Abstract
Study Objectives:
Exposure to low ambient temperature (Ta) depresses REM sleep (REMS) occurrence. In this study, both short and long-term homeostatic aspects of REMS regulation were analyzed during cold exposure and during subsequent recovery at Ta 24°C.
Design:
EEG activity, hypothalamic temperature, and motor activity were studied during a 24-h exposure to Tas ranging from 10°C to −10°C and for 4 days during recovery.
Setting:
Laboratory of Physiological Regulation during the Wake-Sleep Cycle, Department of Human and General Physiology, Alma Mater Studiorum-University of Bologna.
Subjects:
24 male albino rats.
Interventions:
Animals were implanted with electrodes for EEG recording and a thermistor to measure hypothalamic temperature.
Measurements and Results:
REMS occurrence decreased proportionally with cold exposure, but a fast compensatory REMS rebound occurred during the first day of recovery when the previous loss went beyond a “fast rebound” threshold corresponding to 22% of the daily REMS need. A slow REMS rebound apparently allowed the animals to fully restore the previous REMS loss during the following 3 days of recovery.
Conclusion:
Comparing the present data on rats with data from earlier studies on cats and humans, it appears that small mammals have less tolerance for REMS loss than large ones. In small mammals, this low tolerance may be responsible on a short-term basis for the shorter wake-sleep cycle, and on long-term basis, for the higher percentage of REMS that is quickly recovered following REMS deprivation.
Citation:
Amici R; Cerri M; Ocampo-Garcés A; Baracchi F; Dentico D; Jones CA; Luppi M; Perez E; Parmeggiani PL; Zamboni G. Cold exposure and sleep in the rat: REM sleep homeostasis and body size. SLEEP 2008;31(5):708–715.
Keywords: REM sleep, low ambient temperature, REM sleep homeostasis, REM sleep rebound, body size, theta power density
MANY STUDIES HAVE SHOWN THAT A SLEEP DEFICIT INDUCES A SUBSEQUENT INCREASE IN THE DURATION AND/OR IN THE INTENSITY OF SLEEP AND THAT the occurrence of sleep reduces sleep propensity. The outcome of the regulation of such a balance between sleep and wake has been addressed as “sleep homeostasis”.1,2
The major hindrance in a quantitative approach to sleep homeostasis lies in the fact that sleep consists of two different states, NREM sleep (NREMS) and REM sleep (REMS), which cyclically alternate on an ultradian basis. In particular: (1) it is not possible to carry out a selective NREMS deprivation without interfering with REMS occurrence; (2) selective REMS deprivation procedures have been shown to affect to some extent the quality of NREMS during deprivation3,4; (3) a complex interaction between NREMS and REMS rebounds has been observed following different sleep deprivation protocols5,6; and (4) NREMS and REMS regulation are influenced differently by circadian rhythmicity.7
In spite of this, many different “short-term” (minutes/hours) or “long-term” (hours/days) sleep deprivation studies seeking a homeostatically regulated sleep parameter have shown that NREMS is substantially regulated in terms of intensity, and REMS in terms of duration.2 However, some contradictory aspects arising from “extended” (days/weeks) sleep deprivation studies and/or from the comparison of animal and human studies still leave this topic open to discussion.2,8–11
As far as NREMS is concerned, the power density in the delta band (approximately, 0.5–4.5 Hz) of the electroencephalogram (EEG), not NREMS duration, is considered to be the homeostatically regulated parameter in NREMS and is commonly taken as an index of NREMS intensity.1,2,12 Such a regulation appears to be disrupted following an extended period of either sleep deprivation or sleep restriction.9,13
REMS appears to be precisely regulated in terms of its duration on both a short-term and long-term basis. The short-term component is expressed as a function of the ultradian wake-sleep cycle. Within a species, the duration of the interval between two consecutive REM episodes (REMS interval) appears to be directly related to the duration of the preceding REMS episode, but not to the duration of the subsequent REMS episode.14–17 The long-term component of REMS regulation is expressed in the total amount of REMS during the days following a total sleep or selective REM sleep deprivation. A precise REMS conservation has been observed in both the cat and the rat, since the rebound in REMS has been found to be proportional to the total loss of REMS during the deprivation period. 11,18–22 This precise quantitative regulation of total REMS amount may not occur following an extended period of sleep deprivation or sleep restriction.9,13,23 Whereas changes in EEG power are an important component of NREMS rebound, they are considered to be of smaller relevance for REMS rebound.12,19,24
