Abstract
Study objectives:
Previous investigators have suggested that quiet sleep (QS) in rats develops rapidly upon the emergence of cortical delta activity around postnatal day (P)11 and that the presence of “half-activated” active sleep (AS) suggests that infant sleep is initially disorganized. To address these issues, we examined the temporal organization of sleep states during the second postnatal week in rats as delta activity emerges.
Design:
Subjects were P9, P11, and P13 Sprague-Dawley rats. Electroencephalogram and nuchal electromyogram electrodes were implanted, and data were recorded at thermoneutrality for 2 hours.
Results:
At all ages, using electromyogram and behavioral criteria, QS (defined as nuchal atonia and behavioral quiescence) dominated the first third of each sleep period, whereas AS (defined as nuchal atonia accompanied by myoclonic twitching) dominated the last third. When delta activity, which was first detected at P11, could be added to the definition of QS, gross assessments of sleep-state organization were not altered, although it was now possible to identify brief periods of QS interposed between periods of AS. No evidence of “half-activated” AS was found. Finally, “slow activity transients” were detected and were primarily associated with QS; their rate of occurrence declined as delta activity emerged.
Conclusions:
When delta activity emerges at P11, it integrates smoothly with periods of QS, as defined using electromyogram and behavioral criteria alone. Delta activity helps to refine estimates of QS duration but does not reflect a significant alteration of sleep-state organization. Rather, this organization is expressed much earlier in ontogeny as fluctuations in muscle tone and associated phasic motor activity.
Citation:
Seelke AMH; Blumberg MS. The microstructure of active and quiet sleep as cortical delta activity emerges in infant rats. SLEEP 2008;31(5):691–699.
Keywords: Development, EEG, behavior, cortex, slow activity transient, early network oscillation, GABA
SLEEP AND WAKEFULNESS ARE BEHAVIORAL STATES COMPRISING TONIC AND PHASIC CHANGES IN MUSCLE AND BRAIN ACTIVITY. TRADITIONALLY, THESE states are identified by monitoring muscle tone, (electromyogram [EMG]), eye movements, (electrooculogram [EOG]), and neocortical activity (electroencephalogram [EEG]).1 In infant rats, state-dependent EEG activity—in the form of 1- to 4-Hz delta waves—does not emerge until around postnatal day (P)11.2–5 Nonetheless, nuchal EMG and EOG activity reliably mirror behavioral assessments of state, including quiet sleep (QS) and active sleep (AS), as early as P2-3,6,7 and is sufficient for demonstrating state-dependent neural activity within the brainstem as early as P8.8,9
Thus, significant progress has been made in our understanding of sleep development at ages during which EEG measures are unavailable. Nonetheless, rightly or not, for many the EEG remains the gold standard of sleep measurement. This emphasis on the EEG has engendered some confusion concerning the definition, quantity, and structure of infant sleep before and after the emergence of delta activity. For example, Jouvet-Mounier et al5 reported that AS is prominent soon after birth but that the quantity of QS is negligible during the early postnatal period and increases explosively with the emergence of delta activity at P11. This observation suggested to them that the brain mechanisms needed to express QS develop later than those needed to express AS. Furthermore, Jouvet-Mounier et al5 reported that, in the days after their emergence, bouts of delta activity are often intermixed with AS-related myoclonic twitching, which they designated as “half-activated” AS. Subsequent investigators have reported similar results.3,10,11 The presence of such ambiguous sleep states provided a foundation for Frank and Heller's “presleep hypothesis.”3,12
In contrast, in a previous study, we found no evidence for half-activated AS in P14 rats.7 Nonetheless, because we did not examine younger infants as delta activity was emerging, it remained unclear how delta integrates with already-existing QS and AS at the very earliest moments of its developmental expression. Therefore, here we provide a detailed analysis of sleep-state organization in P9, P11, and P13 rats, that is, during the 5-day period surrounding the emergence of delta activity. At all ages, nuchal EMG and behavioral criteria were used to identify bouts of QS and AS, and, for comparison, these criteria were supplemented with EEG activity at P11 and P13. We found no evidence of disorganization (i.e., half-activated AS) or reorganization (i.e., explosive increase in QS) of sleep structure across the emergence of delta activity. All together, these results reveal general principles of state organization that hold true across an important developmental transition.
METHODS
Subjects
A total of 18 P9, P11, and P13 rats (n = 6 at each age) from 18 litters were used. At the time of testing, body weights ranged from 20.9 to 37.0 grams. All pups were born to Harlan Sprague-Dawley rats housed in the animal colony at the University of Iowa. The pups were raised in litters that were culled to 8 pups within 3 days of birth (day of birth = P0). Litters and mothers were raised in standard laboratory cages (48 cm long × 20 cm wide × 26 cm high), in which food and water were available ad libitum. All rats were maintained on a 12-hr light-dark schedule with lights on at 0700.
Test Environment
Pups were tested inside an electrically shielded double-walled glass chamber (height = 17.0 cm; i.d. = 12.5 cm) with a Plexiglas lid. Air temperature inside the chamber was regulated using a temperature-controlled water circulator. Access holes in the side and lid of the chamber allowed for the flow of air through the chamber as well as the passage of EMG and EEG electrodes. A round platform constructed of polyethylene mesh was fitted inside the chamber, and a perforated felt pad was placed on top of the mesh.
