | (44.7) | (49.9) | (31.5) | (26.8) | (44.1) | (25.7) | (45.0) | (31.1) | (35.9) | (15.9) | (18.4) | (80.5) | (57.1) | (109) | (84.7) |
| Sleep Latency (min) | |||||||||||||||
| N=21 | N=20 | N=21 | N=19 | N=21 | N=20 | N=21 | N=20 | N=21 | N=20 | N=20 | N=20 | N=20 | N=20 | N=20 | N=19 |
| 29.0 | 27.8 | 18.2 | 14.5 | 12.2 | 7.8 | 8.9 | 6.8 | 8.4 | 5.7 | 8.2 | 5.7 | 29.7 | 14.8 | 50.0 | 44.0 |
| (27.0) | (20.3) | (12.6) | (9.4) | (7.9) | (5.3) | (8.1) | (4.7) | (12.8) | (4.5) | (16.1) | (4.7) | (42.0) | (14.1) | (52.0) | (46.0) |
| Wake After Sleep Onset (min) | |||||||||||||||
| N=19 | N=20 | N=19 | N=18 | N=19 | N=17 | N=20 | N=13 | N=16 | N=14 | N=18 | N=15 | N=20 | N=20 | N=20 | N=17 |
| 27.2 | 16.8 | 13.5 | 21.2 | 2.9 | 1.9 | 3.0 | 2.3 | 6.0 | 2.5 | 3.6 | 5.1 | 30.2 | 35.3 | 42.1 | 45.9 |
| (38.6) | (13.8) | (15.7) | (30.6) | (2.8) | (3.4) | (5.1) | (3.1) | (15.0) | (3.6) | (3.9) | (15.3) | (53.9) | (37.2) | (54.0) | (60.0) |
| Number of Awakenings | |||||||||||||||
| N=21 | N=20 | N=21 | N=18 | N=21 | N=20 | N=21 | N=20 | N=21 | N=20 | N=21 | N=19 | N=20 | N=20 | N=20 | N=19 |
| 2.9 | 3.0 | 2.9 | 2.3 | 1.4 | 0.3 | 1.0 | 0.3 | 0.9 | 0.4 | 1.1 | 0.3 | 3.1 | 2.4 | 2.8 | 2.7 |
| (1.7) | (2.3) | (2.8) | (1.3) | (1.6) | (0.6) | (1.3) | (0.5) | (1.4) | (0.6) | (1.2) | (0.5) | (2.4) | (1.7) | (2.4) | (1.7) |
| Sleep Quality Rating# | |||||||||||||||
| N=21 | N=20 | N=21 | N=19 | N=21 | N=20 | N=21 | N=20 | N=21 | N=20 | N=21 | N=20 | N=20 | N=20 | N=20 | N=19 |
| 55.0 | 51.5 | 63.6 | 67.7 | 73.3 | 71.5 | 73.4 | 77.9 | 67.5 | 74.1 | 66.9 | 69.6 | 69.7 | 77.0 | 65.0 | 56.5 |
| (21.4) | (19.9) | (23.1) | (17.3) | (18.9) | (18.1) | (17.4) | (14.6) | (23.1) | (19.6) | (19.8) | (22.5) | (19.3) | (14.8) | (21.8) | (24.4) |
| Refreshed Upon Awakening Rating# | |||||||||||||||
| N=21 | N=20 | N=21 | N=19 | N=21 | N=20 | N=21 | N=20 | N=21 | N=20 | N=21 | N=20 | N=20 | N=20 | N=20 | N=19 |
| 67.6 | 65.6 | 62.6 | 60.6 | 57.6 | 44.8 | 57.0 | 41.1 | 33.5 | 34.0 | 37.1 | 23.4 | 75.8 | 76.8 | 79.4 | 64.5 |
| (21.6) | (17.8) | (25.1) | (21.2) | (22.5) | (22.3) | (25.5) | (25.2) | (18.3) | (20.6) | (18.3) | (21.9) | (18.1) | (16.3) | (21.1) | (27.0) |
100 mm analog scale rating; higher values are more positive
Table 4.
Observed Mean (SD) PVT Variables for Gaboxadol (GBX) and Placebo (PBO) Groups on Days 1,2,5,6, and 7
| Baseline |
Sleep Restriction |
Recovery |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 |
Day 2 |
Day 5 |
Day 6 |
Day 7 |
||||||
| PBO | GBX | PBO | GBX | PBO | GBX | PBO | GBX | PBO | GBX | |
| N | 21 | 20 | 21 | 20 | 21 | 20 | 20 | 20 | 20 | 20 |
| Lapses, no. | 0.8 (1.7) | 0.7 (1.1) | 1.2 (2.6) | 0.7 (1.2) | 1.5 (2.2) | 1.7 (2.8) | 1.8 (2.4) | 1.6 (2.3) | 0.6 (1.3) | 0.8 (1.5) |
| Transformed lapses | 1.7 (1.0) | 1.7 (0.8) | 1.9 (1.4) | 1.7 (0.9) | 2.1 (1.3) | 2.2 (1.5) | 2.3 (1.4) | 2.2 (1.2) | 1.5 (1.0) | 1.7 (1.1) |
| Reaction time, msec | 257.1 (41.0) | 243.3 (30.4) | 253.3 (43.2) | 253.5 (36.0) | 270.0 (43.4) | 273.7 (50.6) | 276.4 (44.7) | 274.8 (38.2) | 248.9 (37.7) | 249.8 (37.9) |
| 1/reaction time | 4.1 (0.5) | 4.3 (0.4) | 4.2 (0.6) | 4.2 (0.5) | 4.0 (0.5) | 4.0 (0.5) | 3.9 (0.5) | 3.9 (0.5) | 4.2 (0.5) | 4.2 (0.5) |
| Fastest 10% reaction time, msec | 199.3 (25.3) | 187.8 (18.6) | 196.2 (25.3) | 195.8 (22.3) | 203.5 (24.0) | 202.7 (22.6) | 206.1 (23.7) | 203.9 (25.7) | 192.6 (19.9) | 193.2 (20.1) |
| Slowest 10% reaction time, msec | 368.8 (85.0) | 364.8 (80.6) | 372.3 (94.7) | 379.2 (82.8) | 421.2 (125.0) | 455.2 (232.0) | 431.3 (124.0) | 434.6 (107.0) | 352.8 (91.6) | 377.6 (139.0) |
Table 5.
