Abstract
Study Objectives:
This mini-review considers certain factors related to the developmental decrease in rapid eye movement (REM) sleep, which occurs in favor of additional waking time, and its relationship to developmental factors that may influence its potential role in brain development.
Design:
Specifically, we discuss some of the theories proposed for the occurrence of REM sleep and agree with the classic notion that REM sleep is, at the least, a mechanism that may play a role in the maturation of thalamocortical pathways. The developmental decrease in REM sleep occurs gradually from birth until close to puberty in the human, and in other mammals it is brief and coincides with eye and ear opening and the beginning of massive exogenous activation. Therefore, the purported role for REM sleep may change to involve a number of other functions with age.
Measurements and Results:
We describe recent findings showing that morphologic and physiologic properties as well as cholinergic, gamma amino-butyric acid, kainic acid, n-methyl-d-aspartic acid, noradrenergic, and serotonergic synaptic inputs to mesopontine cholinergic neurons, as well as the degree of electrical coupling between mostly noncholinergic mesopontine neurons and levels of the neuronal gap-junction protein connexin 36, change dramatically during this critical period in development. A novel mechanism for sleep-wake control based on well-known transmitter interactions, as well as electrical coupling, is described.
Conclusion:
We hypothesize that a dysregulation of this process could result in life-long disturbances in arousal and REM sleep drive, leading to hypervigilance or hypovigilance such as that observed in a number of disorders that have a mostly postpubertal age of onset.
Citation:
Garcia-Rill E; Charlesworth A; Heister D; Ye Y; Hayar A. The developmental decrease in REM sleep: the role of transmitters and electrical coupling. SLEEP 2008;31(5):673–690.
Keywords: Acetylcholine, arousal, excitatory amino acids, gamma amino-butyric acid, kainic acid, n-methyl-d-aspartic acid, noradrenaline, serotonin
A NUMBER OF RECENT REVIEWS PROVIDE DETAILED DESCRIPTIONS OF THE ORGANIZATION OF THE NEUROLOGIC SUBSTRATES CONTROLLING SLEEP-WAKE systems.1–6 The reader is also referred to an extensive consideration of rapid eye movement (REM) sleep.7 Our recent mini-review focused on developmental events related to the decrease in REM sleep, including its relationship to other developmental changes, the role of blood flow, some factors that may influence its progress, and its potential role in brain development.8 Briefly, the part of the brain controlling sleep-wake states and most responsible for inducing REM sleep is the reticular activating system (RAS), especially its cholinergic component. The RAS triggers changes in state mainly via its ascending projections to the intralaminar thalamus (ILT) to modulate thalamocortical systems in both waking and REM sleep, while simultaneously modulating REM sleep and postural muscle tone via its descending projections to the pontomedullary reticular formation. Since that review, a more complete survey of the transmitter systems that affect these neurons has been completed, and the role of electrical coupling as a novel mechanism in sleep-wake control has been discovered. We will describe the organization of sleep-wake control systems in the adult and then deal with development.
1.1 Local Organization and Sleep-Wake States in the Adult
The main cell groups of the RAS are the cholinergic pedunculopontine (PPN), the noradrenergic locus coeruleus (LC), and the serotonergic raphe nuclei. The cholinergic input to LC from PPN is excitatory, 9–12 and the noradrenergic input to PPN from LC is inhibitory, via α2-adrenergic receptors.13,14 The serotonergic raphe inhibits the PPN and LC.15–19 The PPN receives inhibitory cholinergic input from other mesopontine nuclei,19,20 whereas recurrent collaterals using α2-adrenergic autoreceptors inhibit the LC. The PPN also receives GABAergic input from the substantia nigra and local neurons.1 Classic findings established that electrical stimulation of the RAS induced desynchronization of the electroencephalogram, similar to that seen during waking and REM sleep.21 LC and raphe neurons decrease discharge rates preceding REM sleep and show low to no activity during REM sleep, whereas they are more active during waking.22–25 Many of these do not show a “REM-off” pattern of activity but, rather, a “wake/REM-on” pattern. Putative cholinergic mesopontine neurons increase firing during waking (“Wake-on”) and REM sleep (“REM-on”), or both (“Wake/REM-on”), but decrease during slow-wave sleep (SWS).26–28 That is, monoamine-containing neurons act in concert with cholinergic neurons to increase excitability levels in thalamic and cortical cells during waking, but only cholinergic neurons also induce activation during REM sleep.
1.2 Descending Projections in the Adult
The PPN sends projections throughout the pontomedullary reticular formation,1–3 including the anterior pontine region.29,30 Injections of cholinergic agonists into a region called the pontine inhibitory area induce a REM-sleep–like state (atonia and ponto-geniculo-occipital [PGO] waves),31–37 an effect mediated by muscarinic blockade of an outward, G-protein–coupled, potassium current.38 After pontine injections of carbachol to induce REM sleep, increased c-fos expression is seen in GABAergic and non-GABAergic/noncholinergic neurons in the PPN.39 Lesioning of this pontine area, termed the subcoeruleus, can produce REM sleep without atonia,31,34,40–42 but these lesions may also damage neurons/axons responsible for REM sleep initiation and maintenance.43 Recent studies on subcoeruleus cells reported neurons excited by the cholinergic agonist carbachol (presumed to be REM-on cells) with low threshold spikes (LTS) and cells inhibited by carbachol, some with LTS, some with Ia current.44 Neurons in this region were depolarized by nicotinic and muscarinic agonists,45–47 although some cells were hyperpolarized by muscarinic agonists.48 Medial reticular neurons are also activated by excitatory amino acids,47,49 and injections of glutamate into the pontine inhibitory area also induce atonia.50 Projections to this area from the PPN may be both cholinergic and glutamatergic.51 Descending cholinergic projections of mesopontine neurons trigger the atonia of REM sleep, which is marked by hyperpolarization of motoneurons.52
1.3 Ascending Projections in the Adult
Comprehensive reviews of thalamic mechanisms involved in the control of changes in state are available.53,54 Basically, brief PPN stimulation induces a prolonged blockade of slow thalamic neuronal oscillations, promoting a short latency nicotinic activation and a long-lasting tonic (muscarinic) activation.23 Hyperpolarization in thalamic neurons induces reiterative LTS-mediated bursting activity, but, when depolarized, these cells assume tonic firing patterns. Glutamate contributes to this activation, perhaps via metabotropic receptors,53, 54 but both serotonin (5-HT) and noradrenaline can also dampen slow thalamic oscillations.55 Some ILT, specifically centrolateral, neurons were found to discharge very high frequency (800–1000 Hz) bursts during SWS. Interestingly, such bursts were superimposed on fast oscillations (20–80 Hz) triggered during depolarization.56 Stimulation of the PPN potentiates the appearance of fast (20- to 40-Hz) oscillations in the cortical EEG, outlasting stimulation by 10 to 20 seconds,57 indicative of the induction of prolonged responses eliciting changes in state by the ascending cholinergic arm of the RAS to the ILT, in particular, projections to the centrolateral and parafascicular nucleus (Pf).
Ascending PPN projections innervate all thalamic nuclei and the basal forebrain,20,58 some via collateral axons.59 Stimulation of the PPN (which leads to release of acetylcholine in the thalamus 60), or thalamic administration of cholinergic agonists, blocks spindles and delta waves (i.e., blocks SWS).61 Lesions of the PPN62,63 or pharmacologic blockade of PPN efferents 64,6 decrease or eliminate REM sleep and diminish waking. The fast cortical oscillations of waking and REM sleep are triggered as the PPN exercises a push-pull effect by depolarizing thalamocortical relay neurons via muscarinic activation of a potassium conductance23,27,66 to induce synchronization of fast rhythms, while hyperpolarizing reticular thalamic neurons to block spindles.61,67 Although most reports involve recordings of thalamocortical relay neurons in “specific” thalamic nuclei, the majority of PPN neurons actually project to the “nonspecific” ILT thalamus.2 These terminals form both symmetrical and asymmetrical synapses, suggesting that PPN input to the ILT thalamus is mixed. However, there is very little information on the synaptic relationships between the PPN and the ILT, especially during development.
We recently described the properties of developing neurons in one part of the ILT, the Pf.68 We found that Pf cells could be divided into types I and II, both of which differed from thalamic relay neurons in morphology and electrophysiology, particularly in having much lower incidence of LTSs. More recent studies showed that cholinergic input to Pf cells had both excitatory and inhibitory effects (65% of cells were hyperpolarized and 35% depolarized).69 These effects were directly on Pf neurons through activation of muscarinic and nicotinic receptors, but there was no major trend observed in the degree of hyperpolarization or depolarization during development. More type I cells were depolarized by the cholinergic agonist carbachol than type II cells, but both types were hyperpolarized to the same extent. M2 cholinergic receptors (AChR) appear to be responsible for all of the inhibitory modulation. However, the excitatory modulation requires the participation of M1 AChR, nicotinic cholinergic receptors (nAChR) and probably M3 or M5 AChR. Whole cell patch recordings showed that cells with prominent LTS or spikelets (indicative of electrical coupling, see below) tended to be excited by cholinergic agonists, but not all depolarized cells had LTS or spikelets.
1.4 Development and Maturation
The human newborn exhibits an even distribution of waking, REM sleep, and SWS, spending about 8 hours in each state.70 After birth, there is a gradual decrease in REM sleep from about 8 hours at birth to about 1 hour by 15 years of age, beyond which there is a small decrease until senescence.70 After birth, SWS may increase transiently, then gradually decrease from 8 hours per day to 6 to 7 hours per day by 15 years of age. The gain observed in total waking time, from about 8 hours at birth to about 16 hours at maturity, is mostly at the expense of REM sleep duration.
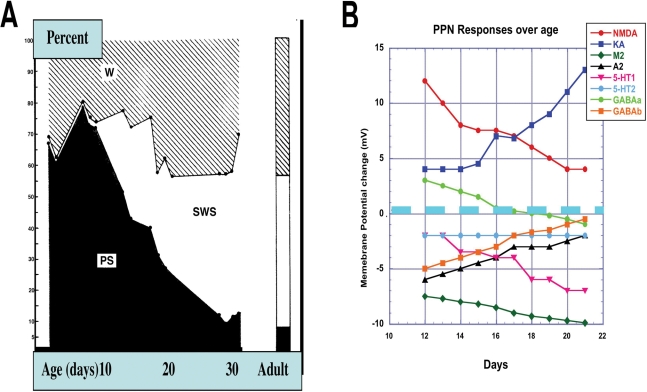
It has been suggested that REM sleep serves to direct the course of brain maturation.71 This is in keeping with the suggestion that activity-dependent development may direct neural connectivity throughout the brain.71,72 REM sleep could provide endogenous activation at a time when the brain has little or no exogenous input. High-frequency brainstem activation, especially in the form of PGO waves, could contribute to the maturation of thalamocortical pathways. If so, what is the role of REM sleep in the adult? Since there is a rebound in REM sleep after it is suppressed, REM sleep appears to be important for the adult. However, REM sleep does not appear to be essential for survival. When the stressful components of REM sleep deprivation are removed, REM sleep suppression is not fatal.73 In fact, tricyclic antidepressants, including imipramine, can lead to an absence of dreams (and reduction of REM sleep) without major consequences.7,74,75 Moreover, clonidine and monoamine inhibitors lead to complete blockade of REM sleep without major repercussions.76,77 In the rat, the decrease in REM sleep occurs between 10 and 30 days of age, declining from more than 75% of total sleep time at birth to about 15% of sleep time by 30 days of age.78 Figure 1A is modified from this early work and shows the dramatic decrement in REM sleep in the rat between 10 and 30 days of age. Recent studies found that REM sleep rebound after REM sleep deprivation in the developing rat was absent at 15 days of age, small at 21 days and larger at 30 days.79 That is, the ontogeny of REM sleep rebound was related to the ontogeny of baseline REM sleep. These data suggest that REM rebound is more important for the developed or adult animal.
Figure 1.
The developmental decrease in REM sleep and transmitter changes. A. Adapted from,78 to show the decrease in REM sleep from ∼80% of sleep time to ∼10% between 10 and 30 days in the rat, after which point it remains stable. B. Changes in the effects of transmitter systems on the membrane potential of PPN neurons. The y axis shows the mean depolarization above resting membrane potential (RMP, 0 mV) or hyperpolarization below RMP. Between 12 and 21 days, the same concentration of 1) NMDA was found to decrease from +12 mV to + 4 mV (red line)118; 2) KA was found to increase from + 4 mV to + 13 mV (blue line)118; 3) a cholinergic agonist increased hyperpolarization from −8 mV to −10 mV127; 4) a α2 adrenergic agonist decreased hyperpolarization from −6 mV to −2 mV121; 5) a 5-HT1 agonist increased hyperpolarization from −2 mV to −7 mV101; 6) a 5-HT2 agonist did not change the degree of hyperpolarization (-2 mV)101; 7) a GABAa agonist first depolarized (+3 mV) then hyperpolarized (-1 mV)131; and 8) a GABAb agonist decreased hyperpolarization from −5 mV to −1 mV.131
1.5 Development and Memory
Various authors have suggested that REM sleep plays a role in memory. One hypothesis suggests that dreams delete unimportant memories through inhibition of sensory information and suppression of movement.80 Others suggest a role for REM sleep in the consolidation of memories,81 that cognitive activity experienced during daily learning is “replayed” to promote long term storage.82,83 Other reports suggest a role for REM sleep in experience-dependent changes.84–86 This suggestion has been questioned because of (a) the absence of “replays” in REM sleep after the behavioral episode involving the novel task, (b) the absence of dream reports linked to recent experiences, (c) the lack of effect of REM sleep deprivation on consolidation, and (d) the lack of effects on consolidation by monoamine oxidase inhibitors that block REM sleep.87, 88
Moreover, cortical blood flow is increased during REM sleep in the brainstem and limbic areas, but decreased in frontal cortex.89 These findings suggest that metabolism decreases during resting sleep and increases during REM sleep in the brainstem but the frontal cortex remains depressed. This “hypofrontality” may be why there is a lack of critical judgment (a function of the working frontal cortex) during dreaming. The decreased blood flow to the frontal lobes may help deafferent the cortex, and may explain why dream content is accepted at face value. It is difficult to imagine how this helps consolidation of accurate memories.
Even if the memory consolidation hypothesis has some foundation, it would mean that the role of REM sleep is different after compared to before birth, emphasizing a dissociation across development. What memories does the fetus consolidate? An attractive theory of conscious perception suggests that thalamocortical projections from specific thalamic nuclei provide the “content” of sensory experience, while thalamocortical projections from non-specific (ILT) thalamic nuclei provide the “context,” and their coincidence leads to binding or conscious perception.90 Since there is no eye or ear opening before birth, it would seem that the “content” of memories to be consolidated by REM sleep would be highly reduced. Therefore, why is there a need for so much REM sleep? This would suggest that the majority of the activity to be consolidated in the fetus would be related to “context”. Therefore, a role for REM sleep in memory consolidation may not apply prenatally, even if it is a function in the adult.
1.6 Evolutionary Considerations
The theory of “advanced wakefulness” proposes that a sleeping mammal has its reptilian brainstem either awake or in a reduced activity state (presumably equivalent to mammalian SWS and REM sleep), but when it awakens it reaches a new state of awareness (cortical functionality). This agrees with what we know about thermoregulation and sleep, which shifts from waking control by the telencephalon (endothermic), to SWS control by the diencephalon (fall in set point), to mesopontine REM sleep control (virtually ectothermic).91 This is in agreement with the idea that the REM sleep patterns generated by the evolutionarily conserved RAS represent a preconscious state which, upon recruitment of the newly-evolved thalamocortical system, reaches full consciousness (waking).92 As mentioned above, the gains made in waking time are at the expense mostly of REM sleep, but also of SWS. This theory suggests that, as far as postnatal sleep time is concerned, phylogeny does reflect ontogeny.
In general, it would seem as if the most obvious explanation for the presence of “early” REM sleep would be to subserve a maturational process. In addition, there may be a reorganization of the role of REM sleep from “early” to “late” versions, such that the neurological mechanisms of REM sleep undergo drastic changes from birth to the end of puberty in the human.