Procedure
On the day of testing, a pup was removed from the litter, weighed, and anesthetized using isoflurane. An incision was made in the scalp, which was then retracted to expose the skull. Holes were drilled over the cerebellum and left parietal and frontal cortices. Electrodes consisting of 3 skull screws (shaft diameter: 1.19 mm; #00-96 × 1/16; Plastics One, Roanoke, VA) attached to insulated silver wires (AG 10T; Medwire, Mt. Vernon, NY) and inserted to a depth of 1.5 mm below the top surface of the skull (at histology, it was found that the screws produced only shallow indentations on the cortical surface). The frontal and parietal electrodes served as the recording and reference electrodes, respectively, and the cerebellar electrode served as the ground. The scalp was closed, and bipolar stainless-steel hook electrodes (50-μm diameter, California Fine Wire, Grover Beach, CA) were bilaterally implanted into the nuchal muscle and secured with collodion. The subject was placed inside the testing chamber, which was maintained at thermoneutrality (i.e., 35°C at P9, 33.5°C at P11, and 32°C at P13), for 2 hours to recover and acclimate. Pups were tested while freely moving. Electrographic (nuchal EMG and cortical EEG) data were collected for 2 hours, 1 hour of which was recorded to digital videotape. Although each subject was separated from its mother for as long as 5 hours, we have shown previously in younger subjects that even longer separations do not significantly alter sleep and wake durations.13 After testing, the subject was euthanized using an overdose of nembutal.
Nuchal EMG and cortical EEG electrodes were connected to differential amplifiers (A-M Systems, Carlsborg, WA), and their signals were amplified (×10,000) and filtered (EMG: 300-5000 Hz; EEG: 0.1-5000 Hz). EMG and EEG data were digitized at 1 kHz using a data-acquisition system (BioPac Systems, Santa Barbara, CA) and simultaneously visualized by the experimenter during the test. A microcamera placed above the chamber lid allowed for the monitoring and recording of behavior. EMG and video data were recorded to digital videotape using a data recorder (DV8; WinTron Technologies, Rebersberg, PA).
Analysis
For each subject, a 30-minute synchronized digital record of behavior, EMG activity, and EEG activity was created. During digitization, the pup's behavior was observed on a monitor as an event recorder was used to identify sleep- and wake-related movements. As described elsewhere,14,15 myoclonic twitches, indicative of AS, were defined as phasic, rapid, and independent movements of the limbs and tail. High-amplitude coordinated movements, indicative of wakefulness, included locomotion, stretching, and yawning.
Mean durations of sleep and waking periods were determined as previously decribed.6 Briefly, nuchal EMG records were integrated and full-wave rectified. The EMG signal was then dichotomized into periods of high tone and atonia. For each pup, the amplitude of five 1-second representative segments of high tone and atonia were measured and averaged, and the midpoint between the 2 means was calculated. A period of muscle atonia (indicative of sleep) was defined as a period in which the mean amplitude of muscle tone was below the midpoint for at least 1 second. Likewise, a period of high tone (indicative of wakefulness) was defined as a period in which the mean amplitude of muscle tone was above the midpoint for at least 1 second. The duration of each high-tone and atonia period was then measured to the nearest 0.1 second.
Periods of nuchal muscle atonia, indicative of sleep, were further divided into QS and AS durations. QS durations were characterized by a period of nuchal muscle atonia coupled with behavioral quiescence and, from P11 on, by the appearance of delta activity in the neocortical EEG. AS durations were characterized by a period of nuchal atonia coupled with myoclonic twitching and, in the EEG after P11, the absence of delta activity. As with periods of high tone and atonia, QS and AS durations were measured to the nearest 0.1 second.
Power spectra for each age and state were generated by identifying 10 periods of wakefulness, QS, and AS with a duration of at least 30 seconds in each subject and performing spectral analyses (Hanning window, fast Fourier transform size = 1024) on each selected period using Spike2 software (CED, Cambridge, UK). For each behavioral state, the resulting values were averaged within each subject, and mean power spectra for each age and state were produced.
The mean amplitude of nuchal EMG during periods of wakefulness, QS, and AS was determined using 10-second samples from 10 separate representative bouts of wakefulness, QS, and AS in each P11 and P13 subject. For this analysis, behavioral states were categorized on the basis of EEG and behavior (thus, P9 subjects were excluded from this analysis). The samples were averaged within each subject. The mean amplitude of the EMG during each behavioral state was analyzed using analysis of variance (ANOVA; Statview, SAS, Cary, NC). Unless otherwise specified, planned comparisons were performed here and elsewhere to assess within-age differences. For all analyses, α < .05.
The distribution of delta activity throughout an atonia period was calculated by finding the first 6 atonia periods in each record that contained periods of both QS and AS. Each atonia period, regardless of length, was divided into thirds. To compute the proportion of total delta activity that occurred within each third of a sleep period, the duration of delta activity within each third was determined and divided by the total duration of delta activity that occurred within the sleep period. The values for each third were averaged first within subjects and then within ages and compared using ANOVA.
Behavioral states were determined using 2 different criteria. Criterion 1 entailed the use of EMG and behavior, but not EEG, to identify behavioral states. Under Criterion 1, periods of QS were defined as the time from the onset of nuchal muscle atonia until the onset of twitching, and periods of AS were defined as the time from the onset of twitching until the onset of wakefulness. Therefore, using this criterion, we assumed that there could be no more than 1 QS and 1 AS bout per sleep period.
Criterion 2 entailed the use of EEG, EMG, and behavior to identify behavioral states. Under Criterion 2, periods of QS were defined on the basis of nuchal atonia, behavioral quiescence, and delta activity. Periods of AS were defined on the basis of nuchal atonia, myoclonic twitching, and the absence of delta activity. Moreover, this criterion allowed for multiple QS and AS bouts within a sleep period. Finally, due to the reliance of this criterion on EEG activity, it was only applied to P11 and P13 subjects.
Using Criteria 1 and 2, the distribution of QS bouts within a sleep period was determined. To do this, 6 sleep periods comprising bouts of QS and AS were identified for each subject, and these periods were divided into thirds (regardless of length). Then, the proportion of time spent in QS during each third of the sleep period was calculated. The values determined using each scoring criteria were compared using unpaired t tests.
Mean bout durations of wakefulness, QS, and AS were determined by first identifying all available periods of wakefulness, QS, and AS in each record using both Criterion 1 and Criterion 2. The proportion of time spent in each state was calculated and averaged for each subject prior to statistical analysis using ANOVA.