Pearson Partial Correlation Coefficients (Adjusted for Age and Sex), for all Subjects Combined and for Both the Gaboxadol (GBX) and Placebo (PBO) Groups Alone, Describing the Association Between Mean Change from Baseline in MSLT and KSS on Day 6 and Change from Baseline in Sleep Stage Amounts and Spectral Power Density in Specific Frequency Bands on Night 6
| All Subjectsa |
GBX Groupa |
PBO Groupa |
||||
|---|---|---|---|---|---|---|
| MSLT | KSS | MSLT | KSS | MSLT | KSS | |
| Power 1 – 5 Hz | 0.331* | −0.437** | 0.443+ | −0.435+ | −0.100 | −0.315 |
| Power 6 - 11 Hz | 0.284+ | −0.426* | 0.265 | −0.452+ | 0.018 | −0.314 |
| Power 12 - 14 Hz | −0.175 | −0.107 | −0.229 | −0.042 | −0.027 | −0.397 |
| Power 15 - 17 Hz | −0.001 | −0.086 | −0.074 | 0.080 | −0.069 | −0.182 |
| Power 18 - 32 Hz | 0.217 | 0.041 | 0.374+ | 0.169 | −0.263 | −0.010 |
| Stage 1 (min) | 0.197 | 0.264 | 0.278 | 0.241 | 0.024 | 0.281 |
| Stage 2 (min) | −0.200 | 0.144 | −0.447+ | 0.183 | 0.158 | 0.110 |
| Slow Wave Sleep (min) | 0.514*** | −0.326* | 0.578* | −0.444+ | 0.181 | −0.181 |
| REM (min) | −0.231 | −0.015 | −0.124 | −0.316 | −0.089 | 0.014 |
sample sizes for all spectral measures were 37 for all subjects, 19 for GBX and 18 for PBO; for sleep stage measures the sample sizes were 40 for all subjects, 20 for GBX and 20 for PBO.
*** P ≤0.001; ** P ≤0.01; * P ≤0.05; + P ≤0.10
Figure 1.
Unadjusted (observed) mean minutes of slow wave sleep (Panel A), total sleep time (Panel B), minutes of stage 1 (Panel C), and shifts to wake or stage 1 (Panel D) on Nights 1–8 for both study groups. Error bars indicate standard deviations. Time in bed varied across Nights (9 h on baseline Night 2, approximately 5 h on Nights 3–6, 12 h on Nights 7 and 8). All subjects received placebo on Nights 1, 2, 7, and 8. Double-blind GBX 15 mg (filled symbols) or placebo (open symbols) were administered on Nights 3–6. SWS was significantly greater and stage 1 and shifts to wake or 1 were significantly less for the GBX group as compared to the placebo group on Nights 3–6 (P < 0.001 for each). Total sleep time did not differ between groups on any night. No variable differed between groups at baseline (Nights 1 and 2) or on recovery nights (7 and 8).
Figure 3.
Observed means for sleep latency on the MSLT on Days 1, 2, 5, 6, and 7 for both groups. Error bars indicate standard deviations. Least square mean sleep latency of Days 5 and 6 was significantly longer (P = 0.047) for the GBX group (filled symbols) than for the placebo group (open symbols).
Mean power spectra were computed for each subject by 1-Hz bin (ranging from 1 to 32 Hz) relative to the average of baseline Nights 1 and 2 during all NREM (Stages 1, 2, 3, and 4) epochs. Power density values at baseline were calculated for the entire (9 h) night and for the first 5 h of sleep and comparisons to sleep restriction nights were made with both. No correction was made for multiplicity.
Correlation and partial correlations (controlling for age and sex) were computed for changes from baseline in MSLT and changes from baseline in KSS versus changes from baseline in PSG variables including SWS and power density endpoints. Principal components analysis was used to reduce the number of power density ranges by identification of clusters of 1-Hz bins that accounted for significant portions of the variance. The frequency clusters reported in Table 5 are based on the results of this analysis.
MSLT was the a priori primary dependent variable and was used for power calculations. Assuming 20 evaluable subjects per group and a standard deviation of 2.5 min, the study was planned to have 80% power to declare significant (one-sided test, 5% level of significance) a 2-min difference between groups in change from baseline MSLT scores.
Effect sizes (i.e., Cohen's d) were computed using parameter estimates from the regression models for exploratory endpoints such as those in the neurocognitive battery, self-reported sleep measures from the morning and evening diaries, and subscale endpoints from the POMS.