2. MECHANISMS INVOLVED
2.1 REM Sleep Inhibition in Development
It has been suggested that there is an active REM sleep inhibitory process during the first 2 weeks of life in the rat,93 which may or may not be equivalent to the decrease seen in the human across puberty. This model predicts that (1) 1 or more inhibitory process becomes progressively stronger during this period (as outlined above, the PPN receives inhibitory serotonergic, noradrenergic, cholinergic and GABAergic inputs, all likely candidates) and (2) stimulation or blockade of this process will decrease or increase, respectively, the manifestations of REM sleep.79 A number of findings lend support to this idea by indicating that there are a number of changes in (a) the intrinsic properties of, and (b) the neurotransmitter inputs to, mesopontine cholinergic neurons during this developmental window of time. Recent findings suggest that electrical coupling in the RAS also may be involved in the developmental decrease in REM sleep.
2.2 Intrinsic Properties of Developing Mesopontine Neurons
Morphologically, PPN neurons change markedly from birth to 30 days of age in the rat. At birth, the mean cell area is ∼200 sq μm, which increases to > 500 sq μm by 15 days, then decreases to ∼300 sq um by 30 days.94 The hypertrophy observed in the PPN coincides with eye and ear opening at around 15 days of age in the rat.95,96 There is an increase in choline acetyltransferase activity during the developmental decrease in REM sleep percent in the rat,97 simultaneously with the hypertrophy described.
Physiologically, PPN neurons in vivo display both tonic and phasic activity in relation to waking and REM sleep.23 However, intracellular recordings of the guinea pig PPN in vitro show the presence of 3 types of PPN neurons, namely neurons with an LTS (type I), an A current (type II), and both A+LTS (type III).98 Most type II and III neurons were identified as cholinergic. Others99 have reported data from the neonate rat similar to those in the guinea pig, i.e., 3 types. Subsequent analysis confirmed the presence of type I and II neurons, 65% of type II (A) neurons were cholinergic, but only 36% of their type III (A+LTS) neurons were cholinergic.100
We found that type III (A+LTS) neurons were less evident after 17 days in the rat, apparently converting into type I (LTS) neurons,101 suggesting that there is a gradual increase across this stage in the number of PPN neurons with LTS properties, a mechanism that changes the dynamic pattern of activity in this region. LTSs are important calcium-dependent currents that activate (open) on depolarization and inactivate (close) with a slow time course.102,103 This allows a burst of action potentials to be generated, which is similar to the firing patterns of PGO-burst neurons found in PPN.28 The discrepancy in this view is that PGO waves are generated by cholinergic mechanisms,23 but PPN type I (LTS) cells appear to be noncholinergic. How and which PPN cells generate PGO bursts remains controversial. Another confounding factor may be related to species, as recent findings suggest that PGO bursting in the rat may not be as widespread as in other species.104
The other current described above is a voltage-gated, transient (outward potassium, IA) A current,101 which is activated when a neuron is depolarized after a hyperpolarization, and is an outward current which tends to counteract inward currents such as LTS if they occur in the same cells (e.g., type III). The A current displays a delayed return to baseline after hyperpolarization, is rapidly deinactivated, and thus serves to increase interspike interval and slow the discharge rate of these neurons.99 The presence of a high amplitude and long duration, calcium-dependent afterhyperpolarization in type II (IA) cells further limits spontaneous firing rate. Type II cells exhibit higher amplitude and longer duration afterhyperpolarizations than type I or III cells, and are likely to fire at lower rates. We found that, after 15 days, type II cells become segregated into two groups based on long vs short duration afterhyperpolarization.101 Type II neurons also showed significant decreases in action potential duration at approximately 15 days of age, again indicating a change in intrinsic firing properties at this time.101
To date, however, there is no agreement on how or if the types of firing patterns observed in vitro (type I, II, III) correspond to those seen in vivo (“REM-on,” Wake-on,” Wake/REM-on”). Moreover, while a number of intrinsic properties change between 12 and 21 days, it is not yet clear if any or all are responsible for the developmental decrease in REM sleep.
2.3 Inputs to Cholinergic Neurons
Any transmitter that modulates the expression of either A and/or LTS currents will have a dramatic impact on firing frequency and bursting behavior. PPN neurons have excitatory amino acid and GABAergic receptors with ionotropic effectors, and 5-HT, noradrenergic, cholinergic, adenosinergic and histaminergic receptors with a common metabotropic effector.8 Figure 1B summarizes the effects of each of these transmitter systems on the membrane potential of PPN neurons during the developmental decrease in REM sleep.
2.3.1 Serotonin
5-HT induces a tetrodotoxin-resistant hyperpolarization in most PPN neurons15,98 but inhibits only 25% of noncholinergic neurons.8 In the cat, inhibition of serotonergic transmission does not increase REM sleep.25 Moreover, 5-HT neurons in the dorsal raphe cease firing during the “atonic walking state,”105 but not during REM sleep without atonia,106 in keeping with evidence showing that injections of 5-HT into the mesopontine region suppressed REM sleep.107 Conversely, injection of a 5-HT1A agonist into the PPN did not affect PGO wave induction, and the PPN has few 5-HT1A binding sites compared to LDT.108 Earlier studies concluded that only about 12% of the 5-HT terminals in PPN synapse on cholinergic cells.49 5-HT2 receptors were found on cholinergic neurons,17 but others suggested that 5-HT2 receptors were found instead on noncholinergic neurons.109 These findings require more evidence to be reconciled in order to understand specifically how 5-HT modulates PPN function. Our results show that some PPN cells were hyperpolarized by a 5-HT2 agonist, but the effect does not change during the developmental decrease in REM sleep (Figure 1B, light blue).
A recent study described the inhibition of extracellularly recorded “REM-on” neurons in the mesopontine region by a 5-HT1A agonist, which had minimal effect on “Wake/REM-on” neurons.110 They suggested that 5-HT raphe neurons slow their firing in drowsiness and SWS, and less inhibition via 5HT1A receptors on cholinergic PPN “REM-on” cells leads to increased firing to promote REM sleep (see also 111). This suggests that some cholinergic PPN neurons will be inhibited while others will not be affected by 5-HT.
Using the 5-HT1 receptor agonist 5-carboxyamido-tryptamine, we showed that type I and III PPN cells aged 12 to 21 days were depolarized, hyperpolarized, or not affected in about equal numbers. However, 80% of type II PPN cells were hyperpolarized, but only 8% were depolarized, and depolarizing responses were evident up to only day 16, not thereafter.101 These results suggest that there is a reorganization of serotonergic input to the PPN such that (a) it is both excitatory and inhibitory, then purely inhibitory after approximately 15 days and (b) the 5-HT1 inhibition increases between 12 and 21 days (Figure 1B, magenta).
2.3.2 Excitatory Amino Acids
PPN neurons may receive glutamate inputs from the reticular formation,47,49 whereas some cholinergic cells also release glutamate.112 Responses in guinea-pig LDT neurons appear mediated by both n-methyl-d-aspartic acid (NMDA) and non-NMDA receptors.113,114 Injections of glutamate (which activates both NMDA and kainate receptors) into the PPN are known to induce increased duration of REM sleep and wakefulness in the rat.115,116 NMDA receptors appear to be involved in the induction of increased duration of wakefulness,115 whereas kainate receptors may be involved in the induction of increased duration of REM sleep.117
We found that, at day 12, most PPN neurons were strongly depolarized by NMDA, while the same neurons were only slightly depolarized by KA.118 The NMDA effect gradually decreased, whereas the KA effect gradually increased. By 21 days, PPN neurons were only slightly depolarized by NMDA, while the same neurons were strongly depolarized by KA (Figure 1B, crossing red and dark blue lines). These results indicate a switch in NMDA vs KA receptor activation of PPN cells at around 15 days.118 It is not clear if “Wake-on” neurons in the mesopontine region are preferentially driven by NMDA receptor activation while “REM-on” neurons are preferentially driven by KA-receptor activation, but this would be explained by the developmental changes reported. “Wake/REM-on” neurons may represent a subpopulation that is affected by both NMDA and KA-receptor activation.
2.3.3 Noradrenaline
Noradrenaline has been reported to hyperpolarize 7 to 15 day (i.e., during the first half of the developmental decrease in REM sleep) cholinergic mesopontine neurons in the laterodorsal tegmental nucleus (LDT),14 although similar studies have never been carried out in the PPN, or at later stages (15-30 days) of the developmental decrease in REM sleep. Moreover, α2-adrenergic receptor development is known to undergo marked changes in the LC and other mesopontine regions after 15 days of age in the rat.119 Interestingly, in adult cholinergic mesopontine neurons, only about one half showed α2-adrenergic receptor immunocytochemical labeling, whereas one third of cholinergic cells showed α1-adrenergic receptor labeling.120 These authors suggested that there may be differential activation of different populations of cholinergic cells that underlie aspects of behavioral state control. However, they did not perform triple labeling studies in order to determine if different populations of cholinergic cells are differentially modulated by the two receptor sub-types. This would determine if there is indeed differential adrenergic regulation of “Wake-Off/REM-On” vs “Wake-On/REM-On cells.”
Our results show that the α2-adrenergic receptor agonist clonidine hyperpolarized most cholinergic and non-cholinergic PPN cells.121 This hyperpolarization decreased significantly in amplitude from 12 to 21 days (Figure 1B, black line). However, much of these early effects (12–15 days) were indirect and non-cholinergic cells were less hyperpolarized than cholinergic cells. These results suggest that the α2-adrenergic receptor on cholinergic PPN neurons activated by clonidine may play only a modest role, if any, in the developmental decrease in REM sleep. However, clonidine blocked or reduced the hyperpolarization-activated inward cation conductance Ih, so that its effects on the firing rate of a specific population of PPN neurons could be significant.117
2.3.4 Acetylcholine
The PPN is modulated by cholinergic input.65 The cholinergic cells in the PPN have been hypothesized to be regulated, at least in part, by muscarinic (mAChR) and nicotinic (nAChR) cholinergic receptors located either on the soma and dendrites to modulate action potential discharge, or on pre-synaptic axon terminals to regulate neurotransmitter release.122,123 Studies utilizing in situ hybridization have multiply-labeled cells with choline acetyltransferase mRNA with those containing mRNA for the 5 subtypes of mAChRs (M1 - M5) in the PPN, and found that the cholinergic cells expressed primarily the M2 subtype, but also had detectable levels of M3 and M4 mRNA.123 However, it is not known if these were co-localized on the same cells, if they represented different populations of cells, or if the different subtypes were differentially located on the soma, dendrites and/or presynaptic terminals. PPN neurons receive cholinergic input from the contralateral PPN and bilaterally from the laterodorsal tegmental nuclei.124 Previous electrophysiological studies have demonstrated muscarinic inhibition of PPN neurons, but these were carried out only on the guinea pig, which has an adult-like sleep-wake cycle at birth.19,101
Recent work in the adult PPN using c-Fos expression showed that nicotine, when administered systemically, activated primarily the noncholinergic cells, whereas only a few cholinergic cells were labeled with c-Fos. The authors concluded that the PPN is a primary target for the action of nicotine and hypothesized that the non-cholinergic cells were directly activated by nicotine, which in turn modulate the activity of the cholinergic cells.125 When microinjections of nicotine were made in the medial pontine reticular formation of freely moving cats, REM sleep was induced.65 Compared to controls, these animals showed increased REM sleep at the expense of both waking and stage I slow-wave sleep, while also exhibiting a decrease in the time to REM sleep onset. Nicotine, injected intraperitoneally has been shown to increase waking in wild-type mice while also causing a significant decrease in non-REM and REM sleep during the first hour post-injection. However, in knockout β2−/− mice there was no change in state following similar injections, indicating that the alerting effects of nicotine are probably mediated by the β2-containing nAChRs.126 When nicotine was administered subcutaneously in an acute model, there was a dose-dependent decrease in REM sleep such that the highest doses (0.5 and 1mg/kg) suppressed REM sleep the most when compared to saline or lower amounts of nicotine. However, when nicotine was given repeatedly at low doses (0.1mg/kg) there was an increase in REM sleep following the third injection that lasted until the following day. It should be noted that these effects could be blocked by mecamylamine, indicative of a purely nicotinic effect. These studies show that nicotine may have differing effects on sleep architecture depending on route of administration, dosing and/or species of animal used.
Our results show that the nicotinic agonist 1,1-dimethyl-4-phenyl-piperazinium iodide (DMPP) depolarized PPN neurons early in development, then had dual effects before hyperpolarizing PPN neurons by the end of the period studied.127 Most of the effects of DMPP persisted following application of the sodium channel blocker tetrodotoxin, and in the presence of glutamatergic, serotonergic, noradrenergic, and GABAergic antagonists, but were blocked by application of the nicotinic antagonist mecamylamine. These results suggest that PPN neurons exhibit postsynaptic nicotinic receptors, but additional studies need to verify that this is not a tetrodotoxin-resistant effect. The mixed muscarinic agonist carbachol hyperpolarized all type II PPN cells and depolarized all types I and III PPN cells, but did not change effects during the developmental decrease in REM sleep. These effects persisted in the presence of tetrodotoxin but were mostly blocked by the muscarinic antagonist atropine, and the remainder by mecamylamine. These results suggest that PPN neurons exhibit postsynaptic muscarinic receptors, as previously shown. While the nicotinic and muscarinic inputs to the PPN may modulate the developmental decrease in REM sleep, the muscarinic inputs appear to modulate different types of cells differentially,127 increasing its inhibitory effects during the developmental decrease in REM sleep (Figure 1B, dark green line).
2.3.5 GABA
GABAergic neurons in the PPN are intermingled with cholinergic neurons128 and express c-fos during carbachol-induced REM sleep in the cat.39 In the cat, there is co-localization of GABA and acetylcholine in PPN cells, suggesting possible co-release by PPN neurons involved in the regulation of behavioral states.129 The PPN also receives GABAergic projections from the substantia nigra,2 which terminate mostly on noncholinergic neurons49 but also terminate on cholinergic neurons.130 Our findings suggest that GABAA-receptor activation leads to depolarization of PPN neurons early in development (12–16 days) but to hyperpolarization later (17–21 days) during the developmental decrease in REM sleep (Figure 1B, light green line).131 These effects persisted in tetrodotoxin and were assumed to be postsynaptic. The majority of depolarized cells early in development were non-cholinergic PPN cells, whereas most cholinergic PPN cells were hyperpolarized in both early and later periods. GABAb receptor activation hyperpolarized both cholinergic and noncholinergic PPN neurons early and less so in later periods (Figure 1B, orange line). GABA, the primary inhibitory influence in the adult brain, was found to be excitatory during development due to the delayed expression of chloride co-transporters.132 Our results suggest that PPN neurons, especially non-cholinergic cells, show depolarization early in development shifting to hyperpolarization later during the developmental decrease in REM sleep. We do not know if the differential responses in cholinergic neurons (i.e., mostly hyperpolarization with few cells depolarizing) are due to earlier maturation of chloride transporters in cholinergic compared to non-cholinergic cells, or to other factors. This developmental shift occurred only in response to the GABAa agonist muscimol and not to the GABAB agonist baclofen. It is unknown if a delay in maturation of chloride transporters in PPN cells is limited to channels activated by GABAA and not to GABAB receptors.131
In summary, our pharmacologic findings suggest that there is a developmental shift around 15 days of age in the rat, during the most dramatic decrease in REM sleep duration, towards increased 5-HT1 inhibition, decreased NMDA excitation and increased KA activation, decreased noradrenergic inhibition, and increased cholinergic and GABAergic inhibition of PPN neurons (Figure 1B).
2.4 Electrical Coupling
Electrical coupling in the mammalian brain was first described in the 1970s, with connexin 36 (Cx36) being the only gap junction protein in neurons.133 Connexins oligomerize into hemichannels and are transported to the membrane, where they can be unapposed or apposed, congregate in ‘formation plaques,’ allow the passage of ions and molecules as large as cAMP, and have a half-life of several hours.134,135 Cx36 gap junctions are the least voltage dependent136 and are closed by low intracellular pH and low intracellular calcium.137 Spikelets are stereotypical, usually rhythmic, subthreshold depolarizing potentials thought to reflect firing in the coupled neurons.136 Electrical coupling138–141 and Cx36 labeling142 are present in the reticular nucleus of the thalamus. Electrical coupling is also evident in thalamic relay neurons, but only in the presence of metabotropic glutamate receptor agonists.136 Electrical synapses appear mainly between GABAergic neurons in (a) the thalamic reticular nucleus and may promote synchronization of burst firing,41 (b) the cortex and may enhance the synchrony of gamma oscillations 143–146 (Cx36 knockout mice show disruption of gamma frequency147), and (c) hippocampus, where they may promote seizure activity148,149 and where cholinergic agonist carbachol induces gamma oscillations.150 Does Cx36 in the RAS modulate the manifestation of gamma or lower frequency oscillations with carbachol? If so, gap junctions may play a critical role in the modulation of waking and REM sleep, states marked by high frequency EEG.