The proportion of atonia periods that were composed entirely of QS (QS-only), entirely of AS (AS-only), or a combination of QS and AS bouts (QS-AS) was determined by examining the first 10 sleep periods for each subject. (Because AS-only periods were never found, they are not discussed further.) The proportion and mean duration of QS-only and QS-AS bouts was determined. For QS-AS bouts, the mean duration of the first QS bout (designated QS1) was also determined.
Slow activity transients (SATs), previously described in premature human infants,16,17 were defined here in infant rats as discrete, high-amplitude, cortical events with a duration of approximately 5 seconds (SATs also often contain embedded high-frequency activity). The number, duration, and peak-to-trough amplitude of SATs were assessed and averaged within each subject and then averaged across subjects at each age. The distribution of SATs within QS-AS sleep periods was determined for each subject across the entire recording period and then averaged across subjects at each age. Finally, we compared the SATs recorded using stainless-steel skull screws with DC-coupled recordings obtained in unanesthetized head-fixed subjects using Ag-AgCl electrodes in contact with the cortical surface. Using either method, similar large-scale events were readily identifiable (data not shown), although it should be stressed that DC-coupled recordings provide a more accurate depiction of EEG waveforms.16
For illustrative purposes, wavelet analyses were performed on select EEG data using a Morelet wavelet. For these analyses, custom-written code was implemented using the Matlab wavelet toolbox (The MathWorks, Natick, MA).
RESULTS
Representative Sleep-Wake Cycle
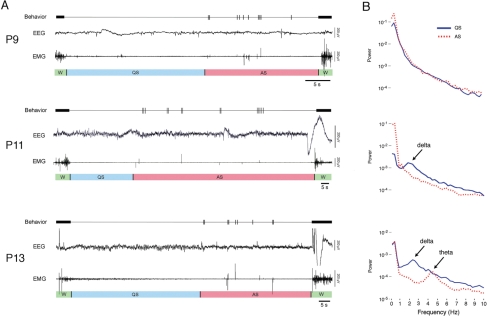
Figure 1A depicts representative sleep-wake cycles in P9, P11, and P13 subjects. For each example, the cycle begins with wakefulness, characterized by high-amplitude coordinated movements and high muscle tone. As the subject visibly relaxes and muscle tone gradually decreases, the subject enters a period of QS. The onset of AS is identified by the presence of twitching in the nuchal muscle, limbs, and tail, along with muscle atonia. Finally, the subject awakens, again exhibiting high muscle tone and coordinated movements.
Figure 1.
(A) Representative data from postnatal day (P)9, P11, and P13 rats spanning 1 sleep-wake cycle (W: wakefulness; QS: quiet sleep; AS: active sleep). For each subject, the top trace depicts behaviorally scored coordinated movements (thick horizontal bars) and myoclonic twitches (vertical tics), the middle trace depicts neocortical electroencephalogram (EEG) activity, and the bottom trace depicts nuchal electromyogram (EMG) activity. Behavioral states are indicated beneath each EMG trace. For these examples, only sleep periods comprising 1 QS and AS bout were chosen. (B) Representative spectral analyses of EEG activity during QS (blue) and AS (red). Each power spectrum was produced from 10 AS and QS bouts from a single subject at each age. The first delta-related spectral peak occurred during QS in the P11 subjects.
At P9, the neocortical EEG record never exhibited any obvious state-dependent activity, and so this measure could not contribute to the determination of behavioral states at this age. At P11, the first evidence of delta activity was detected, thus allowing neocortical EEG activity to be included in the identification of QS. Delta activity was also present at P13 and exhibited higher power than was seen at P11.
Spectral Analysis
Representative results of spectral analyses performed on neocortical EEG activity during periods of QS and AS are shown in Figure 1B (power spectra for wakefulness did not reveal any differences across age and so are not depicted in the figure). At P9, AS and QS exhibited indistinguishable spectral features. In contrast, at P11, there was a peak in the power spectrum of QS between 1 and 4 Hz, indicating the emergence of delta activity. At P13, the delta peak during QS was even more pronounced. Also, there was a peak in the power spectrum between 4 and 6 Hz during AS, which is within the range of theta activity, as seen in adult rats.18,19
EMG Amplitude
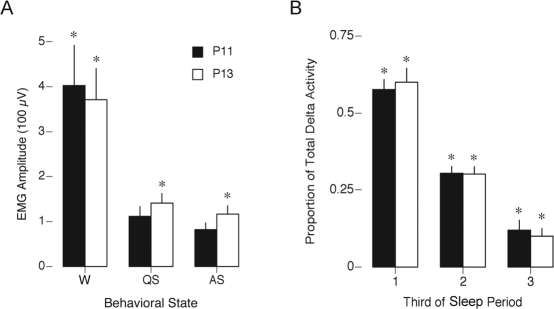
In previous studies,7,9,20 we have used nuchal EMG and behavior to assess sleep and wakefulness in subjects younger and older than P9 (i.e., before and after the emergence of delta activity). Here, we assessed the validity of EMG amplitude as a measure of state using EEG and behavior to categorize periods of wakefulness, QS, and AS and then measuring the associated mean EMG amplitudes. For this analysis, only P11 and P13 subjects could be included. At each age, the mean EMG amplitude was highest during wakefulness and was significantly lower during both QS (t11 = 6.3, p < .0001) and AS (t11 = 6.3, p < .0001) (Figure 2A). At both ages, mean amplitude for QS was slightly higher than that for AS, but this difference was only significant at P13 (P13: t5 = 2.7, p < .05; P11: t5 = 2.2, NS). There was no effect of age on EMG amplitude for any state (wake: F1, 10 = .1, NS; QS: F1, 10 = .9, NS; AS: F1, 10 = 2.1, NS). Thus, as a practical matter for pups at these ages, nuchal EMG amplitude alone is sufficient to discriminate between sleep and wakefulness but not between AS and QS. For such discriminations, behavior, EEG, or twitch-related phasic activity discernible in the EMG record must also be included.7
Figure 2.