RESULTS
Sleep Restriction Period
Polysomnography
Unadjusted (observed) mean PSG data for baseline, sleep restriction, and recovery nights for both groups are shown in Table 2. Groups were numerically very similar at baseline. Least square mean TST during sleep restriction (Nights 3–6) did not differ between groups (PBO = 299.4 ± 0.94; GBX = 300.8 ± 0.96 min; Figure 1) as predicted from the restricted time in bed. During sleep restriction the GBX group demonstrated significantly more stage 4 and SWS (stage 3 plus stage 4) compared to the PBO group (P < 0.001 for both). GBX averaged 20.5 min more SWS on the last 2 sleep restriction nights (5 and 6) and 21.8 min more SWS than PBO on all 4 sleep restriction nights (3 through 6). Compared to baseline, GBX averaged 17.2 more min of SWS on Nights 3–6, whereas the PBO group averaged 1.8 more min than at baseline on Nights 3–6 (Figure 1). During sleep restriction as compared to PBO, GBX also had less stage 1 sleep (7.1 ± 2.4 minutes less on Nights 5-6, P = 0.003 and 9.3 ± 2.1 min less on Nights 3–6, P < 0.001; Figure 1), less REM (9.7 ± 4.2 min less on Nights 5-6, P = 0.014 and 6.7 ± 3.2 min less on Nights 3–6, P = 0.022), fewer shifts to wake or stage 1 (5.4 ± 2.1 fewer on Nights 5-6, P = 0.007 and 5.4 ± 1.8 fewer on Nights 3–6, P < 0.001; Figure 1), and a longer REM latency (13.7 ± 6.1 min longer on Nights 5-6, P = 0.015 and 11.8 ± 5.9 min longer on Nights 3–6, P = 0.027). WASO did not differ between groups on Nights 5-6 (P = 0.26) but averaged 3.0 ± 1.5 min less on Nights 3–6 with GBX (P = 0.03). There were no group differences for latency to persistent sleep (LPS), stage 2, or stage 3.
Spectral Analyses
Spectral analysis of the EEG produced power density findings consistent with the visually scored increase in SWS with GBX. Figure 2 illustrates the group differences in relative (to the mean of baseline Nights 1 and 2) NREM power density during the first 5 h of the recording for each sleep restriction night individually and for the average of Nights 5–6. The specific preplanned statistical comparisons were made between groups for the mean values of Nights 5–6, and differences between groups are shown in the figure. In general, GBX significantly increased relative power density, as compared to PBO, in all 1-Hz frequency bins from 1 to 8 Hz.
Figure 2.
Spectral power density profiles for the GBX group (filled symbols) and the placebo group (open symbols) for nights 3, 4, 5, and 6 (Panels A-D, respectively) and averaged across Nights 5 and 6 (Panel E), relative to baseline (mean of Nights 1 and 2). Geometric mean values (± SD) for each 1-Hz bin are plotted. Baseline night values are represented by the abscissa at 1.0. Statistical comparisons were made only between groups for means of Nights 5 and 6 versus baseline. Horizontal lines above the frequency bin demarcations indicate statistically significant differences between groups (Panel E).
Inspection of the placebo group data show that sleep restriction alone produced mild numerical changes in relative spectral power density, with the spectral profile showing increases and decreases at approximately the same frequencies as seen with GBX, although at slow frequencies the magnitude of change was much smaller.
Group comparisons in relative spectral power density were also conducted using the average of all 4 sleep restriction nights and for each sleep restriction night alone. These analyses were in agreement with the data for Nights 5–6 presented above. Relative power was also calculated using 9 h of baseline and the group differences were essentially identical to those obtained using 5 h of baseline data. In all of these additional analyses, GBX produced relative spectral power increases as compared to PBO at all frequencies up to 8 Hz.
Self-reported Ratings of Sleep
GBX subjects reported fewer awakenings on average during Nights 5 and 6 than PBO (GBX 0.36 ± 0.15; PBO 0.97 ± 0.15; P = 0.003). There were no other group differences in subjective ratings of sleep including ratings of sleep quality and the restorative nature of sleep. Unadjusted (observed) self-report measures of sleep for both groups on each study night are shown in Table 3.
Multiple Sleep Latency Test
Baseline (Days 1 and 2) unadjusted mean MSLT values for the 2 groups were nearly identical and were consistent with normal alertness levels (PBO 12.4 ± 3.9; GBX 12.2 ± 4.2 min). During sleep restriction MSLT latencies were considerably reduced as anticipated; however, the GBX group was significantly less sleepy than the PBO group (P = 0.047; see Figure 3). The PBO group mean for Days 5 and 6 decreased to 5.8 ± 0.81 min during sleep restriction, whereas the GBX group mean latency was 7.8 ± 0.83 min. A supportive analysis on precomputed MSLT daily mean values using a general linear model (controlling for age, sex and baseline value), also indicated that the groups differed significantly (P = 0.033). Exclusion of protocol violators did not change these findings.
Psychomotor Vigilance Test
Table 4 shows unadjusted (observed) mean data for each group for key PVT measures (Figure 4). There were no group differences on most PVT measures. There was a slight decrement in the mean of the fastest 10% reaction time in the GBX group compared to PBO (P = 0.007). This result was no longer significant when the protocol violators were excluded in an ad hoc analysis (P = 0.17).
Figure 4.
Psychomotor Vigilance Task (PVT) data for baseline (Days 1 and 2), sleep restriction (Days 5 and 6), and recovery (Day 7) conditions for the GBX group (filled symbols) and the placebo group (open symbols). Square root transformed lapses (Panel A), mean reaction time (Panel B), and mean of the slowest 10% of reaction times (Panel C) are shown. Error bars indicate standard deviations. There were no group differences at any time during the study. See text and Table 4 for additional PVT data and analyses.
To assess the effects of sleep restriction we examined the PBO group alone. The changes from baseline to sleep restriction were numerically mild with a decline of approximately 5% to 25% depending upon the variable measured. Significant changes from baseline to sleep restriction were seen for mean reaction time (P = 0.006), mean of the slowest 10% of reaction times (P = 0.007), mean of the fastest 10% of reaction times (P = 0.015), and mean 1/RT (P = 0.002). There was no significant increase in number of lapses (P = 0.18); transformed lapses showed a trend toward a significant difference (P = 0.056).