2.4.1 Electrical Coupling in the RAS
Gap junctions can be blocked through membrane fluidization such as that induced by the anesthetics halothane and propofol.151,152 Oleamide promotes sleep and blocks gap junctions.153,154 Anandamide enhances adenosine levels to induce sleep155 and blocks gap junctions.151 One possibility is that a mechanism by which these agents may induce sleep and/or anesthesia is through blockade of electrical coupling in the RAS. Carbenoxolone, a putative gap junction blocker, decreases the synchronicity of gamma oscillations,156 as well as seizure activity.157 18-glycyrrhetinic acid is a glycyrrhetinic acid derivative that blocks gap junctions.158 Quinine and a related compound, mefloquine, also block gap junctions.159–162 Mefloquine is particularly useful because it blocks Cx36 and Cx50 gap junctions at low concentrations but not Cx43, Cx32, or Cx26 gap junctions.161 On the other hand, increased electrical coupling can be induced by low calcium aCSF and trimethylamine, a gap junction opener, perhaps by increasing intracellular pH.150,158,162
Modafinil is approved for use in treating excessive sleepiness in narcolepsy, sleepiness in obstructive sleep apnea, and shift work sleep disorder and is also prescribed in a number of neuropsychiatric conditions. Many publications on this agent acknowledge that the mechanism of action of modafinil is unknown, although there is general agreement that it increases glutamatergic, adrenergic and histaminergic transmission and decreases GABAergic, transmission.163 Recent imaging studies using voltage-sensitive dyes have shown that inhibitory interneurons modulate cortical activation by afferent input.164 Moreover, cortical interneurons exhibit gamma band (∼ 40Hz) oscillations144 that are reduced by pharmacologic blockade of gap junctions. The presence of both electrical coupling and chemical synapses between inhibitory interneuron networks are thought to enhance the timing of action potentials.133,135,145,146 In the cortex, electrical coupling may contribute to action-potential synchronization and network oscillations, to coordination and reinforcement of IPSPs, and to coincidence detection in inhibitory networks.144,165 In the olfactory bulb, the spike-bursting activity in simultaneously recorded pairs of external tufted cells is synchronized by coincident EPSPs, IPSPs, and spikelets, suggesting that both synaptic transmission and gap junction coupling coordinate the spontaneous bursting of external tufted cells.166 There is extensive electrical coupling in the cortex during development,167 but epileptiform activity is virtually absent. Therefore, electrical coupling may result in a “shunting effect” by decreasing the input resistance of coupled cells, thereby reducing the excitability of cortical interneurons. Such a “shunting effect” has been proposed as the mechanism behind the general absence of epileptiform discharges during early postnatal development of the rat neocortex.168
In a landmark study, modafinil was recently found to increase electrical coupling between cortical interneurons, thalamic reticular neurons, and inferior olive neurons.169 Following pharmacologic blockade of connexin permeability, modafinil restored electrotonic coupling. The effects of modafinil were counteracted by the gap junction blocker mefloquine. These authors proposed that modafinil may be acting in a wide variety of cerebral areas by increasing electrotonic coupling in such a way that the high input resistance typical of GABAergic neurons is reduced. These authors proposed that this “shunting effect” of modafinil may activate the whole thalamocortical system by mildly downregulating inhibitory networks while increasing synchronous activation of both interneurons and noninhibitory neurons.169
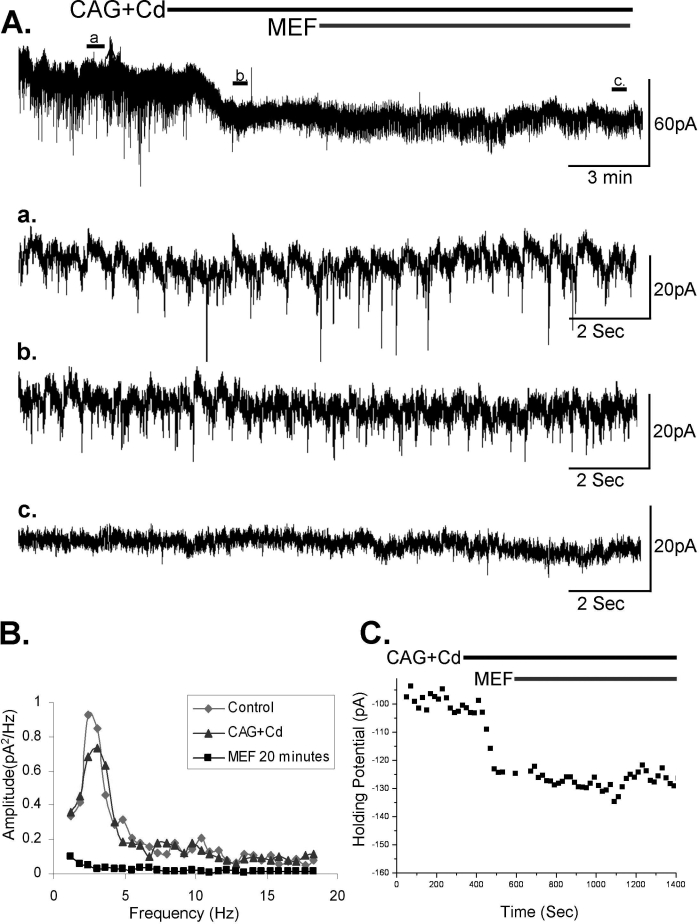
We recently described the presence of dye and electrical coupling in the RAS, specifically in the Pf, PPN and subcoeruleus.170,171 We also found that modafinil decreased the resistance of PPN and subcoeruleus neurons, in keeping with results in the cortex, reticular thalamus, and inferior olive.169 The effects of modafinil were evident in the absence of changes in resting membrane potential or of changes in the amplitude of induced EPSCs, and were blocked by low concentrations of the gap junction blocker mefloquine, also in the absence of changes in resting membrane potential or in the amplitude of induced EPSCs.169,171 This suggests that these compounds do not act indirectly by affecting voltage-sensitive channels such as potassium channels, but rather modulate electrical coupling via gap junctions. Figure 2 shows that the cholinergic agonist carbachol induced oscillations in subcoeruleus cells at specific frequencies within the theta range. To date, higher frequencies of oscillations have not been observed using carbachol alone. This figure also shows that such oscillations were evident spontaneously, could be better distinguished by fast synaptic blockade (indicating mediation by electrical coupling) and blocked by the gap junction blocker mefloquine (without changing resting membrane potential). Mefloquine can block potassium channels, but if that were the case, it would lead to an inward current and increase spikelets, not decrease them. These findings in general suggest that increasing electrical coupling may promote states of synchronization of sleep-wake rhythms, thus controlling changes in state. The presence of coincident rhythmic IPSPs, especially during cholinergic receptor activation, suggests that a syncitium of inhibitory GABAergic neurons is present in the Pf, PPN and/or subcoeruleus, helps generate synchronous oscillations (i.e. ensemble activity, especially in the theta range). Electrical coupling appears to modulate oscillations in different cell types in each structure, suggesting a role for electrical coupling in sleep-wake control.
Figure 2.
Spontaneous spikelets and oscillations in a SubC neuron that were blocked by mefloquine (MEF).171 A. Voltage clamp recording (HP=-50mV) with spontaneous spikelets and excitatory post-synaptic currents (EPSCs). Expanded record at a. depicts both spontaneous EPSCS and underlying spikelets in this cell. Expanded record at b. shows the inhibition of EPSCs following the addition of fast synaptic blockers (CNQX, APV, gabazine, abbreviated as CAG, and the calcium channel blocker cadmium, abbreviated as Cd). Note the continued presence of rhythmic spikelets. Expanded record at c. depicts a complete cessation of PSCs and spikelets after the addition of mefloquine (25 uM). B. Power spectrum analysis showing the rhythmicity present in this cell at baseline (Control, gray diamonds), after CAG+Cd (blue triangles) and after mefloquine (MEF, black squares). Note the decrease in power after the addition of CAG+Cd suggesting that at least some of the previously observed PSCs were also synchronized at this frequency. C. Holding potential necessary to maintain this cell at −50mv. Note the lack of change in holding potential after the addition of MEF, suggesting that this agent does not affect ionic conductances.
We should emphasize that gap junctions are not necessary for generating subthreshold oscillations, rather they are required for clustering coherent oscillatory activity.172 Oscillatory properties of single neurons endow the system with important reset dynamics, while gap junctions are mainly required for synchronized neural activity.173 For example, oscillations in single inferior olive neurons persisted over a limited range of voltages in the presence of gap junction blockers, leading one lab to conclude that gap junctions allow oscillations to persist over a wide range of membrane potentials making their frequency independent of membrane potential.173 This is in keeping with the suggestion that the output from a syncitium of electrically coupled cells may maintain oscillations over a wide range of voltages that would otherwise inactivate rhythmic conductances in single neurons.173 Note: As far as the central nervous system is concerned, the term syncitium has been most often used to describe the network of coupled astrocytes. The linked cells have been found to propagate oscillatory calcium waves over long distances, perhaps representing a long-range signaling system.174 At present, we do not know if the electrically coupled cells in the RAS form a network or exist as clusters of cells.
2.4.2 Connexin 36
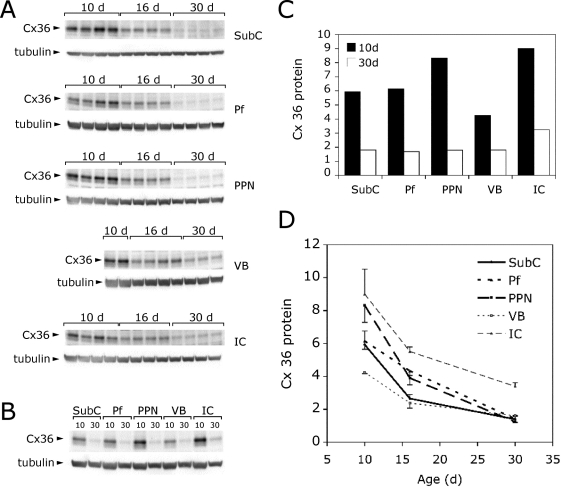
We sampled each of the nuclei of interest for Cx36 protein using 400-μm slices like those used for recordings, and punched 1 mm of Pf, PPN, and subcoeruleus (without including locus coeruleus) on days 10 and 30, spanning the developmental decrease in REM sleep. We also sampled a nearby nucleus, the inferior colliculus, and another nucleus unrelated to sleep-wake control, the ventrobasal thalamus. Figure 3 shows that Cx36 protein levels at the end of the developmental decrease in REM sleep (day 30) were about 25% of those at day 10 in Pf, PPN and subcoeruleus. This suggests the presence of a marked developmental decrease in Cx36 protein levels, with considerable amounts of Cx36 protein still present in the adult, suggesting that this gap junction protein may participate in developmental regulation and contribute to sleep-wake control in the adult. Cx36 protein levels in the inferior colliculus were higher than in the RAS, and also decreased by about one third, but levels at 30 days were higher than in any structure sampled. The ventrobasal thalamus initially showed low levels that decreased by half by 30 days. These studies suggest that there is a developmental decrease in Cx36 protein levels during this time across many regions, but the degree of decrement observed differed between regions.170,171
Figure 3.
Developmental changes in Cx36 protein expres-sion.170,171 A. Western blots comparing Cx36 protein levels at different ages. 15 ug of protein was loaded in each lane. Each sample contains punches from 2-3 littermates. Blots were stripped and re-probed with anti-β tubulin antibodies to verify equal protein loading. All brain regions showed a decrease in Cx36 protein during development. B. Western blot comparing Cx36 expression in different brain regions. Equal amounts of protein from the samples in A were pooled and run on a single gel to allow comparisons to be made between brain regions. The blot was stripped and re-probed for β tubulin as in A. The PPN and inferior colliculus (IC) had the highest levels of Cx36 in 10 day pups, whereas the ventrobasal thalamus (VB) had the lowest. C. Chart showing quantification of data in B. Cx36 protein was quantified and normalized to β tubulin. At 30 days, Cx36 levels in all three nuclei of the RAS dropped to 25% of the level at 10 days. In contrast, VB dropped to 50% and the IC to 30%. D. Chart showing quantification of data in A and B. Cx36 protein was quantified and normalized to β tubulin. Error bars show standard deviation. Cx36 levels decreased at different rates in different brain regions, IC- inferior colliculus, Pf- parafascicular nucleus, PPN- pedunculopontine nucleus, SubC- subcoeruleus nucleus, VB- ventrobasal thalamus.
Our previous study on the SubC only, used real-time polymerase chain reaction to show that both Cx36 mRNA expression as well as protein levels decreased during the developmental decrease in REM sleep in a few samples.171 A similar analysis in the other nuclei remains to be performed. The mechanism that regulates the expression of Cx36 also remains to be investigated. Since the level of Cx36 mRNA decreases over age, regulation of Cx36 protein levels is probably at the level of transcriptional control. Changes in intracellular signaling need to be investigated and how these impact the Cx36 promoter. Other studies need to manipulate Cx36 expression levels and assess the resulting changes in REM sleep drive, thus determining if the developmental decrease in REM sleep is driven in whole or in part by a decrement in Cx36.
A recent study of Cx36 knockout (KO) mice showed that gap junctions in the inferior olive were mostly abolished, but the genetically uncoupled neurons still generated subthreshold oscillations of their membrane potential at a similar amplitude and frequency as those recorded in the wild-type.177 This implies that inferior olive oscillations may be generated by conductances of individual neurons, however, these authors suggested the alternative explanation that oscillations of Cx36 KO animals are qualitatively different from those in wild-type mice, and that these differences are due to structural and physiological changes in the Cx36 KO mouse. That is, single cell oscillations may occur in such Cx36 KO cells as a result of compensatory ionic mechanisms not encountered in the normal animal.177 Others showed instead that oscillatory properties of uncoupled inferior olive neurons are not caused by long-term compensatory changes but are due to the single-cell properties of normal inferior olive cells.173 Thus gap junctions are required for the synchronizing the oscillatory responses of neurons and for clustering of coherent rhythmic activity.
What functional significance can be accorded to electrical coupling if Cx36 KO mice survive, sleep, and walk? Several studies showed that while gross motor activity patterns appeared normal in the Cx36 KO mouse, detailed analysis of motor patterns showed a 10- to 20-millisecond degradation in coordination,178 and a delay of more than 20 milliseconds in the optokinetic reflex.179 These differences appear vital to survival. In terms of consciousness and sleep, 2 laboratories have found that cortical gamma oscillations in vitro are impaired in Cx36 KO mice,148,180 which was reviewed by Buszaki.181 A later study from this lab on Cx36 KO mice showed that Cx36 gap junctions contributed to gamma oscillations,182 whereas others showed that gap junctions play a role in learning and memory.183 All of these studies taken together suggest that gap junctions confer an advantage in timing, probably due to their ability to promote coherence in brain rhythms for optimal performance. The unique ability of coupled cells to maintain synchrony across a wide range of membrane potentials173 probably allows brain rhythms to persist for longer periods without waning.