(A) Mean nuchal electromyogram amplitude during wakefulness (W), quiet sleep (QS), and active sleep (AS) in postnatal day (P)11 and P13 rats. Behavioral states were identified using only behavior and cortical electroencephalogram activity. *Significantly different from other 2 states within an age. (B) Mean proportion of total delta activity occurring within each third of a sleep period. *Significantly different from other two thirds within an age. Mean + SEM.
Distribution of Delta Activity Within a Sleep Period
Previous work indicated that, in infant rats, QS—as determined using nuchal EMG and behavior—occurs predominantly during the first third of the sleep period.7,13 Here, we assessed the linkage between QS and emerging delta activity by quantifying the distribution of delta activity across sleep periods. As shown in Figure 2B, delta was most prevalent during the first third of the sleep period at both P11 and P13, and the proportion of delta activity decreased significantly as the sleep period progressed (F2, 27 = 121.8, p < .0001). There was no significant difference between the distributions of delta activity at P11 and P13.
Organization of AS and QS Within a Sleep Period
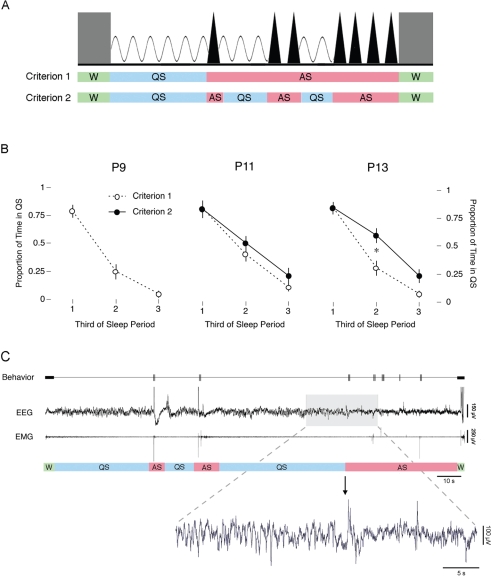
As illustrated in Figure 3A, 2 different scoring criteria were used to analyze the same data. For Criterion 1, nuchal EMG and behavior were used to assess wakefulness, QS, and AS, with the assumption that only 1 QS and AS bout occurs during any given sleep period. For Criterion 2, delta activity was also used, with the result that each sleep period could now comprise more than 1 QS bout. Because of the absence of delta activity at P9, only Criterion 1 could be used at that age.
Figure 3.
(A) Schematic of 2 alternative criteria used to define behavioral states (W: wakefulness; QS: quiet sleep; AS: active sleep). Grey blocks represent periods of W, sinussoidal waves represent delta activity, and triangles represent AS-related bursts of phasic activity. In Criterion 1, behavioral states were identified using nuchal electromyogram (EMG) and behavioral criteria. QS periods were defined as the time from the onset of atonia to the first myoclonic twitch, and AS periods were defined as the time from the first twitch to the onset of high muscle tone (regardless of the occurrence of interposed periods of behavioral quiescence). In Criterion 2, behavioral states were identified using electroencephalogram (EEG), EMG, and behavioral data. This criterion allowed for the occurrence of QS bouts interposed between AS bouts when positive evidence of delta activity was found. (B) Mean proportion of time spent in QS during each third of a sleep period. QS periods were identified using Criterion 1 (open circles) or Criterion 2 (filled circles). *Significant difference between 2 criteria. Mean ± SEM. (C) Top: Representative data from a subject on postnatal day 11 depicting a single sleep period comprising multiple QS and AS bouts. The top trace depicts behaviorally scored coordinated movements (thick horizontal bars) and myoclonic twitches (vertical tics), the middle trace depicts neocortical EEG activity, and the bottom trace depicts nuchal EMG activity. Behavioral states are identified beneath the EMG trace. The shaded region highlights the third QS bout of the sleep period as it transitions to the third AS bout. Bottom: EEG activity during the QS-AS transition highlighted above is depicted in expanded form. Before the transition to AS, bursts of 1- to 4-Hz delta activity can be seen. Once the transition to AS occurs, delta activity disappears.
Figure 3B presents, at each age, the proportion of QS that occurred during each third of the sleep period using Criterion 1 or 2. Regardless of age, QS dominated the first third of the atonia period, whereas AS dominated the final third. This was true whether behavioral state was determined using Criterion 1 (F2, 35 = 29.6, p < .0001) or Criterion 2 (F2, 35 = 47.8, p < .0001). However, the proportion of time spent in QS was different when behavioral states were determined using Criterion 1 versus Criterion 2. This difference was most apparent in the last two thirds of the sleep period, when delta activity could be used to positively identify QS bouts interposed between bouts of AS.
Figure 3C presents an example of delta activity that occurred after a bout of AS in a P11 subject. The top panel presents behaviorally scored coordinated movements and myoclonic twitches, neocortical EEG, and nuchal EMG. Behavioral states, identified using Criterion 2, are indicated below. Bouts of QS are interspersed between bouts of AS. The gray box highlights a section of EEG data which is expanded below. In that expansion, it can be seen that 1- to 4-Hz delta activity ceases when AS-related twitching resumes.
Proportion of Time Spent in Wakefulness, QS, and AS
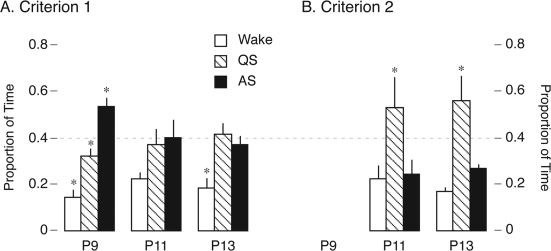
Figure 4 presents the proportion of time within each recording session spent in wakefulness, QS, and AS across age, as determined using Criterion 1 (Figure 4A) or Criterion 2 (Figure 4B). Again, Criterion 2 could not be used at P9. Using Criterion 1, ANOVA revealed a significant main effect of state (F2,45 = 24.6, p < .0001), no effect of age (F2, 45 = .03, NS), and a small but significant age × state interaction (F4,45 = 2.7, p < .05). Using Criterion 2, ANOVA also revealed a main effect of state (F2,30 = 43.2, p < .0001) but no effect of age and no interaction (Fs < 0.7). As a comparison between Figure 4A and Figure 4B makes evident, the addition of delta activity in Criterion 2 increases the estimation of the proportion of time spent in QS.