Introspective Sleepiness and Mood
Mean KSS ratings showed a trend towards lower levels of sleepiness for GBX than PBO during sleep restriction. The mean KSS score for the PBO group averaged over Days 5 and 6 was 6.7 ± 0.30 as compared to 6.0 ± 0.30 for the GBX group (P = 0.058). An ad hoc ANCOVA analysis using the general linear model on the precomputed daily means to compare the groups provided supportive evidence for this observation (P = 0.036). Sleepiness ratings on a visual analog scale made prior to and following the PVT were also lower for GBX than for PBO (PBO = 6.5 ± 0.34, GBX = 5.7 ± 0.34, P = 0.044 for pre-PVT rating; PBO = 7.2 ± 0.33, GBX 6.1 ± 0.34. P = 0.02 for post-PVT rating).
The Fatigue (PBO 12.8 ± 1.4; GBX 9.6 ± 1.5) and Vigor (PBO 8.7 ± 1.2; GBX 11.2 ± 1.3) scales on the POMS tended to favor the GBX group (P = 0.067 and 0.080, respectively). Differences on both scales were more evident (P = 0.030 and 0.024) when the protocol violators were excluded in an ad hoc analysis.
Cognitive Test Battery and Procedural Memory Test
Two of 25 measures made with the 14 tests of the cognitive battery showed a significant difference between the GBX and PBO groups, both in favor of GBX. These findings are consistent with chance observations. The two significant findings were on motor transport time (P = 0.04) and the percent correct on the spatial 1-back memory task (P = 0.02). Examination of the data suggests that few of the measures showed a negative influence of sleep restriction on PBO, leaving little room for improvement with GBX. Additionally, substantial intersubject variability was characteristic of these measures. Because the current study was not powered to detect differences between groups on any neurocognitive measure, the effect size (Cohen's d) for each measure (for the mean of Days 5 and 6) was calculated for 25 endpoints from the 14 tests in the cognitive battery to gain a global comparison of overall neurocognitive function. Seven of 25 measures exceeded a moderate Cohen's d of 0.5 and 6 of those 7 favored GBX. Nevertheless, no clear cognitive domain pattern was noted and no conclusions can be made from this exploratory analysis.
No differences between groups were found on any measure of the Procedural Memory Test. Mild to moderate effect sizes (0.2 to 0.5) on 4 measures of this test were in favor of GBX.
Salivary Cortisol and Urinary Catecholamines
Neither salivary free cortisol nor urinary catecholamine differed between groups during sleep restriction or recovery. Unadjusted mean salivary cortisol values (μg/dL), averaged across the 8 daily samples for the PBO and GBX groups, respectively, were 0.182 (± 0.057) and 0.171 (± 0.09) at baseline and 0.174 (0.05) and 0.165 (± 0.04) on Day 6. Urinary epinephrine/creatinine mean ratios, for the 22:00-10:00 and 10:00-22:00 aliquots, were 6.3 (± 3.6) and 8.9 (± 5.4) at baseline and 5.8 (± 3.3) and 8.4 (± 5.3) on Day 6 for the PBO group and 4.2 (± 2.2) and 7.8 (± 2.6) at baseline and 4.6 (± 2.0) and 6.7 (± 2.2) on Day 6 for the GBX group. Urinary norepinephrine/creatinine mean ratios, for the 22:00-10:00 and 10:00-22:00 aliquots, were 18.2 (± 6.0) and 23.4 (± 8.7) at baseline and 19.5 (± 6.2) and 22.1 (± 6.5) on Day 6 for the PBO group and 16.1 (± 5.7) and 27.2 (± 9.3) at baseline and 18.3 (± 6.3) and 20.6 (± 8.1) on Day 6 for the GBX group.
Recovery Period
There were no group differences on any PSG variable during recovery sleep on either Night 7 or 8. Spectral power density did not differ between groups on Night 7. SWS and spectral power density showed no differences between Night 7 and baseline.
Groups also did not differ on the MSLT, KSS, or other daytime measures following recovery sleep. On Day 7, POMS Fatigue was worse for PBO (8.33 ± 1.41) than for GBX (3.10 ± 1.51; P = 0.008). There were no group differences in other self-report ratings. Following recovery sleep on Night 7, mean fastest 10% of reaction times was slightly slower for GBX than for PBO (P = 0.02), with less evidence of this effect when the protocol violators were excluded (P = 0.06). No other PVT measures differed between groups on Day 7.
Association of MSLT, KSS, Sleep Stages, and Spectral Power Density
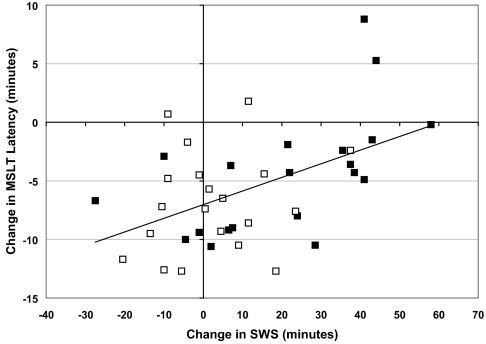
Table 5 contains Pearson partial correlation coefficients (adjusted for age and sex) describing the association of MSLT and KSS with sleep stages and spectral power density. The change from baseline (Day 2) to Day 6 mean MSLT latency was positively and significantly correlated with the change in SWS from baseline (Night 2) to Night 6, for both the GBX group alone (r = 0.58 P < 0.05; see Figure 5), and for all subjects (r = 0.51, P = 0.001), but not for the PBO group (r = 0.18, ns). Change from baseline in MSLT was not significantly correlated with change in TST, as expected given the control over TST in this study, nor with changes in minutes of REM or stage 1. Change in minutes of stage 2 was not associated with change in MSLT for all subjects, and showed a trend toward a negative correlation in the GBX group. When changes in MSLT from baseline to Day 6 were compared with changes in SWS averaged across Nights 3–6 the correlation was lower for all subjects (r = 0.36, P < 0.05) and was not significant for GBX subjects (r = 0.28, ns).
Figure 5.