Great care is needed, however, in the interpretation of findings in Cx36 KO animals, since compensation by other isoforms of connexins is present in other tissues,184 some brain cells can compensate for the absence of cell coupling,185 and novel AMPA currents are absent in these animals.186 On the other hand, these animals have potential for exploring developmental changes since preliminary findings suggest that thalamic relay neurons (TRNs) upregulate gap junctions in development, presumably to compensate for changes in membrane properties in order to maintain the strength of electrical coupling.187 Moreover, gap junction expression appears to undergo changes related to sensory input, switching from purely electrical to electrical and chemical transmission with age.186
2.4.3 System Schematic
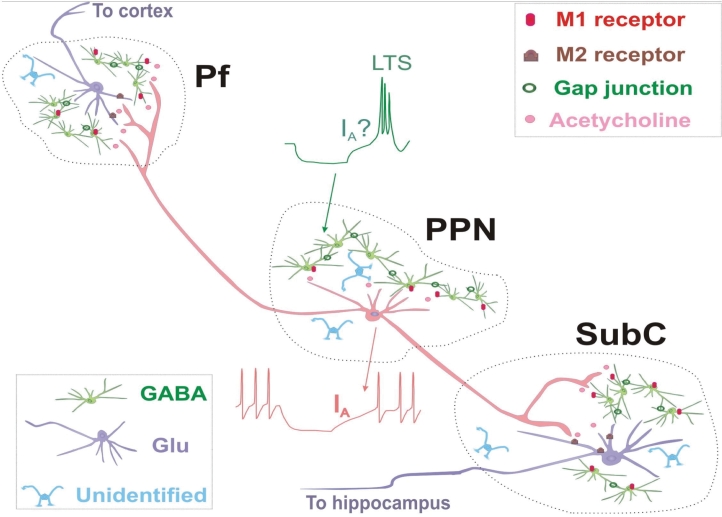
We hypothesize that the Pf, PPN, and subcoeruleus are organized similarly to the specific relay and reticular nuclei of the thalamus (Figure 4). TRNs in specific nuclei project to the cortex and send collaterals to inhibitory GABAergic interneurons (which are electrically coupled) in the reticular nucleus that in turn inhibit TRNs and local circuit neurons. Cortical projections return to the TRNs, local circuit cells, and to inhibitory GABAergic interneurons. Ascending cholinergic input from the PPN excites TRNs and inhibits reticular cells. The manner in which this circuit generates thalamocortical oscillations was reviewed recently.139 In figure 4, for SubC, our data suggests that descending projections from the PPN excite, possibly via M1 muscarinic receptors, at least some GABAergic, coupled interneurons with LTS (could be REM-on cells44), and inhibit, possibly via M2 muscarinic receptors, subcoeruleus output neurons (perhaps glutamatergic188) and perhaps some LTS neurons (unknown transmitter, could be PGO-burst cells44). In turn, GABAergic interneurons may inhibit output neurons (we have little evidence on local circuit cells in subcoeruleus). This circuit may be involved in generating oscillations characteristic of REM sleep. In Pf, we speculate that ascending projections from the PPN excite (perhaps via M1) GABAergic coupled interneurons and inhibit (perhaps via M2) Pf output neurons (could be glutamatergic). In turn, GABAergic interneurons inhibit these output neurons (and local circuit). This circuit may help generate thalamocortical oscillations related to arousal. In PPN, we found that cholinergic inputs inhibit type II mostly cholinergic PPN, perhaps output, neurons (M2?) and excite (M1?) type I (LTS) and III (IA+LTS) mostly noncholinergic (could be GABAergic, some of them coupled) neurons. GABAergic interneurons may inhibit output neurons. This circuit may help generate rhythms related to waking and REM sleep. While the known transmitter systems may serve to regulate the firing patterns of RAS neurons, electrical coupling may provide the ensemble activation to propagate those rhythms, especially at higher frequencies. If there is a decrease in electrical coupling, it is the generation of coherence in these fast rhythms that is most impaired.144 Such a decrement would be expected to lead to a decrease in arousal and performance levels. Conversely, an excess of electrical coupling would be expected to lead to increased vigilance and REM sleep drive.
Figure 4.
System Schematic.170,171 We hypothesize that the Pf, PPN and SubC are organized similarly to the specific relay and reticular nuclei of the thalamus. Thalamic relay neurons (TRNs) in specific nuclei project to the cortex and send collaterals to inhibitory GABAergic interneurons (which are electrically coupled) in the reticular nucleus that in turn inhibit TRNs and local circuit neurons. Cortical projections return to the TRNs, local circuit cells, and to inhibitory GABAergic interneurons. Ascending cholinergic input from the PPN excites TRNs and inhibits reticular cells. The manner in which this circuit generates thalamocortical oscillations was reviewed recently.139 For SubC, our data suggests that descending projections from the PPN excite, possibly via M1 muscarinic receptors, at least some GABAergic, coupled interneurons with LTS (could be REM-on cells), and inhibit, possibly via M2 muscarinic receptors, SubC output neurons (perhaps glutamatergic) and perhaps some LTS neurons (unknown transmitter, may be PGO-burst cells). In turn, GABAergic interneurons may inhibit output neurons (we have little evidence on local circuit cells in SubC). This circuit may be involved in generating oscillations characteristic of REM sleep. In Pf, we speculate that ascending projections from the PPN excite (perhaps via M1 receptors) GABAergic coupled interneurons and inhibit (perhaps via M2 receptors) Pf output neurons (may be Glutamatergic). In turn, GABAergic interneurons inhibit these output neurons (and local circuit). This circuit may help generate thalamocortical oscillations related to arousal. In PPN, we found that cholinergic inputs inhibit type II mostly cholinergic PPN, perhaps output, neurons (via M2 receptors) and excite (via M1 receptors) type I (LTS) and III (IA+LTS) mostly non-cholinergic (may be GABAergic, some may be coupled) neurons. GABAergic interneurons may inhibit output neurons. This circuit may help generate rhythms related to waking and REM sleep. In support of this model, we have considerable evidence showing that virtually all of the coupled neurons in SubC are GABAergic. We also showed that some coupled neurons are depolarized by CAR and most of these are GABAergic, and others are hyperpolarized by CAR and most of these are non-GABAergic. A few non-coupled GABAergic cells are hyperpolarized by CAR, i.e. there may be two populations of GABAergic cells, one coupled, the other not. These suggestions lay the groundwork for the exploration of a novel mechanism for sleep-wake control based not only on known transmitter interactions, but also on superimposed ensemble activation provided by electrical coupling.
As far as the developmental decrease in REM sleep is concerned, it seems more likely that the abundant REM sleep drive present early in development is more related to high levels of electrical coupling (than to changes in chemical transmission), providing increased ensemble activity leading to persistence of high frequency rhythms essential for waking and REM sleep. With a decrement in gap junction levels, ensemble activity would decrease. This speculation needs to be tested by manipulating gap junction levels. Unfortunately, KO animals may compensate in a number of ways (see above), so that knock-in technology may be required to address the issue. If selective Cx36 gene upregulation can be accomplished, adult animals should show increased arousal levels, i.e., hypervigilance, and increased REM sleep drive, with concomitant increases in total REM sleep such as in the early postnatal state.
3. DYSREGULATION IN THE DECREASE IN REM SLEEP
3.1 Thalamocortical Dysrhythmia
The ILT is highly relevant because it is thought to be involved in motor function, receiving input from the motor and premotor cortices, the globus pallidus and brainstem (cochlear-vestibular, solitarius), and projecting heavily to the striatum and associative limbic cortex.189–195 Portions of the ILT undergo significant degeneration in Parkinson disease,196 and are involved in the modulation of pain, auditory and respiratory responses, among others.197,198 The “nonspecific” ILT system is thought to allow sensory input to access the machinery that generates conscious experience, i.e., the thalamocortical 40 Hz rhythm.199 If there is a mismatch between the specific thalamic afferent input and the non-specific thalamic input to cortex, misperception or aberrant “binding” of sensory perception is to be expected. Thalamocortical dysrhythmia, manifested as increased low-frequency theta rhythmicity (instead of normal alpha rhythm), is evident in patients with such disparate disorders as psychosis, neurogenic pain, tinnitus, Parkinson disease, and depression.200 This suggests that all of these conditions a) may be generated by disturbance in the same mechanism but in slightly different cell groups within the ILT, and b) could be ameliorated by surgical lesion of cell groups and blocking such dysrhythmia at the level of the ILT, specifically, portions of the centrolateral.201
Early painful experience leads to chronic changes in pain threshold as well as attentional and cognitive deficits.202,203 A model of early somatic pain (paw formalin injection) leads to sensory and behavioral disturbances in adulthood in all those treated,202 whereas a model of early visceral pain (colonic distention) leads to such deficits in most of those treated.204 Pf neurons recorded from the somatic pain model displayed LTS activity in 95% of cells instead of the normal 18%. Pf neurons recorded from the visceral pain model displayed LTS activity in 65% of cells. Thus, early pain experience appears to induce LTS activity in Pf neurons that normally do not exhibit LTS, which may explain lasting changes resulting from early somatic or visceral pain.205 This may represent the first animal model of thalamocortical dysrhythmia.
Assuming that increased LTS is involved in thalamocortical dysrhythmia, the surgical approach, albeit successful at least in some patients,202 may be replaced by treatments that increase the frequency of oscillations, e.g. from theta to alpha. One such method would be by increasing electrical coupling to drive higher frequency activity, i.e. by using agents that increase coupling such as modafinil. This agent is being tested in a number of the disorders associated with thalamocortical dysrhythmia.
3.2 Disorders of Arousal and REM Sleep Drive
Whether or not some or all of the potential functions of REM sleep discussed above turn out to be correct, the fact remains that certain sleep pathologies have a developmental etiology, and a number of severe disorders are marked by a virtually permanent developmental increase in vigilance and REM sleep drive. That is, certain states are characterized by increased levels of high frequency rhythms in both waking, which are diagnosed as hypervigilance, and during sleep, which manifests during REM sleep as increased drive. In schizophrenia, anxiety disorders and bipolar and unipolar depression, increased vigilance and REM sleep drive (increased REM duration, decreased REM latency, hypervigilance, etc., usually coupled with decreases in SWS) is a major, incapacitating symptom.206,207,208 Changes in REM sleep parameters appear to be relatively specific abnormalities in depression.209 During manic episodes, when high frequency rhythms would be expected, bipolar patients show increased REM sleep drive, but if they instead manifest hypersomnia, when low frequency rhythms would be present, there are no signs of increased REM sleep drive.209 Most patients with schizophrenia, bipolar depression, male obsessive-compulsive disorder, and panic attacks develop the disorder postpubertally (∼80% between the ages of 15 and 25, during the normal decrease in REM sleep shown in humans69), whereas unipolar depression in adolescents is very high.203,206 Interestingly, approximately 80% of narcoleptic patients also have a postpubertal age of onset. Narcolepsy is characterized by sudden shifts into unconsciousness and atonia, similar to arousal-triggered episodes of REM sleep. We hypothesized that, if the developmental decrease in REM sleep does not occur or is reduced, lifelong increases in vigilance and REM sleep drive would ensue.8 One group reported that neonates and endogenous depressives have the same distinctive features of baseline REM sleep,90 and suggested that the REM sleep abnormalities of endogenous depression represent an immature, underdeveloped REM sleep system.210 Is it possible that the increased REM sleep drive in these disorders represents a regression to a more abundant REM sleep state such as that seen soon after birth? In general, the increased REM sleep drive in the disorders mentioned may represent a developmental disturbance of the REM sleep inhibitory process,89 which normally tends to reduce REM sleep drive, perhaps via several mechanisms. One obvious mechanism that needs investigation is the action of hormones on this system.
3.3 Gonadal Steroids
In general, estrogen and testosterone have an excitatory effect on arousal and attention in females and males, respectively. There is considerable evidence suggesting that exogenous administration of estradiol in women will increase cognitive performance and decrease reaction time.211 A similar effect is observed after androgen therapy in men, which also shortens sleep time.212 Anabolic steroids lead not only to increased muscle size and therefore strength, but also to decreased reaction time and increased attention that athletes call “being in the zone.”213 How can gonadal steroids affect brain functions related to gamma band activity such as arousal and attention? If gonadal steroids induce increased electrical coupling, the effect could resemble that of modafinil, i.e., increasing arousal and attention by general disinhibition and RAS upregulation. Given the advantages in timing conferred by electrical coupling, it would not be surprising if anabolic steroids and other agents that increase coupling would also decrease reaction time, allowing for superior performance in sports. On the other hand, excessive use of anabolic steroids is known to lead to psychotic symptoms, including increased vigilance and REM sleep drive (hallucinations), suggesting that overinduction of coupling could lead to dysregulation of sleep-wake states, arousal and attention. These findings also suggest that the reported effects on cognition of gonadal steroid therapy (postmenopause, sexual dysfunction, etc.) could be due to increased electrical coupling in the brain, perhaps promoting longer lasting synchrony, especially of gamma band activity.
In vitro, gonadal steroids are known to increase gap junction expression in a number of somatic cell lines and cultures. In neurons, they alter neurite outgrowth, neuritic spine development, synaptogenesis and increase gap junctions in certain brain regions.214 Spinal cord neurons in sexually dimorphic nuclei loose gap junctions after castration or ovariectomy that return with exogenous androgen or estrogen treatment, respectively.215,216 Estrogen increases Cx36 mRNA expression in the suprachiasmatic nucleus but not the cortex.217 These results suggest that gonadal steroids increase neuronal gap junction expression and probably increase electrical coupling, an effect diminished by castration or ovariectomy. Because we all experience increases in exposure to gonadal steroids during puberty, there must be additional factors that trigger the manifestation of these disorders, therefore, much additional research in this area is needed.
There are 2 estrogen receptors, α and β. The 2 estrogen receptors are expressed in different patterns and at different levels throughout the brain,218 but the expression in the individual nuclei of the RAS has not been described. The androgen receptor is present in a portion of PPN neurons,219 but its presence in the subcoeruleus and Pf has not been described. There are several ways that estrogen could regulate Cx36 expression. The mouse Cx36 promoter220 has an imperfect palindrome with remarkable similarity to the consensus estrogen response element. Although no characterized estrogen response elements are identical to the putative element in the Cx36 promoter, all mismatches are found in other estrogen response elements.221 The putative estrogen response element is conserved in the human Cx36 promoter suggesting that estrogen/estrogen receptor regulate Cx36 expression directly. There are also Sp1-like sites that could synergize with multiple estrogen response element half sites nearby.218 Alternatively, the estrogen receptor could interact with AP1 at the AP1 site in the Cx36 promoter.220,222 If, indeed, gonadal steroids modulate gap junction expression and dysregulation of this mechanism occurs, increased Cx36 function may lead to unwanted synchronization promoting a state of excessive vigilance and increased REM sleep drive.
The evidence presented herein raises exciting possibilities for some of the cellular and transmitter-related changes that might be responsible for the development of the adult REM sleep control system. These considerations help explain how one of our most essential, evolutionarily-conserved systems develops into its predictable adult form, and perhaps how disturbances in its development can explain a number of disorders with devastating circumstances. Future studies will also be essential in determining how such disturbances result in life-long changes in the function of this system. The development of more effective therapeutic strategies for the disorders mentioned is dependent on detailed information to be gained from investigation of these critical periods.
4. ADULT VIGILANCE
If the bountiful REM sleep state in the newborn reorganizes into a limited episodic state, what function or functions are subserved by the adult process? A number of theories for the role of REM sleep have been advanced.2,91,92 We suggest that a major role of REM sleep is related to a constant mechanism of recurring vigilance spanning the entire day. Studies by Kleitman223 concluded that the periodicity of the REM state reflects the operation of a “basic rest-activity cycle” (BRAC), which continues to modulate brain function during waking, i.e. an ultradian BRAC of approximately 90-minute duration. He showed that the sleep-wake cycle of the human neonate was marked by increased activation every 50 to 60 minutes that lengthened gradually to the 90-minute cycle in the adult. Studies recording gross body movements in adults found that subjects showed periods of spontaneous activity every 90 to 120 minutes during sleep and wakefulness (reviewed in224). Other studies have shown a similar periodicity in eye movements, muscle tone and EEG patterns,225 as well as temperature and behavioral measures226 and errors in motor task performance,222 verbal and spatial tasks,226 and other measures.227–234 More recent studies showed that delta wave activity in the electroencephalogram, adrenocorticotropic activity and heart rate variability are coupled at 90 to 110 minutes.235 Similar BRACs have been observed in other species such as primates (REM sleep and electromyogram cycles around 66 minutes236), felines (REM sleep237–239 and operant performance240 cycles around 25 minutes), and rodents (REM sleep cycles from 8–17 minutes241–243). There appears to be a linear relationship between the log of the body weight and the log of the period or amplitude of related measures of arousal.206 Results from our labs have shown that the sleep state-dependent P50 potential in the human, which has been proposed to be an expression of ascending cholinergic activation of ILT (reviewed in208), shows approximately 90-minute cycles in the peak amplitude of this “preattentional/arousal” measure.244 The magnetic equivalent of the P50 potential in the human, the M50, has been shown to localize to fronto-parietal regions, not primary auditory temporal cortex, where the P50 potential exhibits its highest amplitude.245 The rodent equivalent of the human P50 potential, the P13 potential, also was found to show a periodicity in peak amplitude, but at a 13- to 16-minute period, similar to the rodent REM sleep-cycle duration.244
These observations suggest that there is a cyclical endogenous activation of arousal-related systems. That is, the early form of REM sleep contributing to the maturation of thalamocortical pathways may persist as a REM-sleep and vigilance drive in the adult. These findings suggest that we require a periodic shot of vigilance, becoming more alert every 90 minutes. What is the function of a BRAC that transcends the sleep-wake cycle and is expressed as timed oscillations of activity during waking, and as REM sleep periods during sleep? The possibilities include metabolic variation, pacemaker-like activity and/or a fatigue-recovery phenomenon.223 We proposed that one such purpose may be to ensure periodic increases in blood flow in conjunction with increased arousal.8 PPN neurons have high concentrations of the vasodilating agent, nitric oxide, and contain nitric oxide synthase, which is synonymous with NADPH diaphorase. Wherever PPN projects, it can be assumed to vasodilate. The transient increases in blood flow to the frontal lobes during REM sleep in the adult may be present only at the beginning of the REM sleep episode, while continued REM sleep appears to lead ultimately to “hypofrontality.” Another possibility is that the BRAC is related to gap junction expression, turnover and/or exteriorization, which may be reflected in periodic increased shunting of coupled GABAergic neurons and concomitant disinhibition. Other functions subserved by this state, beyond those already proposed by a number of investigators, remain unknown. However, recent understanding of the presence of electrical coupling in the RAS, which may act with known transmitter interactions to generate ensemble activity, provide new and exciting directions for the field of sleep-wake control and anesthesia.