Figure 4.
Mean proportion of time spent by postnatal day (P)9, P11, and P13 rats in wakefulness (W), quiet sleep (QS), and active sleep (AS) determined using Criterion 1 (A) or Criterion 2 (B). See Figure 3A for comparison between 2 criteria. Because delta activity is not found at P9, Criterion 2 cannot be applied to subjects at that age. *Significantly different from other 2 states within an age. Mean + SEM.
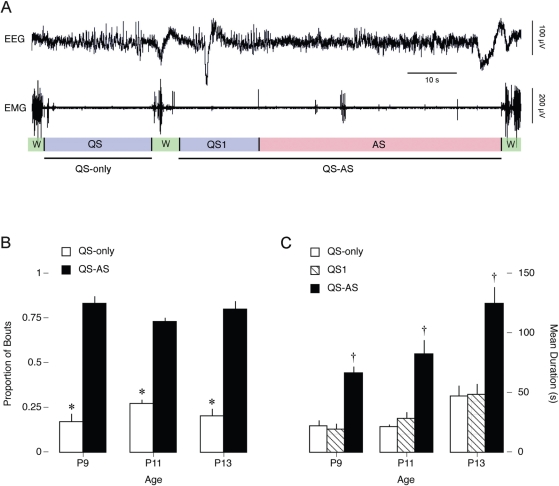
QS-Only and QS-AS Sleep Periods
The typical sleep period comprised at least 1 bout of QS and AS. However, as illustrated in Figure 5A, sleep periods can exhibit a single bout of QS followed directly by wakefulness. We quantified the occurrence of such QS-only sleep bouts in relation to QS-AS sleep periods (Figure 5B). ANOVA revealed that a significantly higher proportion (80% vs 20%) of sleep bouts were of the AS-QS type (F1,30 = 260.4, p < .0001), and there was no effect of age (F2,30 = 0) and no type × age interaction (F2,30 = 2.9).
Figure 5.
(A) Representative data from a rat on postnatal day (P)11 depicting 2 sleep-wake cycles. The top trace depicts neocortical electroencephalogram (EEG) activity and the bottom trace depicts nuchal electromyogram (EMG) activity. Behavioral states are indicated beneath each EMG trace (W: wakefulness; QS: quiet sleep; AS: active sleep). The first sleep period is designated “QS-only” as the single bout of QS transitions directly back into W. The second sleep period is designated “QS-AS” as it is composed of an initial bout of QS (QS1) followed by at least 1 bout of active sleep (AS). (B) Mean proportion of sleep periods consisting of QS-only (white bars) or QS-AS (black bars) bouts. *Significantly different from QS-AS. (C) Mean durations of QS-only (white bars), QS1 (hatched bars), and QS-AS (black bars) bouts. †Significantly different from other 2 bout types. Mean + SEM.
We next assessed the mean durations of QS-only, QS1 (i.e., the first QS bout of a QS-AS sleep period), and QS-AS bouts across subjects (Figure 5C). ANOVA revealed a significant difference in mean duration across bout type (F2, 41 = 42.8, p < .0001), as well as a significant effect of age (F2, 41 = 13.7, p < .0001), but there was no type × age interaction (F4,41 = 1.1). Importantly, the mean durations of QS-only and QS1 bouts were not significantly different.
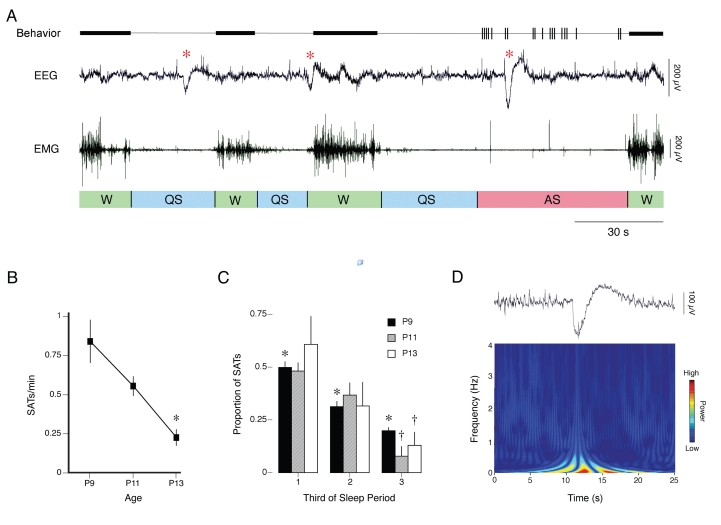
Slow Activity Transients
Examples of SATs in a P9 rat are shown in Figure 6A (red asterisks). SAT amplitude and duration ranged from 396 to 808 μV and 5.2 to 6.6 seconds, respectively, and did not differ significantly across age (F2,6s < 1.7, NS). As shown in Figure 6B, the number of SATs per minute decreased significantly with age (F1,13 = 24.8, p < .0005). SATs were detected during periods of wakefulness, QS, and AS. However, as shown in Figure 6C, when SATs occurred during QS-AS sleep periods, they were significantly more likely to occur during the first third (F2,33 = 30.7, p < .0001), regardless of age (F2,33 = .34, NS); there was no period × age interaction (F4,33 = .95, NS).
Figure 6.