Relationship between change from baseline in mean minutes of slow wave sleep on Night 6 and change from baseline in mean MSLT latency on Day 6 for all subjects. GBX subjects = filled symbols; placebo subjects = open symbols. Solid line is regression line for all subjects. Pearson correlation coefficients adjusted for age and sex are r = 0.514 (P < 0.001) for all subjects, and r = 0.578 (P < 0.05) for GBX subjects.
Change from baseline to Day 6 MSLT scores for all subjects were also positively associated with the change in power density from baseline to Night 6 in the 1-5 Hz band (r = 0.33, P = 0.052), but not for the 6-11 Hz band (r = 0.28, P = 0.099), or other frequency bands. Although the correlation between change from baseline to Day 6 MSLT and change in 1-5 Hz power density from baseline to Night 6 for GBX alone was higher (r = 0.44), it was not significant (P = 0.075), probably due to the smaller sample size in single group analyses. No association was indicated for the PBO group (r = −0.10, ns).
KSS change scores correlated negatively with change in min of SWS (i.e, more SWS was associated with less introspective sleepiness) for all subjects (r = −0.33, P = 0.05); there was a trend for the GBX group (r = −0.44, P = 0.13), but not for the PBO subjects (r = −0.18, ns). No association between the change in minutes of TST, stage 2 or REM and KSS change scores was observed for all subjects or either group separately.
KSS change from baseline to Day 6 scores were significantly correlated with change in power in the 1-5 Hz band for all subjects (r = −0.44, P < 0.01) and there was a trend for the GBX group (r = −0.44, P = 0.081). In addition, KSS change from baseline to Day 6 scores were significantly correlated with change in power in the 6-11 Hz band for all subjects (r = −0.43, P = 0.011); there was a trend for the GBX group, (r = −0.45, P = 0.068), and not in the PBO group (r = −0.31, ns).
Safety Measures
No clinically relevant changes in vital signs, gait and balance, ECG, hematology, blood chemistry, or routine urinalysis were detected. No serious adverse effects were reported and no subject discontinued prematurely due to an adverse effect. Three subjects in each group reported one or more adverse effects. Two subjects reported dizziness with GBX. No other adverse effect was reported by more than one subject.
DISCUSSION
SWS was consistently increased by GBX 15 mg during the 4-night sleep restriction period, relative to both baseline and to PBO values. Increased SWS with GBX has been shown in a number of studies,22,28–30 and we believe that this represents an enhancement of at least some of the normal physiological processes associated with NREM sleep and is not simply an electroencephalographic change. The most compelling finding in support of this interpretation is the significant reduction in the degree of physiologically assessed sleepiness following sleep restriction in the GBX group as compared to the PBO group. The strong correlation between the increase from baseline in SWS and the change in MSLT from baseline further suggests that the SWS effect may contribute to the reduced impact of sleep restriction in a direct fashion.
There were PSG differences between GBX and PBO groups other than the increase in SWS during sleep restriction. Specifically, GBX reduced stage 1, WASO, REM, and number of stage shifts relative to PBO; although TST and stage 2 did not differ between groups. Although statistically significant, the absolute magnitude of the group differences is quite modest and is not likely to affect next day function when TST is held constant. The mean group differences in WASO, stage 1, and REM were 3, 9, and 7 min per night, respectively. The differences in number of stage shifts to wake or stage 1, a measure of sleep fragmentation,38 differed by an average of 7.2 per night, or about 1.4/h of sleep. That degree of difference in sleep fragmentation between groups would not be suspected to affect subsequent waking function when TST is held constant at 5 h. In a population-based study including 483 individuals without sleep disordered breathing the mean number of stage shifts to wake or stage 1 was 4.8 + 2.2/h.38 Thus, our group difference of 1.4/h is approximately 0.6 of a standard deviation of the normal distribution for that measure.
The physiologic significance of the 2-min mean MSLT difference between groups appears to be substantial when compared with the absolute difference on the MSLT with other interventions to reduce sleepiness. For example, modafinil 200 mg administered to narcolepsy or shift work sleep disorder (SWSD) patients, produces significant increases in MSLT latencies with a magnitude between 1-2 min.39,40 The mean increase in MSLT following treatment of sleep apnea with continuous positive airway pressure is approximately 1 minute.41 These increases in MSLT latencies are judged to be clinically relevant because they are accompanied by improvements in quality of life, patient sleepiness ratings, clinician ratings of treatment effectiveness, and ability to maintain wakefulness or sustain attention. The KSS has been utilized infrequently in studies assessing interventions, but in 2 intervention studies, differences of a magnitude comparable to our findings were reported.39,42
Failure to show a beneficial effect of GBX on PVT performance complicates interpretation of overall study results, especially since PVT performance was preserved during sleep restriction in a similar study of SWS enhancement with tiagabine.25 Close inspection of the effect of sleep restriction on PVT measures in the PBO group in the present study reveals a minimal change with sleep restriction. In fact, the impact of sleep restriction was only about one-third as large as that in our prior study. For example, in the prior study 4 nights of sleep restriction produced increases of 17% in mean reaction time (vs 5% in the present study), 44% in mean of the slowest 10% of reaction times (vs 14.4%), and 91% in mean number of transformed lapses (vs 21%). With such a mild deficit produced by sleep restriction, separation of the two groups becomes extraordinarily difficult to demonstrate. Rather than focus on the differences in findings between the present research and our prior study, we believe the more relevant observation is the important similarities in the results. Specifically, in two separate studies, using different pharmacologic manipulations, SWS enhancement during sleep restriction reduced the impact of sleep restriction on one or more metrics known to be sensitive to sleep loss. This supports the hypothesis that increased SWS/SWA has functional benefit.
None of the individual tests in the cognitive battery were sensitive to the degree of sleep restriction imposed in this study. The absence of a deficit on these measures with sleep restriction prevents detection of possible differences between groups.