ABBREVIATIONS
- AChRs
cholinergic receptors
- ACSF
artificial cerebrospinal fluid
- BRAC
basic rest-activity cycle
- CAG
CNQX, APV and gabazine
- CAR
carbachol
- Cd
cadmium
- Cx36
connexin 36
- DMPP
1,1-dimethyl-4-phenyl-piperazinium iodide
- EEG
electroencephalogram
- GABA
gamma amino-butyric acid
- GLUT
glutamate
- ILT
intralaminar thalamus
- KA
kainic acid
- KO
knock-out
- LC
locus coeruleus
- LDT
laterodorsal tegmental nucleus
- LTS
low threshold spikes
- NMDA
n-methyl-d-aspartic acid
- Pf
parafascicular nucleus
- PGO
ponto-geniculo-occipital
- PPN
pedunculopontine nucleus
- RAS
reticular activating system
- REM
rapid eye movement
- RIP
REM sleep inhibitory process
- SubC
subcoeruleus nucleus
- SWS
slow-wave sleep
- TRN
thalamic relay neurons
- TTX
tetrodotoxin
- 5-HT
serotonin
ACKNOWLEDGMENT
Supported by USPHS grants R01 NS20246 (EGR), F30 NS053163 (DSH), and P20 RR20146.
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Garcia-Rill has received research support from and consulted for Sepracor. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Reese NB, Garcia-Rill E, Skinner RD. The pedunculopontine nucleus-auditory input, arousal and pathophysiology. Prog. Neurobiol. 1995;47:105–133. doi: 10.1016/0301-0082(95)00023-o. [DOI] [PubMed] [Google Scholar]
- 2.Rye DB. Contributions of the pedunculopontine region to normal and altered REM sleep. Sleep. 1997;20:757–788. doi: 10.1093/sleep/20.9.757. [DOI] [PubMed] [Google Scholar]
- 3.Scarnati E, Florio T. The pedunculopontine nucleus and related structures. Adv. Neurol, 1997;74:97–110. [PubMed] [Google Scholar]
- 4.Lydic R, Baghdoyan HA. Cellular and molecular mechanisms. New York: CRC Press; 1999. Handbook of Behavioral State Control. [Google Scholar]
- 5.Sakai K, Crochet S, Onoe H. Pontine structures and mechanisms involved in the generation of paradoxical (REM) sleep. Arch Ital Biol. 2001;139:93–107. [PubMed] [Google Scholar]
- 6.Hobson JA, Pace-Schott EP. The cognitive neuroscience of sleep: neuronal systems, consciousness and learning. Nature Rev. 2002;3:679–693. doi: 10.1038/nrn915. [DOI] [PubMed] [Google Scholar]
- 7.Jouvet, M . The Paradox of Sleep: The Story of Dreaming. Cambridge: The MIT Press; 1991. [Google Scholar]
- 8.Garcia-Rill E, Kobayashi T, Good C. The developmental decrease in REM sleep. Thal Related Syst. 2003;2:115–131. [Google Scholar]
- 9.Egan TM, North RA. Acetylcholine acts on M2-muscarinic receptors to excite rat locus coeruleus neurones. Brit. J. Pharmacol. 1985;85:733–735. doi: 10.1111/j.1476-5381.1985.tb11070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egan TM, North RA. Actions of acetylcholine and nicotine on rat locus coeruleus neurons in vitro. Neurosci. 1986;19:565–571. doi: 10.1016/0306-4522(86)90281-2. [DOI] [PubMed] [Google Scholar]
- 11.Ennis M, Shipley MT. Tonic activation of locus coeruleus neurons by systemic or intracoerulear microinjection of an irreversible acetylcholinesterase inhibitor: increased discharge rate and induction of C-Fos. Exp. Neurol. 1992;118:164–177. doi: 10.1016/0014-4886(92)90033-m. [DOI] [PubMed] [Google Scholar]
- 12.El-Etri MM, Ennis M, Jiang M, Shipley MT. Pilocarpine-induced convulsions in rats: evidence for muscarinic receptor-mediated activation of locus coeruleus and norepinephrine release in cholinolytic seizure development. Exp. Neurol. 1993;121:24–39. doi: 10.1006/exnr.1993.1068. [DOI] [PubMed] [Google Scholar]
- 13.Muhlethaler M, Khateb A, Serafin M. 1990. Effects of monoamines and opiates on pedunculopontine neurones. In: Mancia M, Marini G, editors. The Diencephalon and Sleep. New York: Raven Press; 1990. pp. 31–48. [Google Scholar]
- 14.Williams JA, Reiner PB. Noradrenaline hyperpolarizes identified rat mesopontine cholinergic neurons in vitro. J. Neurosci. 1993;13:3878–3883. doi: 10.1523/JNEUROSCI.13-09-03878.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luebke JI, Greene RW, Semba K, Kamondi A, McCarley RW, Reiner PB. Serotonin hyperpolarizes cholinergic low threshold burst neurons in the rat laterodorsal tegmental nucleus in vitro. Proc. Natl. Acad. Sci. USA. 1992;89:743–747. doi: 10.1073/pnas.89.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koyama Y, Kayama Y. Mutual interactions among cholinergic, noradrenergic and serotonergic neurons studied by iontophoresis of these transmitters in rat brainstem nuclei. Neurosci. 1993;55:1117–1126. doi: 10.1016/0306-4522(93)90325-a. [DOI] [PubMed] [Google Scholar]
- 17.Morilak DA, Ciaranello RD. 5-HT2 receptor immuno-reactivity on cholinergic neurons of the pontomesencephalic tegmentum shown by double immunofluorescence. Brain Res. 1993;627:49–54. doi: 10.1016/0006-8993(93)90747-b. [DOI] [PubMed] [Google Scholar]
- 18.Honda T, Semba K. Serotonergic synaptic input to cholinergic neurons in the rat mesopontine tegmentum. Brain Res. 1994;647:299–306. doi: 10.1016/0006-8993(94)91329-3. [DOI] [PubMed] [Google Scholar]
- 19.Leonard CS, Llinas R. Serotonergic and cholinergic inhibition of mesopontine cholinergic neurons controlling REM sleep: An in vitro electrophysiological study. Neurosci. 1994;59:309–330. doi: 10.1016/0306-4522(94)90599-1. [DOI] [PubMed] [Google Scholar]
- 20.Semba K. 1999. The mesopontine cholinergic system: a dual role in REM sleep and wakefulness. In: Lydic R, Baghdoyan HB, editors. Handbook of Behavioral State Control. Cellular and molecular mechanisms. New York: CRC Press; 1999. pp. 161–180. [Google Scholar]
- 21.Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephlogr. Clin. Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- 22.Hobson JA, Lydic R, Baghdoyan HA. Evolving concepts of sleep cycle generation: From brain centers to neuronal population. Behav. Brain Sci. 1986;9:371–448. [Google Scholar]
- 23.Steriade M, McCarley RW. Brainstem Control of Wakefulness and Sleep. New York: Plenum Press; 1990. [Google Scholar]
- 24.Sakai K, Crochet S. Differentiation of presumed serotonergic dorsal raphe neurons in relation to behavior and sleep-wake states. Neurosci. 2001;104:1141–1155. doi: 10.1016/s0306-4522(01)00103-8. [DOI] [PubMed] [Google Scholar]
- 25.Sakai K, Crochet S. Role of dorsal raphe neurons in paradoxical sleep generation in the cat: no evidence for a serotonergic mechanism. Eur. J. Neurosci. 2001;13:103–112. [PubMed] [Google Scholar]
- 26.Sakai K, El Mansari M, Jouvet M. Inhibition by carbachol microinjections of presumptive cholinergic PGO-on neurons in freely moving cats. Brain Res. 1990;527:213–223. doi: 10.1016/0006-8993(90)91140-c. [DOI] [PubMed] [Google Scholar]
- 27.Steriade M, Datta S, Pare D, Oakson G, Curro Dossi R. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J. Neurosci. 1990;10:2541–2559. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steriade M, Paráe D, Datta S, Oakson G, Curro Dossi R. Different cellular types in mesopontine cholinergic nuclei related to ponto-geniculo-occipital waves. J. Neurosci. 1990;10:2560–2579. doi: 10.1523/JNEUROSCI.10-08-02560.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitani A, Ito K, Hallanger AE, Wainer BH, Kataoka K, McCarley RW. Cholinergic projections from the laterodorsal and pedunculopontine tegmental field to the pontine gigantocellular tegmental field in the cat. Brain Res. 1988;451:397–402. doi: 10.1016/0006-8993(88)90792-5. [DOI] [PubMed] [Google Scholar]
- 30.Shiromani P, Armstrong DM, Gillin JC. Cholinergic neurons from the dorsolateral pons project to the medial pons: a WGA-HRP and choline acetyltransferase immunohistochemical study. Neurosci. Lett. 1988;95:12–23. doi: 10.1016/0304-3940(88)90625-8. [DOI] [PubMed] [Google Scholar]
- 31.Mouret J, Delorme F, Jouvet M. Lesions of the pontine tegmentum and sleep in rats. CR Seances Soc Biol Fil. 1967;161:1603–1606. [PubMed] [Google Scholar]
- 32.Mitler MM, Dement WC. Cataplectic-like behavior in cats after micro-injections of carbachol in pontine reticular formation. Brain Res. 1974;68:335–343. doi: 10.1016/0006-8993(74)90402-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marks GA, Farber J, Roffwarg HP. Metencephalic localization of ponto-geniculo-occipital waves in the albino rat. Exp Neurol. 1980;69:667–677. doi: 10.1016/0014-4886(80)90059-x. [DOI] [PubMed] [Google Scholar]
- 34.Morrison AR. Paradoxical sleep without atonia. Arch ital Biol. 1988;126:275–289. [PubMed] [Google Scholar]
- 35.Vanni-Mercier G, Debilly G. A key role for the caudoventral pontine tegmentum in the simultaneous generation of eye saccades in bursts and associated ponto-geniculo-occipital waves during paradoxical sleep in the cat. Neurosci. 1989;86:571–585. doi: 10.1016/s0306-4522(98)00045-1. [DOI] [PubMed] [Google Scholar]
- 36.Baghdoyan HB, Rodrigo-Angulo RW, McCarley RW, Hobson JA. Site-specific enhancement and suppression of desynchronized sleep signs following cholinergic stimulation of three brainstem regions. Brain Res. 1984;306:39–52. doi: 10.1016/0006-8993(84)90354-8. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto K, Mamelak AN, Quattrochi JJ, Hobson JA. A cholinoceptive desynchronized sleep induction zone in the anterolateral pontine tegmentum: locus of the sensitive region. Neurosci. 1990;39:279–293. doi: 10.1016/0306-4522(90)90267-8. [DOI] [PubMed] [Google Scholar]
- 38.Shuman S, Capece M, Baghdoyan H, Lydic R. Pertussis toxin-sensitive G proteins mediate charbacol-induced REM sleep and respiratory depression. Am J Physiol. 1995;269:308–317. doi: 10.1152/ajpregu.1995.269.2.R308. [DOI] [PubMed] [Google Scholar]
- 39.Torterolo P, Yamuy J, Sampogna S, Morales FR, Chase MH. GABAergic neurons of the laterodorsal and pedunculopontine tegmantal nuclei of the cat express c-fos during carbachol-induced active sleep. Brain Res. 2001;892:309–319. doi: 10.1016/s0006-8993(00)03264-9. [DOI] [PubMed] [Google Scholar]
- 40.Jouvet M, Delorme F. Locus coeruleus et sommeil paradoxal. C R Soc Biol Paris. 1965;159:895–899. [Google Scholar]
- 41.Sanford LD, Morrison AR, Graziella LM, Harris JS, Yoo L, Ross RJ. Sleep patterning and behavior in cats with pontine lesions creating REM without atonia. J Sleep Res. 1994;3:233–240. doi: 10.1111/j.1365-2869.1994.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 42.Karlsson KA, Gall AJ, Mohns EJ, Seelke AM, Blumberg MS. The neural substrates of infant sleep in rats. PloS Biol. 2005;3:e143. doi: 10.1371/journal.pbio.0030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henley K, Morrison AR. A re-evaluation of the effects of lesions of the pontine tegmentum and locus coeruleus on phenomena of paradoxical sleep in the cat. Acta Neurobiol Exp. 1974;34:215–232. [PubMed] [Google Scholar]
- 44.Brown RE, Winston S, Basheer R, Thakkar MM, McCarley RW. Electrophysiological characterization of neurons in the dorsolateral pontine rapid-eye-movement sleep induction zone of the rat: intrinsic membrane properties and responses to carbachol and orexins. Neurosci. 2006;143:739–755. doi: 10.1016/j.neuroscience.2006.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imon H, Ito K, Dauphin L, McCarley RW. Electrical stimulation of the cholinergic laterodorsal tegmental nucleus elicits scopolamine-sensitive excitatory postsynaptic potentials in medial pontine reticular formation neurons. Neurosci. 1996;74:393–401. doi: 10.1016/0306-4522(96)00134-0. [DOI] [PubMed] [Google Scholar]
- 46.Gerber U, Stevens DR, McCarley RW, Greene RW. Muscarinic agonists activate an inwardly rectifying potassium conductance in medial pontine reticular formation neurons of the rat in vitro. J. Neurosci. 1991;11:3861–3867. doi: 10.1523/JNEUROSCI.11-12-03861.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stevens DR, McCarley RW, Greene RW. Excitatory amino acid-mediated responses and synaptic potentials in medial pontine reticular formation neurons of the rat in vitro. J. Neurosci. 1992;12:4188–4194. doi: 10.1523/JNEUROSCI.12-11-04188.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greene RW, Gerber U, McCarley RW. Cholinergic activation of medial pontine reticular formation neurons in vitro. Brain Res. 1989;476:154–159. doi: 10.1016/0006-8993(89)91549-7. [DOI] [PubMed] [Google Scholar]
- 49.Steininger TL, Rye DB, Wainer BH. Afferent projections to the cholinergic pedunculopontine nucleus and adjacent midbrain extrapyramidal area in the albino rat. J. Comp. Neurol. 1992;321:515–543. doi: 10.1002/cne.903210403. [DOI] [PubMed] [Google Scholar]
- 50.Lai YY, Siegel JM. Medullary regions mediating atonia. J. Neurosci. 1988;8:4790–4796. doi: 10.1523/JNEUROSCI.08-12-04790.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lai YY, Clements JR, Siegel JM. Glutamatergic and cholinergic projections to the pontine inhibitory area identified with horseradish peroxidase retrograde transport and immunohistochemistry. J. Comp. Neurol. 1993;336:321–330. doi: 10.1002/cne.903360302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chase MH, Chandler SH, Nakamura Y. Intracellular determination of membrane potential of trigeminal motoneurons during sleep and wakefulness. J. Neurophysiol. 1980;44:349–358. doi: 10.1152/jn.1980.44.2.349. [DOI] [PubMed] [Google Scholar]
- 53.McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Ann. Rev. Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- 54.Steriade M. Cellular substrates of oscillations in corticothalamic systems during states of vigilance. In: Lydic R., Baghdoyan H.A., editors. Handbook of Behavioral State Control. Cellular and molecular mechanisms. New York: CRC Press; 1999. pp. 327–347. [Google Scholar]
- 55.McCormick DA, Pape HC. Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay cells. J. Physiol. 1990;431:319–342. doi: 10.1113/jphysiol.1990.sp018332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steriade M, Curro Dossi R, Contreras D. Electrophysiological properties of intralaminar thalamocortical cells discharging rhythmic (approximately 40 Hz) spike-bursts at approximately 1000 Hz during waking and rapid eye movement sleep. Neurosci. 1993;56:1–9. doi: 10.1016/0306-4522(93)90556-u. [DOI] [PubMed] [Google Scholar]