It is well known that exposure to low ambient temperature (Ta) deeply influences REMS occurrence in different species.25,26 In particular, REMS is depressed according to either the duration or the depth of Ta lowering,20,21,25,27 while the return to normal laboratory conditions is characterized by an intense REMS rebound that is quantitatively related to the degree of the previous REMS loss.18,20,21,28,29 However, in all the reported studies the quantitative approach to the study of REMS regulation was limited either by the incomplete recording of wake-sleep parameters during cold exposure or the insufficient duration of the recovery period. Under the same experimental conditions, NREMS was affected to a lesser extent, no relevant rebound of NREMS being found in either cats or rats,27,28 and only a slight increase in delta power was observed in the rat during the recovery period.28
The rather selective effects of cold exposure on REMS make this environmental challenge a powerful physiological tool for the study of the homeostatic aspects of REMS regulation. Because cold exposure has a limited effect on the occurrence of NREMS, it might reduce the possible impact of accumulated NREMS propensity on REMS expression during recovery.2 Moreover, cold exposure allows researchers to modulate the intensity of deprivation, leading to the possibility of inducing different degrees of deprivation without changing the duration of the exposure and, as a consequence, avoiding circadian confounds.
On this basis, we recently carried out a study in which four groups of rats were exposed for 24 h to 4 different low Tas (10°C, 5°C, 0°C, −10°C) and then were allowed to recover at normal laboratory Ta (24°C) for 4 consecutive days.30 The results showed that the depression of REMS during cold exposure and the subsequent enhancement during the recovery period were directly related to the degree of Ta lowering. On the contrary, smaller effects were observed on NREMS amount and delta power during cold exposure and the recovery period. The present analysis represents the second part of the aforementioned study. 30 In particular, in the present study short-term and long-term aspects of REMS regulation were separately addressed and a quantitative analysis of the relationship between REMS loss and REMS gain were carried out. The results of such an analysis confirm that REMS is homeostatically regulated in terms of its duration. Furthermore, a model which relates both short and long-term REMS homeostasis in different species to either body or brain size has been proposed in the present study. Preliminary results of this analysis have been submitted in abstract form.31
METHODS
Methods are briefly summarized, since they have been extensively published previously. 30
The subjects in this study were twenty-four male Sprague-Dawley rats (Charles River), which had free access to food and water and were kept at 24 ± 0.5°C ambient temperature (Ta), under a 12h:12h light-dark (LD) cycle (Light: 09:00–21:00; 100 lux at cage level). The experiments were carried out according to the European Union Directive (86/609/EEC) and were under the supervision of the Central Veterinary Service of the University of Bologna and the National Health Authority.
The animals were placed under deep general anaesthesia (diazepam, Valium, Roche, 5 mg kg−1 i.m.; ketamine-HCl, Ketalar, Parke-Davis, 100 mg kg−1 i.p.) and were implanted epidurally with 2 stainless steel electrodes for electroencephalographic (EEG) recording. Also, a thermistor (B10KA303N, Thermometrics) mounted inside the tip of a stainless steel needle was implanted to measure hypothalamic temperature (Thy). The plugs to connect EEG electrodes and the thermistor to the recording apparatus were embedded in acrylic dental resin anchored to the skull by small stainless steel screws. The animals were allowed to recover from surgery and to adapt to the recording apparatus for at least one week. The rats were individually kept in Plexiglas cages which were placed in a thermoregulated and sound-attenuated box, which, in its turn, was placed in a sound-attenuated room. EEG, Thy and the motor activity (MA) of each animal were continuously recorded during each experimental session except between 09:00 to 09:15, when cage bedding, water and food were changed.
The experimental protocol consisted of 7 consecutive 24-h recording sessions. Following 2 days of recording for the baseline condition (BL1 and BL2), the animals were exposed for 24 h to different low ambient temperatures (E1). The exposure started at the onset (09:00) of the L period of the LD cycle. Four experimental groups of animals (n = 6 in each group) were studied. Each group was exposed to a different Ta, i.e. 10 ± 1°C, 5 ± 1°C, 0 ± 1°C, and −10 ± 1°C [E10, E5, E0, and E-10, respectively]. Following cold exposure, the animals were returned to Ta 24°C and allowed to recover for 4 consecutive days (R1–4).