The reduction of time spent in SWS which occurs with aging needs to be considered when discussing the function of SWS, since a parallel decline in the restorative value of sleep has not been definitively documented. Investigators have described that the SWS decline with healthy aging is predominantly, if not exclusively, the result of changes in EEG amplitude whereas EEG frequency patterns remain constant. As discussed recently by Bliwise43 the loss in EEG amplitude may reflect age-related changes in neuroendocrine or other humorally-mediated factors rather than a change in the restorative capacity of sleep. On the other hand, the EEG frequency pattern (which does not change with age), which is closely associated with neuronal synchrony may relate to certain aspects of restoration. Similarly, drug-related reductions in SWS such as that seen with benzodiazepines are predominantly the result of EEG wave amplitude reduction rather than EEG frequency alterations.44
With respect to the postulated restorative role of NREM sleep, it is quite interesting that in the current study both GBX and sleep restriction (i.e., PBO) produce power density increases at frequencies from 1 to 8 Hz. In fact, the shape of the spectral curve (see Figure 2) is very comparable between the two groups although the absolute amplitude is not similar. The difference in amplitude may be a dose effect, with dose being either amount of GBX or the amount of prior wakefulness. In any case, the shape of the spectral power density curve is consistent with the hypothesis that GBX produces EEG synchrony changes similar to those seen with naturally increased homeostatic sleep drive.
It is interesting to speculate that a sleep-promoting agent that elicits spectral changes similar to those observed during sleep following the development of a sleep debt, may be promoting sleep through mechanisms involved in homeostatic sleep regulation. Consistent with that hypothesis is the dose response characteristic of the increase in SWA with GBX described elsewhere,29 similar to the increasing enhancement of SWA with increasing severity of sleep deprivation. Another observation which relates the neural activity of GBX to those of natural sleep is the activation of c-Fos expression by ventrolateral preoptic area (VLPO) neurons by GBX, at a level similar to the activation seen with spontaneous sleep.45 Other GABAergic drugs produce much lower levels of c-Fos expression in VLPO neurons when administered at sleep-promoting doses.46
Several lines of evidence support the concept that NREM sleep is generated locally, in neuronal networks or columns that are loosely coupled, rather than being reflective of a unitary brain state.47 In certain animals unihemispheric sleep reflects local sleep regulation.48,49 In humans, parasomnia disorders have informed us that sleep is not necessarily manifest in the entire brain.50 Experimental studies have linked waking experience to local sleep measures.51 Moreover, the amount of SWS/SWA in brain areas has been demonstrated to reflect prior stimulation of that area during prior waking episodes.52–56 With the concept of local sleep regulation, (and specifically SWS regulation) in mind, it is remarkable that a SWS-behavior association was identified in the present study given the relatively gross measure of SWS/SWA employed (as it is in most human sleep research).
In conclusion, the MSLT data and introspective sleepiness and fatigue findings from this study are consistent with the hypothesis that enhanced SWS, in this study produced by GBX, reduces the physiological and introspective impact of sleep restriction. Moreover, the spectral power density changes seen with GBX are similar to those seen with homeostatic increases in sleep drive.
ACKNOWLEDGMENTS
This research was funded by Merck & Co., Inc., Whitehouse Station, NJ. and Lundbeck, A/S, Copenhagen, Denmark Dr. William Ball (formerly of Merck) and Dr. Steve Deacon (Lundbeck) provided valuable input and support for the conduct of the study. Dr. Yan-Ping Zheng and Donna DiGravio (both from Merck Research Laboratories) made important contributions to study implementation, and Junshui Ma and Vladimir Svetnik (both from Merck Research Laboratories) developed the spectral analysis software used and performed the spectral analysis. The authors especially appreciate the dedication of the technical and research personnel of the Sleep Medicine and Research Center, as well as the time and contributions of the research participants.
APPENDIX
The cognitive testing battery consisted of the following tests (end points in parentheses): complex reaction time (decision and transport time), digit symbol substitution (number attempted, percent correct), goal neglect (compliance with instruction before, after change in goal set and overall), lexical decision (decision time for primed and unprimed words, and non-word stimuli), motor tracking (Euclidian error), paced visual serial addition (number correct), simple reaction time (decision and transport time), spatial 1-back memory (number correct), spatial 2-back memory (number correct), sustained attention to response (number errors of omission and errors of commission, accuracy), serial reaction time (time to complete sequences and random trial blocks), verbal 1-back memory (number correct), verbal 2-back memory (number correct), verbal fluency (number correctly generated words, number incorrectly generated words).
Footnotes
Disclosure Statement
This research was funded by Merck & Co., Inc., Whitehouse Station, NJ. and Lundbeck, A/S, Copenhagen, Denmark. Dr. Walsh has consulted for Pfizer, Sanofi-Aventis, Cephalon, Organon, Neurocrine Biosciences, Takeda, Actelion, Sepracor, Jazz, Elan, Guilford, Respironics, TransOral, Neurogen, GlaxoSmithKline, SleepTech, Somaxon, Eli Lilly, Evotec Neuroscience, Concert, and Merck. Dr. Snyder is an employee of Merck. Dr. Groeger has received research support from GlaxoSmithKline, Lundbeck H/S, and Merck and has consulted for Lundbeck H/S and Merck. Dr. Schweitzer has been a principal investigator on research projects funded by Merck, Somaxon, Evotec Neurosciences, and Jazz. The other authors have indicated no financial conflicts of interest.
Editor's Footnote
The clinical development program for gaboxadol was discontinued by Merck and Lundbeck because of an overall unfavorable therapeutic profile, including lack of efficacy in a three-month study and a higher incidence of psychiatric side effects.