Characterization of slow activity transients (SATs). (A) Representative data from a rat on postnatal day (P)9 depicting SATs occurring within 3 sleep-wake cycles (W: wakefulness; QS: quiet sleep; AS: active sleep). For each subject, the top trace depicts behaviorally scored coordinated movements (thick horizontal bars) and myoclonic twitches (vertical tics), the middle trace depicts neocortical electroencephalogram (EEG) activity, and the bottom trace depicts nuchal electromyogram (EMG) activity. Behavioral states are indicated beneath each EMG trace. SATs (identified by red asterisks) occur during QS, the transition between QS and W, and AS. (B) Mean number of SATs per minute at P9, P11, and P13. *Significantly different from other 2 ages. Mean ± SEM. (C) Mean proportion of SATs occurring during each third of QS-AS sleep periods at P9 (black bars), P11 (gray bars), and P13 (white bars). *Significantly different from other 2 periods. †Significantly different from first period. Mean + SEM. (D) Top: representative SAT from a P11 subject. Bottom: time-frequency wavelet analysis of the waveform depicted above, showing an SAT-related burst of low-frequency activity. Note the embedded 1- to 4-Hz delta activity.
The top panel of Figure 6D depicts an SAT that occurred during QS in a P11 subject. The bottom panel presents a wavelet analysis of that waveform, showing the low-frequency SAT event. Finally, embedded 1- to 4-Hz delta activity can be seen in the raw waveform as well as in the wavelet.
DISCUSSION
In this study, we examined the microstructure of infant rat sleep across a significant developmental transition: the emergence of state-dependent neocortical EEG in the form of delta activity. In agreement with previous studies2–5 and contrary to a recent conjecture concerning strain differences and EEG development,12,21 we found that delta activity emerges around P11 in Sprague-Dawley rats. Furthermore, as delta activity emerged, it was found exclusively during periods of behavioral quiescence. Thus, consistent with a previous report in which scoring epochs were also not used,7 we have again found no evidence of half-activated AS, as has been reported by others.3,5,10,11 We conclude that if half-activated AS truly exists in infants, then it is an exceedingly rare phenomenon. In any event, it certainly does not offer a firm foundation for redefining infant sleep.12
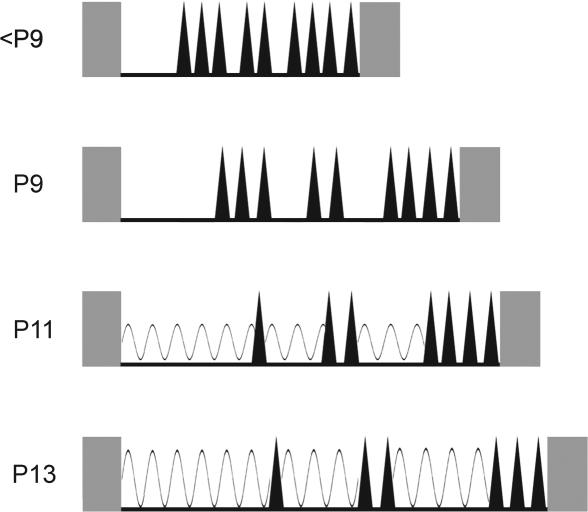
The main results of this experiment can be summarized using the schematic in Figure 7. The atonia periods depicted in the figure are composed of an initial bout of QS followed by bouts of AS-related myoclonic twitching. This basic pattern was found in P9, P11, and P13 subjects (the pattern for subjects younger than P9 is included for comparison). When delta activity emerges at P11, it predominates early in the sleep period but can also be found during periods of behavioral quiescence interposed between bouts of AS. Thus, upon the developmental emergence of delta activity, we can positively identify multiple cycles of QS and AS, as in adults.22 This pattern becomes even clearer by P13.
Figure 7.
Schematic depiction of the developmental emergence of delta activity. Grey rectangles represent periods of high muscle tone, indicative of wakefulness. Interposed periods of sleep are defined by nuchal atonia, depicted as black lines. Phasic bursts of myoclonic twitching, indicative of active sleep, are depicted as black triangles. At postnatal day (P)9 and earlier, each sleep period comprises an initial bout of quiet sleep (QS) followed by bursts of phasic activity interrupted by brief bouts of behavioral quiescence. By P11, delta activity (depicted as sinusoidal waves) is detected during the first QS episode as well as during some of the subsequent periods of quiescence between bouts of active sleep (AS). By P13, delta power has increased and is more reliably expressed during periods of behavioral quiescence. Overall, with age, sleep durations increase and the intervals between bouts of AS also increase, thus providing greater opportunity for the expression of delta activity during the final third of a sleep bout.
Given what we now know about the expression of QS and AS at P13, we can reexamine the structure of sleep at earlier ages. As already mentioned, here and elsewhere,7,13 we have used EMG and behavioral criteria alone to identify QS. Within days after birth, one can identify sleep periods comprising bursts of phasic activity within the nuchal and extraocular EMG, as well as observable twitches of the limbs and tail. These bursts begin shortly after the onset of atonia and continue throughout the duration of the sleep period. These bursts of activation consist of synchronized activity in multiple muscle groups throughout the body, resulting in discrete bouts of activity with interposed periods of behavioral quiescence. These periods of quiescence are initially very brief: during the first postnatal week, they often last less than 2 seconds. At later ages, as shown here, these periods of quiescence elongate and, at P11, begin to occasionally exhibit associated delta activity.
As long as QS is defined by the presence of delta activity—as opposed to merely the absence of phasic activity—we cannot be certain whether a brief bout of quiescence in “pre-delta” animals should be designated as QS. Nonetheless, it is clear that alternations between active and quiet sleep periods are expressed similarly before, during, and after the emergence of delta.7 What appear to change are the durations of AS-related bursts of phasic activity and intervening periods of quiescence. Still, future investigations of sleep development would benefit from additional measures of cortical or subcortical activity that might be used to discern QS independently of delta across early development and into adulthood. SATs may provide one such measure—as shown here, they are linked with QS prior to the emergence of delta—but their rapid diminution after P11 precludes their use for establishing connections between infant and adult QS.