- 57.Steriade M, Curro Dossi R, Pare D, Oakson G. Fast oscillations (20–40 Hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nuclei in the cat. Proc. Nat. Acad. Sci. USA. 1991;88:4396–4400. doi: 10.1073/pnas.88.10.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones BE, Cuello AC. Afferents to the basal forebrain cholinergic cell area from pontomesencephalic-catecholamine, serotonin, and acetylcholine neurons. Neurosci. 1989;31:37–61. doi: 10.1016/0306-4522(89)90029-8. [DOI] [PubMed] [Google Scholar]
- 59.Losier B, Semba K. Dual projections of single cholinergic and monoaminergic brainstem neurons to the basal forebrain and the thalamus in the rat. Brain Res. 1993;604:41–52. doi: 10.1016/0006-8993(93)90350-v. [DOI] [PubMed] [Google Scholar]
- 60.Williams J, Comissarow J, Day J, Fibiger H, Reiner P. 1994. State-dependent release of acetylcholine in rat thalamus measured by in vivo microdialysis. J. Neurosci. 1994;14:5236–5242. doi: 10.1523/JNEUROSCI.14-09-05236.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 62.Webster H, Jones BE. Neurotoxic lesions of the dorsolateral pontomesencephalic tegmentum-cholinergic cell area in the cat. II. Effects upon sleep-waking states. Brain Res. 1988;458:285–302. doi: 10.1016/0006-8993(88)90471-4. [DOI] [PubMed] [Google Scholar]
- 63.Shouse M, Siegel JM. Pontine regulation of REM sleep components in cats: integrity of the pedunculopontine tegmentum (PPT) is important for phasic events but unnecessary for atonia during REM sleep. Brain Res. 1992;571:50–63. doi: 10.1016/0006-8993(92)90508-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laurent JP, Guerrero F. Reversible suppression of ponto-geniculo-occipital waves by localized cooling during paradoxical sleep in cat. Exp. Neurol. 1975;49:356–369. doi: 10.1016/0014-4886(75)90094-1. [DOI] [PubMed] [Google Scholar]
- 65.Velazquez-Moctezuma J, Gillin J, Shiromani P. Effect of specific M1, M2 muscarinic receptor agonists on REM sleep generation. Brain Res. 1989;503:128–131. doi: 10.1016/0006-8993(89)91712-5. [DOI] [PubMed] [Google Scholar]
- 66.McCormick DA. 1992. Cellular mechanisms underlying cholinergic and noradrenergic modulation of neuronal firing mode in the cat and guinea pig dorsal lateral geniculate nucleus. J. Neurosci. 1992;12:278–289. doi: 10.1523/JNEUROSCI.12-01-00278.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCormick DA, Prince DA. 1986. Pirenzepine discriminates among ionic responses to acetylcholine in guinea-pig cerebral cortex and reticular nucleus of thalamus. Trends Pharmacol. Sci. 1986;7:72–77. [Google Scholar]
- 68.Phelan KD, Mahler HR, Deere T, Cross CB, Good C, Garcia-Rill E. Postnatal maturational properties of rat parafascicular thalamic neurons recorded in vitro. Thal. & Rel. Syst. 2005;3:1–25. doi: 10.1017/S1472928805000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ye M, Wilkes S, Garcia-Rill E. Development of cholinergic responses in parafascicular (Pf) neurons. Sleep. 2007;30:A12. [Google Scholar]
- 70.Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152:604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- 71.Marks GA, Shaffery JP, Oksenberg A, Speciale SG, Roffwarg HP. A functional role for REM sleep in brain maturation. Behav. Brain Res. 1995;69:1–11. doi: 10.1016/0166-4328(95)00018-o. [DOI] [PubMed] [Google Scholar]
- 72.Llinas R. 1984. Possible role of tremor in the organization of the nervous system. In: Findley LJ, Capildeo R, editors. International Neurological Symposium on Tremor. London: MacMillan Press; 1984. pp. 473–478. [Google Scholar]
- 73.Jouvet M. Recherches sur les structures nerveuses et mechanismes responsables des differentes phases du sommeil physiologique. Arch. Ital. Biol. 1962;100:125–206. [PubMed] [Google Scholar]
- 74.Suzuki H, Yamadera H, Nakamura S, Endo S. Effects of trezadone and imipramine on the biological rhythm: an analysis of sleep EEG and body core temperature. J Nippon Med Sch. 2002;69:333–341. doi: 10.1272/jnms.69.333. [DOI] [PubMed] [Google Scholar]
- 75.Kupfer DJ, Ehlers CL, Frank E, Grochocinski VJ, McEachran AB, Buhari A. Persistent effects of antidepressants: EEG sleep studies in depressed patients during maintenance treatment. Biol Psychiat. 1994;35:781–793. doi: 10.1016/0006-3223(94)91140-1. [DOI] [PubMed] [Google Scholar]
- 76.Schittecatte M, Dumont F, Machowski R, Fontaine E, Cornil C, Mendlewicz J, Wilmotte J. Mirtazapine, but not fluvoxamine, normalizes the blunted REM sleep response to clonidine in depressed patients: implications for subsensitivity of alpha(2)-adrenergic receptors in depression. Psychiat Res. 2002;109:1–8. doi: 10.1016/s0165-1781(01)00362-6. [DOI] [PubMed] [Google Scholar]
- 77.Landolt HP, Gillin JC. Different aspects of phenelzine treatment on EEG topography in waking and sleep in depressed patients. Neuropsychophramacol. 2002;27:462–469. doi: 10.1016/S0893-133X(02)00322-6. [DOI] [PubMed] [Google Scholar]
- 78.Jouvet-Mounier D, Astic L, Lacote D. Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Dev. Psychobiol. 1970;2:216–239. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- 79.Feng P, Ma Y, Vogel GW. Ontogeny of REM rebound in postnatal rats. Sleep. 2001;24:645–653. doi: 10.1093/sleep/24.6.645. [DOI] [PubMed] [Google Scholar]
- 80.Crick F, Mitchison G. The function of dream sleep. Nature. 1983;304:111–114. doi: 10.1038/304111a0. [DOI] [PubMed] [Google Scholar]
- 81.Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science. 1996;271:1870–1873. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- 82.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 83.Stickgold R, Hobson JA, Fosse R, Fosse M. Sleep, learning, and dreams: off-line memory reprocessing. Science. 2001;294:1052–1057. doi: 10.1126/science.1063530. [DOI] [PubMed] [Google Scholar]
- 84.Maquet P, Laureys S, Peigneux P, et al. Experience-dependent changes in cerebral activation during human REM sleep. Nature Neurosci. 2000;3:831–836. doi: 10.1038/77744. [DOI] [PubMed] [Google Scholar]
- 85.Frank MG, Issa NP, Stryker MP. Sleep enhances plasticity in the developing visual cortex. Neuron. 2001;30:275–287. doi: 10.1016/s0896-6273(01)00279-3. [DOI] [PubMed] [Google Scholar]
- 86.Gais S, Molle M, Helms K, Born J. Learning-dependent increases in sleep spindle density. J. Neurosci. 2002;22:6830–6834. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–1052. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- 88.Siegel JM. The REM sleep-memory consolidation hypothesis. Science. 2001;294:1058–1063. doi: 10.1126/science.1063049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maquet P, Peters JM, Aerts J, Delfiore G, Degueldre C, Luxen A, Franck G. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature. 1966;383:163–166. doi: 10.1038/383163a0. [DOI] [PubMed] [Google Scholar]
- 90.Llinas R, Ribary U, Joliot M, Wang XJ. Content and context in temporal thalamocortical binding. In: Buzsaki G, Christen Y, editors. Temporal Coding in the Brain. Berlin: Springer-Verlag; 1994. pp. 251–272. [Google Scholar]
- 91.Siegel JM. 1999. The evolution of REM sleep. In: Lydic R, Baghdoyan HB, editors. Handbook of Behavioral State Control. Cellular and molecular mechanisms. New York: CRC Press; 1999. pp. 87–100. [Google Scholar]
- 92.Pare D, Llinas R. Conscious and pre-conscious processes as seen from the standpoint of sleep-waking cycle neurophysiology. Neuropsychol. 1995;33:1155–1168. doi: 10.1016/0028-3932(95)00055-8. [DOI] [PubMed] [Google Scholar]
- 93.Vogel GW, Feng P, Kinney GG. Ontogeny of REM sleep in rats: possible implications for endogenous depression. Physiol. Behav. 2000;68:453–461. doi: 10.1016/s0031-9384(99)00207-3. [DOI] [PubMed] [Google Scholar]
- 94.Skinner RD, Conrad N, Henderson V, Gilmore S, Garcia-Rill E. Development of NADPH diaphorase positive pedunculopontine neurons. Exp. Neurol. 1989;104:15–21. doi: 10.1016/0014-4886(89)90003-4. [DOI] [PubMed] [Google Scholar]
- 95.Sheets LP, Dean KF, Reiter LW. Ontogeny of the acoustic startle response and sensitization to background noise in the rat. Behav. Neurosci. 1988;102:706–713. doi: 10.1037//0735-7044.102.5.706. [DOI] [PubMed] [Google Scholar]
- 96.Kungel M, Koch M, Friauf E. Cysteamine impairs the development of acoustic startle response in rats: possible role of somatostatin. Neurosci. Lett. 1996;202:181–184. doi: 10.1016/0304-3940(95)12244-3. [DOI] [PubMed] [Google Scholar]
- 97.Ninomiya Y, Koyama Y, Kayama Y. Postnatal development of choline acetyltransferase activity in the rat laterodorsal tegmental nucleus. Neurosci. Lett. 2001;308:138–140. doi: 10.1016/s0304-3940(01)01990-5. [DOI] [PubMed] [Google Scholar]
- 98.Leonard CS, Llinas R. 1990. Electrophysiology of mammalian pedunculopontine and laterodorsal tegmental neurons in vitro: implications for the control of REM sleep. In: Steriade M, Biesold D, editors. Brain Cholinergic Systems. Oxford: Oxford Science; 1990. pp. 205–223. [Google Scholar]
- 99.Kamondi A, Williams J, Hutcheon B, Reiner P. Membrane properties of mesopontine cholinergic neurons studied with the whole-cell patch-clamp technique: implications for behavioral state control. J. Neurophysiol. 1992;68:1359–1372. doi: 10.1152/jn.1992.68.4.1359. [DOI] [PubMed] [Google Scholar]
- 100.Takakusaki K, Kitai ST. Ionic mechanisms involved in the spontaneous firing of tegmental pedunculopontine nucleus neurons of the rat. Neurosci. 1997;78:771–794. doi: 10.1016/s0306-4522(96)00540-4. [DOI] [PubMed] [Google Scholar]
- 101.Kobayashi T, Homma Y, Good C, Skinner RD, Garcia-Rill E. Developmental changes in the effects of serotonin on neurons in the region of the pedunculopontine nucleus. Dev. Brain Res. 2002;140:57–66. doi: 10.1016/s0165-3806(02)00575-8. [DOI] [PubMed] [Google Scholar]
- 102.Greene RW, Rainnie DG. Mechanisms affecting neuronal excitability in brainstem cholinergic centers and their impact on behavioral state. In: Lydic R, Baghdoyan HA, editors. Handbook of Behavioral State Control. Cellular and molecular mechanisms. New York: CRC Press; 1991. pp. 277–309. [Google Scholar]
- 103.Keele NB, Neugebauer V, Shinnick-Gallagher P. Differential effects of metabotropic glutamate receptor antagonists on bursting activity in the amygdala. J. Neurophysiol. 1991;81:2056–2065. doi: 10.1152/jn.1999.81.5.2056. [DOI] [PubMed] [Google Scholar]
- 104.Datta S, Siwek DF. Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. J. Neurosci. Res. 2002;70:79–82. doi: 10.1002/jnr.10405. [DOI] [PubMed] [Google Scholar]
- 105.Steinfels GF, Heym J, Strecker RE, Jacobs BL. Raphe unit activity in freely moving cats is altered by manipulations of central but not peripheral motor systems. Brain Res. 1983;279:77–84. doi: 10.1016/0006-8993(83)90164-6. [DOI] [PubMed] [Google Scholar]
- 106.Trulson ME, Jacobs BL, Morrison AR. Raphe unit activity during REM sleep in normal cats and in pontine lesioned cats displaying REM sleep without atonia. Brain Res. 1981;226:74–91. doi: 10.1016/0006-8993(81)91084-2. [DOI] [PubMed] [Google Scholar]
- 107.Horner RL, Sanford LD, Annis D, Pack AI, Morrison AR. Serotonin at the laterodorsal tegmental nucleus suppresses rapid-eye-movement sleep in freely behaving rats. J. Neurosci. 1997;17:7541–7552. doi: 10.1523/JNEUROSCI.17-19-07541.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sanford LD, Tejani-Butt SM, Ross RJ, Morrison AR. Elicited PGO waves in rats: lack of 5-HT1a inhibition in putative pontine generator region. Pharmacol. Biochem. Behav. 1996;53:323–327. doi: 10.1016/0091-3057(95)02029-2. [DOI] [PubMed] [Google Scholar]
- 109.Fay RA, Kubin L. 5-HT 2A receptor-like immunoreactivity is present in cells and cellular processes adjacent to mesopontine nitric oxide synthase-containing neurons. Sleep. 2000;23:A108. [Google Scholar]
- 110.Thakkar MM, Strecker RE, McCarley RW. Behavioral state control through differential serotonergic inhibition in the mesopontine cholinergic nuclei: a simultaneous unit recording and microdialysis study. J. Neurosci. 1998;18:5490–5497. doi: 10.1523/JNEUROSCI.18-14-05490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Strecker RE, Thakkar M, Porkka-Heiskanen M, Dauphin LJ, Bjorkum AA, McCarley RW. Behavioral state-related changes of extracellular serotonin concentration in the pedunculopontine tegmental nucleus: a microdialysis study in freely moving animals. Sleep Res. Online. 1999;2:21–27. [PubMed] [Google Scholar]
- 112.Clements JR, Grant S. Glutamate-like immunoreactivity in neurons of the laterodorsal tegmental and pedunculopontine nuclei in the rat. Neurosci. Lett. 1990;120:70–71. doi: 10.1016/0304-3940(90)90170-e. [DOI] [PubMed] [Google Scholar]
- 113.Sanchez R, Leonard CS. NMDA receptor-mediated synaptic input to nitric oxide synthase-containing neurons of the guinea-pig mesopontine tegmentum in vitro. Neurosci. Lett. 1994;179:141–144. doi: 10.1016/0304-3940(94)90954-7. [DOI] [PubMed] [Google Scholar]
- 114.Sanchez R, Leonard CS. NMDA-receptor-mediated synaptic currents in guinea pig laterodorsal tegmental neurons in vitro. J. Neurophysiol. 1996;76:1101–1111. doi: 10.1152/jn.1996.76.2.1101. [DOI] [PubMed] [Google Scholar]
- 115.Datta S, Siwek DF. Excitation of the brain stem pedunculopontine tegmentum cholinergic cells induces wakefulness and REM sleep. J. Neurophysiol. 1997;77:2975–2988. doi: 10.1152/jn.1997.77.6.2975. [DOI] [PubMed] [Google Scholar]
- 116.Datta S, Spoley EE, Patterson EH. Microinjection of glutamate into the pedunculopontine tegmentum induces REM sleep and wakefulness in the rat. Amer. J. Physiol. Reg. Integ. Comp. Physiol. 2001;280:R752–R759. doi: 10.1152/ajpregu.2001.280.3.R752. [DOI] [PubMed] [Google Scholar]
- 117.Datta S. Evidence that REM sleep is controlled by the activation of brain stem pedunculopontine tegmental kainate receptor. J. Neurophysiol. 2002;87:1790–1798. doi: 10.1152/jn.00763.2001. [DOI] [PubMed] [Google Scholar]
- 118.Kobayashi T, Skinner RD, Garcia-Rill E. Developmental decrease in REM sleep: the shift to kainate receptor regulation. Thalamus & Rel. Syst. 2004;2:315–324. [Google Scholar]
- 119.Happe HK, Coulter CL, Gerety ME, Sanders JD, O'Rourke M, Bylund DB, Murrin LC. Alpha-2 adrenergic receptor development in rat CNS: an autoradiographic study. Neurosci. 2004;123:157–178. doi: 10.1016/j.neuroscience.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 120.Hou RH, Freeman C, Langley RW, Szabadi E, Bradshaw CM. Does modafinil activate the locus coeruleus in man? Comparison of modafinil and clonidine on arousal and autonomic functions in human volunteers. Psychopharmacol. 2005;181:537–549. doi: 10.1007/s00213-005-0013-8. [DOI] [PubMed] [Google Scholar]