REFERENCES
- 1.Horne J. Human slow wave sleep: a review and appraisal of recent findings, with implications for sleep functions, and psychiatric illness. Experientia. 1992;48:941–54. doi: 10.1007/BF01919141. [DOI] [PubMed] [Google Scholar]
- 2.Bennington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45:347–60. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- 3.Dijk DJ, Brunner DP, Beersma DGM, et al. Electroencephalogram power density and slow wave sleep as a function of prior waking and circadian phase. Sleep. 1990;13:430–40. doi: 10.1093/sleep/13.5.430. [DOI] [PubMed] [Google Scholar]
- 4.Werth E, Dijk DJ, Achermann P, Borbely AA. Dynamics of the sleep EEG after an early evening nap: experimental data and simulations. Am J Physiol. 1996;271:R501–10. doi: 10.1152/ajpregu.1996.271.3.R501. [DOI] [PubMed] [Google Scholar]
- 5.Borbely AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep deprivation: Effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51:483–93. doi: 10.1016/0013-4694(81)90225-x. [DOI] [PubMed] [Google Scholar]
- 6.Bonnet MH. Sleep restoration as a function of periodic awakening, movement, or electroencephalographic change. Sleep. 1987;10:364–73. doi: 10.1093/sleep/10.4.364. [DOI] [PubMed] [Google Scholar]
- 7.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 8.Horne J. Human slow wave sleep: a review and appraisal of recent findings, with implications for sleep functions, and psychiatric illness. Experientia. 1992;48:941–54. doi: 10.1007/BF01919141. [DOI] [PubMed] [Google Scholar]
- 9.Bennington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45:347–60. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- 10.Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–50. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Agnew HW, Webb WB, Williams RL. Comparison of stage four and 1-REM sleep deprivation. Percept Motor Skills. 1967;24:851–8. doi: 10.2466/pms.1967.24.3.851. [DOI] [PubMed] [Google Scholar]
- 12.Bonnet MH. Performance and sleepiness following moderate sleep disruption and slow wave sleep deprivation. Physiol Behav. 1986;37:915–8. [PubMed] [Google Scholar]
- 13.Lubin A, Moses JM, Johnson LC, Naitoh P. The recuperative effects of REM sleep and stage 4 sleep on human performance after complete sleep loss: Experiment I. Psychophysiol. 1974;11:133–46. doi: 10.1111/j.1469-8986.1974.tb00834.x. [DOI] [PubMed] [Google Scholar]
- 14.Johnson LC, Naitoh P, Moses JM, Lubin A. Interaction of REM deprivation and stage 4 deprivation with total sleep loss: Experiment 2. Psychophysiology. 1974;11:147–59. doi: 10.1111/j.1469-8986.1974.tb00835.x. [DOI] [PubMed] [Google Scholar]
- 15.Adam K, Oswald I. Effects of repeated ritanserin on middle-aged poor sleepers. Psychopharmacology (Berl) 1989;99:219–21. doi: 10.1007/BF00442811. [DOI] [PubMed] [Google Scholar]
- 16.Dijk DJ, Beersma DG, Daan S, van den Hoofdakker RH. Effects of seganserin, a 5-HT2 antagonist, and temazepam on human sleep stages and EEG power spectra. Eur J Pharmacol. 1989;171:207–18. doi: 10.1016/0014-2999(89)90109-x. [DOI] [PubMed] [Google Scholar]
- 17.Foldvary-Schaefer N, De Leon Sanchez I, Karafa M, Mascha E, Dinner D, Morris HH. Gabapentin increases slow-wave sleep in normal adults. Epilepsia. 2002;43:1493–7. doi: 10.1046/j.1528-1157.2002.21002.x. [DOI] [PubMed] [Google Scholar]
- 18.Kubota T, Fang J, Meltzer LT, Krueger JM. Pregabalin enhances nonrapid eye movement sleep. J Pharmacol Exp Ther. 2001;299:1095–1105. [PubMed] [Google Scholar]
- 19.Mathias S, Wetter TC, Steiger A, Lancel M. The GABA uptake inhibitor tiagabine promotes slow wave sleep in normal elderly subjects. Neurobiol Aging. 2001;22:247–53. doi: 10.1016/s0197-4580(00)00232-3. [DOI] [PubMed] [Google Scholar]
- 20.Walsh JK, Zammit GK, Schweitzer PK, Ondrasik J, Roth T. Tiagabine enhances slow wave sleep and sleep maintenance in primary insomnia. Sleep Med. 2006;29:433–43. doi: 10.1016/j.sleep.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Walsh JK, Randazzo AC, Frankowski S, Shannon K, Schweitzer PK, Roth T. Dose response effects of tiagabine on the sleep of older adults. Sleep. 2005;28:673–6. doi: 10.1093/sleep/28.6.673. [DOI] [PubMed] [Google Scholar]
- 22.Lancel M, Wetter TC, Steiger A, Mathias S. Effect of the GABAA agonist GBX on nocturnal sleep and hormone secretion in healthy elderly subjects. Am J Physiol Endocrinol Metab. 2001;281:E130–7. doi: 10.1152/ajpendo.2001.281.1.E130. [DOI] [PubMed] [Google Scholar]
- 23.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- 24.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 25.Walsh JK, Randazzo AC, Stone K, et al. Tiagabine is associated with sustained attention during sleep restriction: evidence for the value of slow-wave sleep enhancement? Sleep. 2006;29:433–43. [PubMed] [Google Scholar]
- 26.Ebert B, i Storustovu S, Mortensen M, Frolund B. Characterization of GABA(A) receptor ligands in the rat cortical wedge preparation: evidence for action at extrasynaptic receptors? Br J Pharmacol. 2002;137:1–8. doi: 10.1038/sj.bjp.0704846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandra D, Jia F, Liang J, et al. GABAA receptor α4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of GBX. Proc Nat Acad Sci U S A. 2006;103:15230–5. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathias S, Zihl J, Steiger A, Lancel M. Effect of repeated GBX administration on night sleep and next-day performance in healthy elderly subjects. Neuropsychopharmacology. 2005;30:833–41. doi: 10.1038/sj.npp.1300641. [DOI] [PubMed] [Google Scholar]