It is clear that QS dominates the first third of sleep periods in rats as early as P37 and that this pattern continues up until the emergence of delta activity at P11.13 These results seem inconsistent with the findings of Jouvet-Mounier et al, who reported that QS in infant rats is virtually nonexistent through P10.5 The discrepancy between these findings is likely due to the restrictive criteria used by those earlier investigators for defining QS. Specifically, 30 seconds of behavioral quiescence were required before the designation of QS was applied. When we consider that the mean sleep-bout duration during the first postnatal week ranges from 20 seconds at P3 to 45 seconds at P820 and that, in this study, mean QS duration only exceeded 30 seconds at P13, a 30-second criterion effectively precludes the detection of QS bouts in early infancy.
We have compared here 2 alternative criteria for assessing QS and AS durations in infant rats. Criterion 1 relied exclusively upon nuchal EMG and behavioral measures and assumed that a single bout of QS could only be followed by a single bout of AS. This criterion has been used in a previous study of P1 rats.13 In contrast, Criterion 2 allowed for the expression of multiple QS bouts, but only when delta activity was detected. These criteria resulted in different estimates of the distributions of QS during sleep periods (Figures 3 and 4). In effect, they provide upper and lower estimates of the proportion of time spent in QS and AS at these ages.
We did not attempt here to modify Criterion 1 to include periods of behavioral quiescence interposed between bouts of AS. Such a modification to Criterion 1 might be valid in older subjects and might lead to estimates of the microstructure of AS and QS that are more similar to those obtained using Criterion 2. However, such a modification to Criterion 1 becomes increasingly impractical in younger “pre-delta” subjects in which pauses between bouts of twitching are less easily distinguishable from pauses within bouts of twitching (as illustrated in the top panel of Figure 7). Importantly, because delta activity is the only positive indicator of QS, we cannot distinguish between QS and “tonic AS” before the emergence of delta at P11. Therefore, the question of whether bouts of QS are expressed between bouts of AS in neonatal rats may be properly described as a problem of measurement, not phenomenology. What is needed, then, are even better methods for describing bouts of phasic activity in relation to bouts of quiescence. Until such indicators are discovered, we should be aware that we might be overestimating the quantity of AS and underestimating the quantity of QS during the early postnatal period.
Approximately 20% of the sleep periods consisted of only a single bout of QS. These QS-only bouts were of similar duration to the first QS bout of sleep periods comprising 1 or more bouts of AS. Thus, it appears that the termination of the first QS bout entails a “decision” as to whether the subsequent state will be AS or wakefulness. This point was reached on average after 25 seconds at P9 and 50 seconds at P13. The factors that determine whether a transition will be made into AS or wakefulness are not known at this time.
In addition to delta, we found evidence of very low-frequency cortical events that appear similar to the SATs described in premature human infants.16,17 The developmental disappearance of SATs has been associated with the upregulation of the neuronal chloride extruder K+-Cl− cotransporter 2 (KCC2) and the associated emergence of the hyperpolarizing effects of GABA.17,23 Using calcium imaging, similar high-amplitude, low-frequency events have been observed in infant rat cortical tissue in vitro24 and neonatal mice in vivo25 and are referred to as “early network oscillations” (ENOs). As with SATs, the developmental disappearance of ENOs may also depend upon emerging GABAergic inhibition.24 Thus, the disappearance of SATs/ENOs is mirrored by the appearance of delta activity and all may be influenced by developmental changes in the expression of GABAergic inhibition.26,27
Both SATs in human infants17 and ENOs in freely moving neonatal mice25 occur during sleep. Interestingly, as shown here in infant rats, the predominance of SATs during the first third of the sleep period suggests that these events are QS-related. Nonetheless, SATs occurred during wakefulness and also appeared to occur in temporal proximity to AS-related twitching. For example, the third SAT identified in Figure 6A coincides with behaviorally scored twitches; however, it is unclear whether this SAT was indeed triggered simultaneously with a bout of twitching or during the preceding period of quiescence (that could, in turn, be indicative of QS). Regardless, the predominantly QS-related expression of SATs and their decline as delta activity emerges provides further evidence for a mechanistic link between these events and oscillations.
As we and others have argued, the EEG derives its usefulness in relation to behavior.28,29 The current study reinforces this notion by showing how the fundamental structure of sleep and wake states is already established at the time when delta activity first emerges. Thus, the absence of delta activity should not lead us to reinterpret the nature of sleep in early infancy. Rather, its presence provides additional confidence in the designations that we are able to make when it is available as a measure of QS. Finally, the smooth integration of delta activity into the existing organizational structure of infant sleep—as evidenced by tonic and phasic EMG activity and associated behaviors—once again shows how, early in infancy, coordinated brainstem activities unify behavioral states from muscle to neocortex.7,9
ACKNOWLEDGMENTS
We thank Ethan Mohns, Amy Jo Marcano-Reik, Andrew Gall, and William Todd for helpful comments and suggestions. Else Tolner and Kai Kaila provided valuable technical advice and, with Sebastian Schuchmann, generously provided helpful comments on an earlier draft of this manuscript.
Supported by a research grant (MH50701) and an Independent Scientist Award (MH66424) from the National Institute of Mental Health (to MSB).