- 121.Bay K, Mamiya K, Good C, Skinner RD, Garcia-Rill E. Alpha-2 adrenergic regulation of pedunculopontine nucleus (PPN) neurons during development. Neuroscience. 2006;141:769–779. doi: 10.1016/j.neuroscience.2006.04.045. [DOI] [PubMed] [Google Scholar]
- 122.Tribollet E, Bertrand D, Marguerat A, Raggenbass M. Comparative distribution of nicotinic receptor subtypes during development, adulthood and aging: An autoradiographic study in the rat brain. Neurosci. 2004;12:405–420. doi: 10.1016/j.neuroscience.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 123.Vilaro MT, Palacios JM, Mengod G. Multiplicity of muscarinic autoreceptor subtypes? Comparison of the distribution of cholinergic cells and cells containing mRNA for five subtypes of muscarinic receptors in the rat brain. Molec Brain Res. 1994;21:30–46. doi: 10.1016/0169-328x(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 124.Semba K, Fibiger HC. Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: a retro- and antero-grade transport and immunocytochemical study. J Comp Neurol. 1992;323:387–410. doi: 10.1002/cne.903230307. [DOI] [PubMed] [Google Scholar]
- 125.Lanca JA, Sanelli TR, Corrigall WA. Nicotine-induced fos expression in the pedunculopontine mesencephalic tegmentum in the rat. Neuropharmacol. 2000;39:2808–2817. doi: 10.1016/s0028-3908(00)00129-5. [DOI] [PubMed] [Google Scholar]
- 126.Lena C, Popa D, Grailhe R, Escourrou P, Changeux J-P, Adrien J. β2-containing nicotinic receptors contribute to the organization of sleep and regulate putative micro-arousals in mice. J Neurosci. 2004;24:5711–5718. doi: 10.1523/JNEUROSCI.3882-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Good CH, Bay KD, Buchanan R, Skinner RD, Garcia-Rill E. Muscarinic and nicotinic responses in the developing pedunculopontine nucleus (PPN) Brain Res. 2007;1129:147–155. doi: 10.1016/j.brainres.2006.10.046. [DOI] [PubMed] [Google Scholar]
- 128.Ford B, Holmes CJ, Mainville L, Jones BE. GABAergic neurons in the rat mesencephalic tegmentum: codistribution with cholinergic and other tegmental neurons projecting to the lateral hypothalamus. J Comp Neurol. 1995;363:177–196. doi: 10.1002/cne.903630203. [DOI] [PubMed] [Google Scholar]
- 129.Jia HG, Yamuy J, Sampogna S, Morales FR, Chase MH. Colocalization of γ-amino-butyric acid and acetylcholine in neurons in the laterodorsal and pedunculopontine tegmental nuclei in the cat: a light and electron microscopy study. Brain Res. 2003;992:205–219. doi: 10.1016/j.brainres.2003.08.062. [DOI] [PubMed] [Google Scholar]
- 130.Grofova I, Zhou M. Nigral innervation of cholinergic and glutamatergic cells in the rat mesopontine tegmentum: light and electron microscopic anterograde tracing and immunohistochemical studies. J. Comp. Neurol. 1998;395:359–379. [PubMed] [Google Scholar]
- 131.Bay KD, Beck P, Skinner RD, Garcia-Rill E. GABAergic modulation of developing pedunculopontine nucleus (PPN) NeuroReport. 2007;18:249–253. doi: 10.1097/WNR.0b013e328011e6c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cossart R, Bernard C, Ben-Ari Y. Multiple facets of GABAergic neurons and synapses: multiple fates of GABA signaling in epilepsies. Trends Neurosci. 2005;28:108–115. doi: 10.1016/j.tins.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 133.Connors BW, Long MA. Electrical synapses in the mammalian brain. Annu. Rev. Neurosci. 2004;27:393–418. doi: 10.1146/annurev.neuro.26.041002.131128. [DOI] [PubMed] [Google Scholar]
- 134.Bukauskas FF, Verselis VK. Gap junction channel gating. Biochim. Biophys. Acta. 2004;1662:42–60. doi: 10.1016/j.bbamem.2004.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Laird DW. Life cycle of connexins in health and disease. Biochem. J. 2006;394:527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hughes SW, Blethyn KL, Cope DW, Crunelli V. Properties and origin of spikelets in thalamocortical neurons in vitro. Neurosci. 2002;3:395–401. doi: 10.1016/s0306-4522(01)00577-2. [DOI] [PubMed] [Google Scholar]
- 137.Srinivas M, Rozental R, Kojima T, Dermietzel R, Mehler M. Functional properties of channels formed by the neuronal gap junction protein connexin 36. J. Neurosci. 1999;19:9848–9855. doi: 10.1523/JNEUROSCI.19-22-09848.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Long MA, Landisman CE, Connors BW. Small clusters of electrically coupled neurons generate synchronous rhythms in the thalamic reticular nucleus. J. Neurosci. 2004;24:341–349. doi: 10.1523/JNEUROSCI.3358-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fuentealba P, Steriade M. The reticular nucleus revisited: Intrinsic network properties of a thalamic pacemaker. Prog. Neurobiol. 2005;75:125–141. doi: 10.1016/j.pneurobio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 140.Landisman CE, Connors BW. Long-term modulation of electrical synapses in the mammalian thalamus. Science. 2005;310:1809–1813. doi: 10.1126/science.1114655. [DOI] [PubMed] [Google Scholar]
- 141.Deleuze C, Huguenard JR. Distinct electrical and chemical connectivity maps in the thalamic reticular nucleus. J. Neurosci. 2006;26:8633–8645. doi: 10.1523/JNEUROSCI.2333-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu XB, Jones EG. Fine structural localization of Connexin-36 immunoreactivity in mouse cerebral cortex and thalamus. J. Comp. Neurol. 2003;466:457–467. doi: 10.1002/cne.10901. [DOI] [PubMed] [Google Scholar]
- 143.Hestrin S, Galarreta M. Electrical synapses define networks of neocortical GABAergic neurons. Trends Neurosci. 2005;28:304–309. doi: 10.1016/j.tins.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 144.Traub RD, Kopell N, Bibbig A, Buhl EH, LeBeau FEN, Whittington MA. Gap junctions between interneuron dendrites can enhance synchrony of gamma oscillations in distributed networks. J. Neurosci. 2001;21:9476–9486. doi: 10.1523/JNEUROSCI.21-23-09478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneuronsdrives synchronized inhibition in neocortex. Nature Neurosci. 2000;3:904–910. doi: 10.1038/78809. [DOI] [PubMed] [Google Scholar]
- 146.Galarreta M, Hestrin S. Electrical synapses between GABA-releasing interneurons. Nature Neurosci. 2001;2:425–433. doi: 10.1038/35077566. [DOI] [PubMed] [Google Scholar]
- 147.Hormudzi SG, Pais I, LeBeau FEN, et al. Impaired electrical signaling disrupts gamma frequency oscillations in connexin-deficient mice. Neuron. 2001;31:487–495. doi: 10.1016/s0896-6273(01)00387-7. [DOI] [PubMed] [Google Scholar]
- 148.Yang Q, Michelson HB. Gap junctions synchronize the firing of inhibitory interneurons in guinea pig hippocampus. Brain Res. 2001;907:139–143. doi: 10.1016/s0006-8993(01)02582-3. [DOI] [PubMed] [Google Scholar]
- 149.Kohling R, Gladwell SJ, Bracci E, Vreugdenhil M, Jefferys JGR. Prolonged epileptiform bursting induced by 0 Mg++ in rat hippocampal slices depends on gap junctional coupling. Neurosci. 2001;105:579–587. doi: 10.1016/s0306-4522(01)00222-6. [DOI] [PubMed] [Google Scholar]
- 150.Traub RD, Bibbig A, Fisahn A, LeBeau FEN, Whittington MA, Buhl EH. A model of gamma-frequency network oscillations induced in the rat CA3 region by carbachol in vitro. Eur. J. Neurosci. 2000;12:4093–4106. doi: 10.1046/j.1460-9568.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- 151.Evans WH, Boitano S. Connexin mimetic peptides: specific inhibitors of gap-junctional intercellular communication. Bioch. Soc. Trans. 2001;29:606–612. doi: 10.1042/bst0290606. [DOI] [PubMed] [Google Scholar]
- 152.He DS, Burt JM. Mechanism and selectivity of the effects of halothane on gap junction channel function. Circ. Res. 2000;86:1–10. doi: 10.1161/01.res.86.11.e104. [DOI] [PubMed] [Google Scholar]
- 153.Boger DL, Henriksen SJ, Cravatt BF. Oleamide: an endogenous sleep-inducing lipid and prototypical member of a new class of biological signaling molecules. Curr. Pharm. Des. 1998;4:303–314. [PubMed] [Google Scholar]
- 154.Guan X, Cravatt BF, Ehring GR, Hall JE, Boger DL, Lerner RA, Gilula NB. The sleep-inducing lipid oleamide deconvolutes gap junction communication and calcium wave transmission in glial cells. J. Cell. Biol. 1997;139:1785–1792. doi: 10.1083/jcb.139.7.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Murillo-Rodriguez E, Blanco-Centurion C, Sanchez C, Piomelli D, Shiromani PJ. Anandamide enhances extracellular levels of adenosine and induces sleep: an in vivo microdialysis study. Sleep. 2003;26:943–947. doi: 10.1093/sleep/26.8.943. [DOI] [PubMed] [Google Scholar]
- 156.Gigout S, Louvel J, Kawasaki H, et al. Effects of gap junction blockers on human neocortical synchronization. Neurobiol. Dis. 2006;22:496–508. doi: 10.1016/j.nbd.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 157.Gareri P, Condorelli D, Belluardo N, et al. Anticonvulsant effects of carbenoxolone in genetically epilepsy prone rats (GEPRs) Neuropharmacol. 2004;47:1205–1216. doi: 10.1016/j.neuropharm.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 158.Rozental R, Srinivas M, Spray DC. How to close a gap junction channel. In: Bruzzone R, Giaume C, editors. Methods in Molecular Biology, Vol. 154, Connexin methods and protocols. New jersey: Humana Press; 2000. pp. 447–477. [DOI] [PubMed] [Google Scholar]
- 159.Srinivas M, Hopperstad MG, Spray DC. Quinine blocks specific gap junction channel subtypes. Proc. Natl. Acad. Sci. 2001;98:10942–10947. doi: 10.1073/pnas.191206198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gajda Z, Szupera Z, Blaszo G, Szente M. Quinine, a blocker of neuronal Cx36 suppresses seizure activity in rat neocortex in vivo. Epilepsia. 2005;46:1581–1591. doi: 10.1111/j.1528-1167.2005.00254.x. [DOI] [PubMed] [Google Scholar]
- 161.Cruikshank SJ, Hopperstad M, Younger M, Connors BW, Spray DC. Potent block of Cx36 and Cx50 gap junction channels by mefloquine. Proc. Natl. Acad. Sci. 2004;101:12364–12369. doi: 10.1073/pnas.0402044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pais I, Hormudzi SG, Monyer H, et al. Sharp wave-like activity in the hippocampus in vitro in mice lacking the gap junction protein connexin 36. J. Neurophysiol. 2003;89:2046–2054. doi: 10.1152/jn.00549.2002. [DOI] [PubMed] [Google Scholar]
- 163.Ballon JS, Feifel D. A systematic review of Modafinil: Potential clinical uses and mechanisms of action. J. Clin. Psychiat. 2006;67:554–566. doi: 10.4088/jcp.v67n0406. [DOI] [PubMed] [Google Scholar]
- 164.Contreras D, Llináas R. Voltage-sensitive dye imaging of neocortical spatiotemporal dynamics to afferent activation frequency. J. Neurosci. 2001;21:9403–9413. doi: 10.1523/JNEUROSCI.21-23-09403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fricker D, Miles R. Interneurons, spike timing, and perception. Neuron. 2001;32:771–774. doi: 10.1016/s0896-6273(01)00528-1. [DOI] [PubMed] [Google Scholar]
- 166.Hayar A, Shipley MT, Ennis M. Olfactory bulb external tufted cells are synchronized by multiple intraglomerular mechanisms. J. Neurosci. 2005;25:8197–208. doi: 10.1523/JNEUROSCI.2374-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Peinado A, Yuste R, Katz LC. Extensive dye coupling between rat neocortical neurons during the period of cortical formation. Neuron. 1993;10:103–114. doi: 10.1016/0896-6273(93)90246-n. [DOI] [PubMed] [Google Scholar]
- 168.Sutor B, Hablitz JJ, Rucker F, ten Bruggencate G. Spread of epileptiform activity in the immature rat neocortex studied with voltage-sensitive dyes and laser scanning microscopy. J. Neurophysiol. 1994;72:1756–1768. doi: 10.1152/jn.1994.72.4.1756. [DOI] [PubMed] [Google Scholar]
- 169.Urbano FJ, Leznik E, Llinas R. Modafinil enhances thalamocortical activity by increasing neuronal electrotonic coupling. Proc. Natl. Acad. Sci. 2007;104:12554–12559. doi: 10.1073/pnas.0705087104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Garcia-Rill E, Heister DS, Ye M, Charlesworth A, Hayar A. Electrical coupling: novel mechanism for sleep-wake control. Sleep. 2007;30:1405–1414. doi: 10.1093/sleep/30.11.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Heister DS, Hayar A, Charlesworth A, Yates C, Zhou Y, Garcia-Rill E. Evidence for electrical coupling in the SubCoeruleus (SubC) nucleus. J. Neurophysiol. 2007;97:3142–3147. doi: 10.1152/jn.01316.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Leznik E, Llinas R. Role of gap junctions in synchronized oscillations in the inferior olive. J. Neurophysiol. 2005;94:2447–2456. doi: 10.1152/jn.00353.2005. [DOI] [PubMed] [Google Scholar]
- 173.Llinas R, Yarom Y. Oscillatory properties of guinea-pig inferior olivary neurons and their pharmacological modulation: an in vitro study. J. Physiol. 1986;376:163–182. doi: 10.1113/jphysiol.1986.sp016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cornell-Bell AH, Finkbeiner SM. Ca+2 waves in astrocytes. Cell Calcium. 1991;12:185–204. doi: 10.1016/0143-4160(91)90020-f. [DOI] [PubMed] [Google Scholar]
- 175.Long MA, Deans MR, Paul DL, Connors BW. Rhythmicity without synchrony in the electrically uncoupled inferior olive. J. Neurosci. 2002;22:10898–10905. doi: 10.1523/JNEUROSCI.22-24-10898.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Charlesworth A, Yates C, Ye M, Zhou Y, Garcia-Rill E. Developmental changes in Connexin 36 expression and protein in the SubCoeruleus nucleus. Sleep. 2007;30:A10. [Google Scholar]
- 177.De Zeeuw CI, Chorev E, Devor A, et al. Deformation of network connectivity in the inferior olive of connexin 36-deficient mice is compensated by morphological and electrophysiological changes at the single neuron level. J. Neurosci. 2003;23:4700–4711. doi: 10.1523/JNEUROSCI.23-11-04700.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Placatonakis DG, Bukovsky AA, Zeng XH, Kiem HP, Welsh JP. Fundamental role of inferior olive connexin 36 in muscle coherence during tremor. Proc. Nat. Acad. Sci. 2004;101:7164–7169. doi: 10.1073/pnas.0400322101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kistler WM, De Jeu MT, Elgersma Y, et al. Analysis of Cx36 knockout does not support tenet that olivary gap junctions are required for complex spike synchronization and normal motor performance. Ann. NY Acad. Sci. 2002;978:391–404. doi: 10.1111/j.1749-6632.2002.tb07582.x. [DOI] [PubMed] [Google Scholar]