- 29.Walsh JK, Deacon S, Dijk DJ, Lundahl J. The selective extrasynaptic GABAA agonist, GBX, improves traditional hypnotic efficacy measures and enhances slow wave activity in a model of transient insomnia. Sleep. 2007;30:593–602. doi: 10.1093/sleep/30.5.593. [DOI] [PubMed] [Google Scholar]
- 30.Deacon S, Staner L, Staner C, Legters A, Loft H, Lundahl J. Effect of short-term treatment with GBX on sleep maintenance and initiation in patients with primary insomnia. Sleep. 2007;30:281–7. doi: 10.1093/sleep/30.3.281. [DOI] [PubMed] [Google Scholar]
- 31.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 32.Monk TH, Buysse DJ, Kennedy KS, Pods JM, DeGrazia JM, Miewald JM. Measuring sleep habits without using a diary: the sleep timing questionnaire. Sleep. 2003;26:208–12. doi: 10.1093/sleep/26.2.208. [DOI] [PubMed] [Google Scholar]
- 33.Rechtschaffen A, Kales A, editors. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. [Google Scholar]
- 34.Mitler MM, Carskadon MA, Hirshkowitz M. Evaluating sleepiness. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. fourth edition. Philadelphia: Elsevier Saunders; 2005. pp. 1417–23. [Google Scholar]
- 35.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods Instrum Comput. 1985;17:652–5. [Google Scholar]
- 35.McNair DM, Lorr M, Droppleman L. Boston University School of Medicine; 1981. Profile of mood states. [Google Scholar]
- 37.Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 38.Morrell MJ, Finn L, Kim H, Peppard PE, Badr S, Young T. Sleep fragmentation, awake blood pressure, and sleep-disordered breathing in a population-based study. Am J Respir Crit Care Med. 2000;162:2091–6. doi: 10.1164/ajrccm.162.6.9904008. [DOI] [PubMed] [Google Scholar]
- 39.Czeisler CA, Walsh JK, Roth T, et al. Modafinil for excessive sleepiness associated with shift-work sleep disorder. New Engl J Med. 2005;353:476–86. doi: 10.1056/NEJMoa041292. [DOI] [PubMed] [Google Scholar]
- 40.US Modafinil in Narcolepsy Multicenter Study Group. Randomized trial of modafinil as a treatment for the excessive daytime somnolence of narcolepsy. Neurology. 2000;54:1166–75. doi: 10.1212/wnl.54.5.1166. [DOI] [PubMed] [Google Scholar]
- 41.Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med. 2003;163:565–71. doi: 10.1001/archinte.163.5.565. [DOI] [PubMed] [Google Scholar]
- 42.Schweitzer PK, Randazzo AC, Stone K, Erman M, Walsh JK. Laboratory and field studies of naps and caffeine as practical countermeasures for sleep/wake problems associated with night work. Sleep. 2006;29:39–50. doi: 10.1093/sleep/29.1.39. [DOI] [PubMed] [Google Scholar]
- 43.Bliwise D. Normal Aging. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Philadelphia: Elsevier Saunders; 2005. pp. 24–38. [Google Scholar]
- 44.Feinberg I, Maloney T, Campbell IG. Effects of hypnotics on the sleep EEG of healthy young adults: new data and pharmacological implications. J Psychiatr Res. 2000;34:423–38. doi: 10.1016/s0022-3956(00)00038-8. [DOI] [PubMed] [Google Scholar]
- 45.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–63. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 46.Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, Maze M. The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat Neurosci. 2002;5:979–84. doi: 10.1038/nn913. [DOI] [PubMed] [Google Scholar]
- 47.Krueger JM, Rector DM, Churchill L. Sleep and cytokines. Sleep Med Clin. 2007;2:161–70. doi: 10.1016/j.jsmc.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mukhametov LM. Sleep in marine mammals. Exp Brain Res. 1984;8:227–38. [Google Scholar]
- 49.Rattenborg NC, Amlaner CJ, Lima SL. Unilateral eye closure and interhemispheric EEG asymmetry during sleep in the pigeon (Columba livia) Brain Behav Evol. 2001;58:323–32. doi: 10.1159/000057573. [DOI] [PubMed] [Google Scholar]
- 50.Mahowald MW, Schenck CH. Insights from studying human sleep disorders. Nature. 2005;437:1279–85. doi: 10.1038/nature04287. [DOI] [PubMed] [Google Scholar]
- 51.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 52.Miyamoto H, Katagiri H, Hensch T. Experience-dependent slow-wave sleep development. Nat Neurosci. 2003;6:553–4. doi: 10.1038/nn1064. [DOI] [PubMed] [Google Scholar]
- 53.Vyazovskiy V, Borbely AA, Tobler I. Unilateral vibrissae stimulation during waking induces interhemispheric EEG asymmetry during subsequent sleep in the rat. J Sleep Res. 2000;9:367–71. doi: 10.1046/j.1365-2869.2000.00230.x. [DOI] [PubMed] [Google Scholar]
- 54.Cottone LA, Adamo D, Squires NK. The effect of unilateral somatosensory stimulation on hemispheric asymmetries during slow wave sleep. Sleep. 2004;27:63–8. doi: 10.1093/sleep/27.1.63. [DOI] [PubMed] [Google Scholar]
- 55.Ferrara M, De Gennaro L, Curcio G, Cristiani R, Bertini M. Interhemispheric asymmetry of human sleep EEG in response to selective slow-wave sleep deprivation. Behav Neurosci. 2002;116:976–81. doi: 10.1037//0735-7044.116.6.976. [DOI] [PubMed] [Google Scholar]
- 56.Yasuda T, Yasuda K, Brown RA, Krueger JM. State-dependent effects of light-dark cycle on somatosensory and visual cortex EEG in rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1083–9. doi: 10.1152/ajpregu.00112.2005. [DOI] [PubMed] [Google Scholar]