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Rechtschaffen A, Kales A, editors. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. [Google Scholar]
- 2.Corner MH, Mirmiran M. Spontaneous neuronal firing patterns in the occipital cortex of developing rats. Int J Dev Neurosci. 1990;8:309–16. doi: 10.1016/0736-5748(90)90037-3. [DOI] [PubMed] [Google Scholar]
- 3.Frank MG, Heller HC. Development of REM and slow wave sleep in the rat. Am J Physiol. 1997;272:R1792–R9. doi: 10.1152/ajpregu.1997.272.6.R1792. [DOI] [PubMed] [Google Scholar]
- 4.Gramsbergen A. The development of the EEG in the rat. Dev Psychobiol. 1976;9:501–15. doi: 10.1002/dev.420090604. [DOI] [PubMed] [Google Scholar]
- 5.Jouvet-Mounier D, Astic L, Lacote D. Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Dev Psychobiol. 1970;2:216–39. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- 6.Karlsson KÆ, Blumberg MS. The union of the state: Myoclonic twitching is coupled with nuchal muscle atonia in infant rats. Behav Neurosci. 2002;116:912–7. doi: 10.1037//0735-7044.116.5.912. [DOI] [PubMed] [Google Scholar]
- 7.Seelke AMH, Karlsson KÆ, Gall AJ, Blumberg MS. Extraocular muscle activity, rapid eye movements, and the development of active and quiet sleep. Eur J Neurosci. 2005;22:911–20. doi: 10.1111/j.1460-9568.2005.04322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karlsson KÆ, Blumberg MS. Active medullary control of atonia in week-old rats. Neuroscience. 2005;130:275–83. doi: 10.1016/j.neuroscience.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karlsson KÆ, Gall AJ, Mohns EJ, Seelke AMH, Blumberg MS. The neural substrates of infant sleep in rats. PLoS Biol. 2005;3:891–901. doi: 10.1371/journal.pbio.0030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGinty D, Stevenson M, Hoppenbrouwers T, Harper RM, Sterman MB, Hodgman J. Polygraphic studies of kitten development: Sleep state patterns. Dev Psychobiol. 1977;10:455–69. doi: 10.1002/dev.420100506. [DOI] [PubMed] [Google Scholar]
- 11.Vogel GW, Feng P, Kinney GG. Ontogeny of REM sleep in rats: possible implications for endogenous depression. Physiol Behav. 2000;68:453–61. doi: 10.1016/s0031-9384(99)00207-3. [DOI] [PubMed] [Google Scholar]
- 12.Frank MG, Heller HC. The ontogeny of mammalian sleep: a reappraisal of alternative hypotheses. J Sleep Res. 2003;12:25–34. doi: 10.1046/j.1365-2869.2003.00339.x. [DOI] [PubMed] [Google Scholar]
- 13.Seelke AMH, Blumberg MS. Thermal and nutritional modulation of sleep in infant rats. Behav Neurosci. 2005;19:603–11. doi: 10.1037/0735-7044.119.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gramsbergen A, Schwartze P, Prechtl HFR. The postnatal development of behavioral states in the rat. Dev Psychobiol. 1970;3:267–80. doi: 10.1002/dev.420030407. [DOI] [PubMed] [Google Scholar]
- 15.Blumberg MS, Lucas DE. Dual mechanisms of twitching during sleep in neonatal rats. Behav Neurosci. 1994;108:1196–202. doi: 10.1037//0735-7044.108.6.1196. [DOI] [PubMed] [Google Scholar]
- 16.Vanhatalo S, Tallgren P, Andersson S, Sainio K, Voipio J, Kaila K. DC-EEG discloses prominent, very slow activity patterns during sleep in preterm infants. Clin Neurophysiol. 2002;113:1822–5. doi: 10.1016/s1388-2457(02)00292-4. [DOI] [PubMed] [Google Scholar]
- 17.Vanhatalo S, Palva JM, Andersson S, Rivera C, Voipio J, Kaila K. Slow endogenous activity transients and developmental expression of KCC2 in the immature human cortex. Eur J Neurosci. 2005;22:199–206. doi: 10.1111/j.1460-9568.2005.04459.x. [DOI] [PubMed] [Google Scholar]
- 18.Jouvet M. Biogenic amines and the states of sleep. Science. 1969;163:32–41. doi: 10.1126/science.163.3862.32. [DOI] [PubMed] [Google Scholar]
- 19.Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–40. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 20.Blumberg MS, Seelke AM, Lowen SB, Karlsson KA. Dynamics of sleep-wake cyclicity in developing rats. Proc Natl Acad Sci. 2005;102:14860–4. doi: 10.1073/pnas.0506340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frank MG, Heller HC. Unresolved issues in sleep ontogeny: a response to Blumberg et al. J Sleep Res. 2005;14:98–101. doi: 10.1111/j.1365-2869.2004.00430_2.x. [DOI] [PubMed] [Google Scholar]
- 22.Zepelin H, Siegel JM, Tobler I. Mammalian sleep. In: Kryger MH, Roth T, Dement W, editors. Principles and Practice of Sleep Medicine. 4th ed. Philadelphia: Elsevier Saunders; 2005. pp. 91–100. [Google Scholar]
- 23.Payne JA, Rivera C, Voipio J, Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003;26:199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 24.Garaschuk O, Linn J, Eilers J, Konnerth A. Large-scale oscillatory calcium waves in the immature cortex. Nat Neurosci. 2000;3:452–9. doi: 10.1038/74823. [DOI] [PubMed] [Google Scholar]
- 25.Adelsberger H, Garaschuk O, Konnerth A. Cortical calcium waves in resting newborn mice. Nat Neurosci. 2005;8:988–90. doi: 10.1038/nn1502. [DOI] [PubMed] [Google Scholar]
- 26.Steriade M, Curro Dossi R, Nunez A. Network modulation of a slow intrinsic oscillation of cat thalamocortical neurons implicated in sleep delta waves: Cortically induced synchronization and brainstem cholinergic suppression. J Neurosci. 1991;11:3200–17. doi: 10.1523/JNEUROSCI.11-10-03200.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terman D, Bose A, Kopell N. Functional reorganization in thalamocortical networks: Transition between spindling and delta sleep rhythms. Proc Natl Acad Sci. 1996;93:15417–22. doi: 10.1073/pnas.93.26.15417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegel JM. The evolution of REM sleep. In: Lydic R, Baghdoyan HA, editors. Handbook of Behavioral State Control. Boca Raton: CRC Press; 1999. pp. 87–100. [Google Scholar]
- 29.Blumberg MS, Karlsson KÆ, Seelke AMH, Mohns EJ. The ontogeny of mammalian sleep: A response to Frank and Heller (2003) J Sleep Res. 2005;14:91–101. doi: 10.1111/j.1365-2869.2004.00430_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]