- 180.Deans MR, Gibson JR, Sellitto C, Connors BW, Paul DL. Synchronous activity of inhibitory networks in neocortex requires electrical synapses containing connexin36. Neuron. 2001;31:477–485. doi: 10.1016/s0896-6273(01)00373-7. [DOI] [PubMed] [Google Scholar]
- 181.Buszaki G. Electrical wiring of the oscillating brain. Neuron. 2001;31:342–344. doi: 10.1016/s0896-6273(01)00378-6. [DOI] [PubMed] [Google Scholar]
- 182.Buhl DL, Harris KD, Hormudzi SG, Monyer H, Buszaki G. Selective impairment of hippocampal gamma oscillations in connexin-36 knock-out mouse in vivo. J. Neurosci. 2003;23:1013–1018. doi: 10.1523/JNEUROSCI.23-03-01013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Frisch C, De Souza-Silva MA, Sohl G, et al. Stimulus complexity dependent memory impairment and changes in motor performance after deletion of the neuronal gap junction protein connexin36 mice. Behav. Brain Res. 2005;157:177–185. doi: 10.1016/j.bbr.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 184.Kibschull M, Magin TM, Traub O, Winterhager E. Cx31 and Cx43 double-deficient mice reveal independent functions in murine placental and skin development. Dev Dyn. 2005;233:853–863. doi: 10.1002/dvdy.20424. [DOI] [PubMed] [Google Scholar]
- 185.He S, Weiler R, Vaney DI. Endogenous dopaminergic regulation of horizontal cell coupling in the mammalian retina. J Comp Neurol. 2000;418:33–40. doi: 10.1002/(sici)1096-9861(20000228)418:1<33::aid-cne3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 186.Maher BJ, Westbrook GL. Sensory deprivation and the development of dendrodendritic excitation in olfactory glomeruli. Neurosci. Abst. 2007;33:503–15. [Google Scholar]
- 187.Parker PRL, Cruikshank SJ, Connors BW. Development of electrical synapses in the thalamic reticular nucleus: Gap junctional conductances increase and compensate for changes in intrinsic membrane properties. Neurosci. Abst. 2007;33:581–15. [Google Scholar]
- 188.Datta S. Cellular basis of pontine ponto-geniculo-occipital wave generation and modulation. Cell Molec. Neurobiol. 1997;17:341–365. doi: 10.1023/A:1026398402985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sadikot AF, Parent A, Francois C. The centre median and parafascicular thalamic nuclei project respectively to the sensorimotor and associative-limbic striatal territories in the squirrel monkey. Brain Res. 1990;510:161–165. doi: 10.1016/0006-8993(90)90746-x. [DOI] [PubMed] [Google Scholar]
- 190.Sadikot AF, Parent A, Francois C. Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a PHA-L study of subcortical projections. J. Comp. Neurol. 1992;315:137–159. doi: 10.1002/cne.903150203. [DOI] [PubMed] [Google Scholar]
- 191.Vogt LJ, Vogt BA, Sikes RW. Limbic thalamus in rabbit: architecture, projections to cingulate cortex and distribution of muscarinic acetylcholine, GABAA and opioid receptors. J. Comp. Neurol. 1992;319:205–217. doi: 10.1002/cne.903190203. [DOI] [PubMed] [Google Scholar]
- 192.Sidibe M, Bevan MD, Bolam JP, Smith Y. Efferent connections of the internal globus pallidus in the squirrel monkey: I. topography and synaptic organization. J. Comp. Neurol. 1997;382:323–347. [PubMed] [Google Scholar]
- 193.Ruggiero DA, Anwar S, Kim J, Glickstein SB. Visceral afferent pathways to the thalamus and olfactory tubercle: behavioral implications. Brain Res. 1998;799:159–171. doi: 10.1016/s0006-8993(98)00442-9. [DOI] [PubMed] [Google Scholar]
- 194.Oakman SA, Faris P, Cozzari C, Hartman BK. Characterization of the extent of pontomesencephalic cholinergic neurons projecting to the thalamus: comparison with projections to midbrain dopaminergic groups. Neurosci. 1999;94:529–547. doi: 10.1016/s0306-4522(99)00307-3. [DOI] [PubMed] [Google Scholar]
- 195.Chiba T, Kayahara T, Nakano K. Efferent projections of infralimbic and prelimbic areas of the medial prefrontal cortex in the japanese monkey, macaca fuscata. Brain Res. 2001;888:83–101. doi: 10.1016/s0006-8993(00)03013-4. [DOI] [PubMed] [Google Scholar]
- 196.Henderson JM, Carpenter K, Cartwright H, Halliday GM. Degeneration of the centre-median-parafascicular complex in parkinson's disease. Ann. Neurol. 2000;47:345–352. [PubMed] [Google Scholar]
- 197.Koos BJ, Chau A, Matsuura M, Punla O, Kruger L. Thalamic lesions dissociate breathing inhibition by hypoxia and adenosine in fetal sheep. Amer. J. Physiol. Reg. Integ. Comp. Physiol. 2000;278:R831–R837. doi: 10.1152/ajpregu.2000.278.4.R831. [DOI] [PubMed] [Google Scholar]
- 198.Craig AD, Dostrovsky JO. Differential projections of thermoreceptive and nociceptive lamina I trigeminothalamic and spinothalamic neurons in the cat. J. Neurophysiol. 2001;86:856–870. doi: 10.1152/jn.2001.86.2.856. [DOI] [PubMed] [Google Scholar]
- 199.Llinas R, Ribary U. Consciousness and the Brain. The thalamocortical dialogue in health and disease. Ann. N.Y. Acad. Sci. 2001;929:166–175. [PubMed] [Google Scholar]
- 200.Llinas R, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc. Nat. Acad. Sci. 1999;96:15222–15227. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jeanmonod D, Magnin M, Morel A, et al. Thalamocortical dysrhythmia. II. Clinical and surgical aspects. Thal. & Related Syst. 2001;1:237–244. [Google Scholar]
- 202.Anand KJS, Garg S, Rovnaghi CR, Narisinghani U, Bhutta AT, Hall RW. Ketamine reduces the cell death following inflammatory pain in newborn rat brain. Pediatr. Res. 2007;62:283–290. doi: 10.1203/PDR.0b013e3180986d2f. [DOI] [PubMed] [Google Scholar]
- 203.Lidow MS. Long-term effects of neonatal pain on nociceptive systems. Pain. 2002;99:377–83. doi: 10.1016/S0304-3959(02)00258-0. [DOI] [PubMed] [Google Scholar]
- 204.Saab CY, Park YC, Al-Chaer E. Thalamic modulation of visceral nociceptive processing in adult rats with neonatal colon irritation. Brain Res. 2004;1008:186–192. doi: 10.1016/j.brainres.2004.01.083. [DOI] [PubMed] [Google Scholar]
- 205.Bay KD, Good C, Anand KJS, Al-Chaer E, Rovnaghi C, Garcia-Rill E. Effects of early somatic pain on parafascicular neurons. Sleep. 2006;29:A16. [Google Scholar]
- 206.Garcia-Rill E. Disorders of the Reticular Activating System. Med. Hypoth. 1997;49:379–387. doi: 10.1016/s0306-9877(97)90083-9. [DOI] [PubMed] [Google Scholar]
- 207.Garcia-Rill E. Mechanisms of sleep and wakefulness. In: Lee-Chiong T, Sateia MJ, Carskadon MA, editors. Sleep Medicine. Philadelphia: Hanley & Belfus; 2002. pp. 31–39. [Google Scholar]
- 208.Garcia-Rill E, Skinner RD. 2002. The sleep state-dependent P50 midlatency auditory evoked potential. In: Lee-Chiong T, Sateia MJ, Carskadon MA, editors. Sleep Medicine. Philadelphia: Hanley & Belfus; 2002. pp. 697–704. [Google Scholar]
- 209.Peterson MJ, Benca RM. Sleep in mood disorders. Psychiatr Clin North Am. 2006;29:1009–1032. doi: 10.1016/j.psc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 210.Schiff R, Bulpitt CJ, Wesnes KA, Rajkumar C. Short-term transdermal estradiol therapy, cognition and depressive symptoms in healthy older women. A randomized placebo controlled pilot cross-over study. Psychoneuroendoc. 2005;30:309–315. doi: 10.1016/j.psyneuen.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 211.Liu PY, Yee B, Wishart SM, et al. The short-term effects of high-dose testosterone on sleep, breathing, and function in older men. J Clin. Endoc. Metab. 2003;88:3605–3613. doi: 10.1210/jc.2003-030236. [DOI] [PubMed] [Google Scholar]
- 212.Canway AJ, Handelsman DJ, Lording DW, Stuckey B, Zajac JD. Use, misuse and abuse of androgens. The Endocrine Society of Australia consensus guidelines for androgen prescribing. Med. J. Aust. 2000;172:220–224. doi: 10.5694/j.1326-5377.2000.tb123913.x. [DOI] [PubMed] [Google Scholar]
- 213.Lustig RH. Sex hormone modulation of neural development in vitro. Horm. Behav. 1994;28:383–395. doi: 10.1006/hbeh.1994.1035. [DOI] [PubMed] [Google Scholar]
- 214.Matsumoto A, Arnold AP, Zampighi GA, Micevych PE. Androgenic regulation of gap junctions between motoneurons in the spinal cord. J. Neurosci. 1988;8:4177–4183. doi: 10.1523/JNEUROSCI.08-11-04177.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Coleman AM, Sengelaub DR. Patterns of dye coupling in lumbar motor nuclei of the rat. J. Comp. Neurol. 2002;454:34–41. doi: 10.1002/cne.10438. [DOI] [PubMed] [Google Scholar]
- 216.Shinohara K, Funabashi T, Nakamura TJ, Kimura F. Effects of estrogen and progesterone on the expression of connexin-36 mRNA in suprachiasmatic nucleus of female rats. Neurosci. Lett. 2001;309:37–40. doi: 10.1016/s0304-3940(01)02022-5. [DOI] [PubMed] [Google Scholar]
- 217.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J. Comp. Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 218.Greco B, Edwads DA, Michael RP, Zumpe D, Clancy AN. Colocalization of androgen receptors and mating-induced FOS immunoreactivity in neurons that project to the central tegmental field in male rats. J. Comp. Neurol. 1999;408:220–236. doi: 10.1002/(sici)1096-9861(19990531)408:2<220::aid-cne6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 219.Cicirata F, Parenti R, Spinella F, et al. Genomic organization and chromosomal localization of the mouse Connexin36 (mCx36) gene. Gene. 2000;251:123–130. doi: 10.1016/s0378-1119(00)00202-x. [DOI] [PubMed] [Google Scholar]
- 220.Klinge CM. Estrogen receptor interaction with estrogen response elements. Nuc. Acid. Res. 2001;29:2905–2919. doi: 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.O'lone R, Frith MC, Karlsson EK, Hansen U. Genomic targets of nuclear estrogen receptors. Mol. Endocrinol. 2004;18:1859–1875. doi: 10.1210/me.2003-0044. [DOI] [PubMed] [Google Scholar]
- 222.Kleitman N. Sleep and Wakefulness. Chicago: University of Chicago Press; 1963. [Google Scholar]
- 223.Sterman MB, Hoppenbrouwers T. The development of sleep-waking and rest-activity patterns from fetus to adult in man. In: Sterman MB, McGinty DJ, Adinolfi AM, editors. Brain Development and Behavior. New York: Academic Press; 1971. pp. 203–227. [Google Scholar]
- 224.Othmer E, Hayden MP, Segelbaum R. Encephalic cycles during sleep and wakefulness: a 24 hour pattern. Science. 1969;164:447–449. doi: 10.1126/science.164.3878.447. [DOI] [PubMed] [Google Scholar]
- 225.Kripke DF, O'Donoghue JP. Perpetual deprivation, REM sleep and ultradian biological rhythm. Psychophysiol. 1968;5:231–232. [Google Scholar]
- 226.Globus GG, Drury RL, Phoebus E, Boyd R. Ultradian rhythms in human performance. Percept. Motor Skills. 1971;33:1171–1174. doi: 10.2466/pms.1971.33.3f.1171. [DOI] [PubMed] [Google Scholar]
- 227.Klein R, Armitage R. Rhythms in human performance: 1 1/2- hour oscillations in cognitive style. Science. 1979;204:1326–1328. doi: 10.1126/science.451541. [DOI] [PubMed] [Google Scholar]
- 228.Rechstaffen A, Wolpert E, Dement W, Mitchell S, Fisher S. Nocturnal sleep in narcoleptics. Electroenceph. Clin. Neurophysiol. 1963;15:599–609. doi: 10.1016/0013-4694(63)90032-4. [DOI] [PubMed] [Google Scholar]
- 229.Friedman S, Fisher C. On the presence of a rhythmic, diurnal, oral instinctual drive cycle in man. Amer. Psychoanal. Assoc. 1967;15:317–343. doi: 10.1177/000306516701500205. [DOI] [PubMed] [Google Scholar]
- 230.Oswald I, Merrington J, Lewis H. Cyclical ‘on demand’ oral intake by adults. Nature. 1970;225:959–960. doi: 10.1038/225959a0. [DOI] [PubMed] [Google Scholar]
- 231.Orr WC, Hoffman HJ. A 90-min cardiac biorhythm: methodology and data analysis using modified periodograms and complex demodulation. IEEE Trans. Biomed. Eng. 1974;21:130–143. doi: 10.1109/TBME.1974.324299. [DOI] [PubMed] [Google Scholar]
- 232.Lavie P, Kripke DF. Ultradian circa 1 1/2 hour rhythm: a multioscillatory system. Life Sci. 1981;29:2445–2450. doi: 10.1016/0024-3205(81)90698-6. [DOI] [PubMed] [Google Scholar]
- 233.Monk TH, Buysse DJ, Reynolds CF, et al. Circadian rhythms in human performance and mood under constant conditions. J. Sleep Res. 1997;6:9–18. doi: 10.1046/j.1365-2869.1997.00023.x. [DOI] [PubMed] [Google Scholar]
- 234.Gronfier C, Simon C, Piquard F, Ehrhart J, Brandenberger G. Neuroendocrine processes underlying ultradian sleep regulation in man. J. Clin. Endoc. Metab. 1999;84:2686–2690. doi: 10.1210/jcem.84.8.5893. [DOI] [PubMed] [Google Scholar]
- 235.Kripke DF, Halberg F, Crowley TJ, Pegram VG. Ultradian spectra in monkeys. Int. J. Chronobiol. 1976;3:193–204. [Google Scholar]
- 236.Delorme F, Vimont P, Jouvet M. Etude statistique du cycle veille-sommeils chez le chat. C. R. Soc. Biol. (Paris) 1964;58:2128–2130. [PubMed] [Google Scholar]
- 237.Sterman MB, Knauss T, Lehmann D, Clemente CD. Circadian sleep and waking patterns in the laboratory cat. Electroenceph. Clin. Neurophysiol. 1965;19:509–517. doi: 10.1016/0013-4694(65)90191-4. [DOI] [PubMed] [Google Scholar]
- 238.Ursin R. The two stages of slow wave sleep in the cat and their relation to REM sleep. Brain Res. 1968;11:347–356. doi: 10.1016/0006-8993(68)90030-9. [DOI] [PubMed] [Google Scholar]
- 239.Sterman MB, Lucas EA, MacDonald LR. Periodicity within sleep and operant performance in the cat. Brain Res. 1972;38:327–341. doi: 10.1016/0006-8993(72)90716-0. [DOI] [PubMed] [Google Scholar]
- 240.Roldan E, Weiss T, Fifkova E. Excitability changes during the sleep cycle of the rat. Electroenceph. Clin. Neurophysiol. 1963;15:775–785. doi: 10.1016/0013-4694(63)90168-8. [DOI] [PubMed] [Google Scholar]
- 241.Van Twyler H. Sleep patterns of five rodent species. Physiol. Behav. 1969;4:901–905. [Google Scholar]
- 242.Timo-Iaria C, Negrao N, Schmidek WR, Hishino K, De Menezes EL, Da Rocha TL. Phases and states of sleep in the rat. Physiol Behav. 1970;5:1057–1062. doi: 10.1016/0031-9384(70)90162-9. [DOI] [PubMed] [Google Scholar]
- 243.Garcia-Rill E, Miyazato H, Skinner RD, Williams K. Periodicity in the amplitudes of the sleep state-dependent, midlatency auditory evoked P1 potential in the human and P13 potential in the rat. Neuroscience. 1999;25:627. [Google Scholar]
- 244.Garcia-Rill E, Moran K, Garcia J, Findley M, Strotman B, Llinas R. Magnetic sources of the M50 response are localized to frontal cortex. Clin Neurophysiol. 2007;119:388–398. doi: 10.1016/j.clinph.2007.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]