User software was developed (QuickBASIC) to handle the data. In each experiment the EEG signal was amplified, filtered (high-pass filter: −40 dB at 0.35 Hz; low-pass filter: −6 dB at 60 Hz; digital Notch filter: −40 dB at 50 Hz) and after AD conversion (sampling rate: EEG, 128 Hz) were stored on a PC (486/100 DX-4). The EEG signal was subjected to on-line Fast Fourier Transform (FFT) and EEG power values were obtained for 4-s epochs in the delta (DPW, 0.75–4.0 Hz), theta (TPW, 5.5–9.0 Hz), and sigma (SPW, 11.0–16.0 Hz) band. Thy signal was amplified (1°C/1V) before AD conversion (sampling rate: 8 Hz). MA was monitored by means of a passive infrared detector (Siemens, PID10) placed at the top of each cage. The signal was amplified and integrated before AD conversion (sampling rate: 8 Hz), to make the output proportional to the amplitude and the duration of movement.
EEG, Thy, and MA signals were visually scored to determine the beginning and end of each REMS episode. The main criteria used for this assessment were based on the analysis of EEG, MA, and Thy.30 Particular consideration was given to the changes in Thy. For example, a REMS episode was considered to have begun only if the EEG changes associated with this sleep stage (i.e., high TPW, low DPW and SPW) were concomitant with an increase in Thy. Moreover, a REMS episode was considered to be over only if the EEG and postural changes were associated with a decrease in Thy. On the basis of these criteria the transition periods from either NREMS to REMS or REMS to Wake were not scored as REMS. The time for the minimal duration of a REMS episode was fixed at 8 s. The energy within the theta band of the EEG (theta energy) for REMS was calculated for 2-h intervals as the product of the average TPW density levels in the 4-s epochs scored as REMS multiplied by the time spent in REMS (number of 4-s epochs scored as REMS).
Following the removal of REMS epochs and that of the 4-s epochs which showed EEG artifacts, an automatic procedure allowed us to separate Wake from NREMS, according to a procedure that has been extensively discussed previously.30
Statistical analysis was carried out by means of SPSS 9.0. For the present analysis of the results, linear regression procedure was used to calculate regression coefficients and Pearson correlation coefficient. Moreover, curve estimation regression procedure for power regression model was used in order to calculate regression coefficients and r-square.
RESULTS
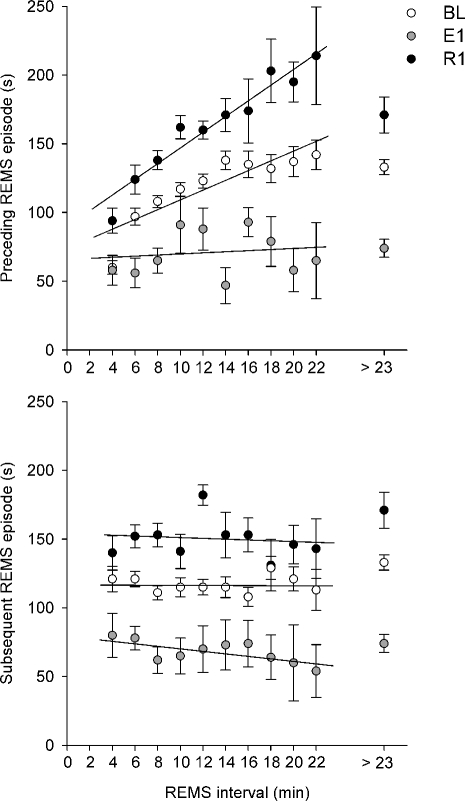
The relationships between the duration of the REMS interval and that of either the REMS episode preceding (Preceding REMS episode) or the one following (Subsequent REMS episode) the interval, for either BL, E1 or R1 are shown in Figure 1 (upper and lower diagram, respectively). Data relative to the 4 different experimental groups (E10, E5, E0, E-10) have been pooled.
Figure 1.
Relationship between the duration of the interval between two consecutive REMS episodes (REMS interval, classes of 2 min) and that of the episode that either precedes (Preceding REMS episode, upper diagram) or follows (Subsequent REMS episode, lower diagram) the interval, during the L period (09:00–21:00) of either baseline (BL; white), 24-h exposure to different low Tas (from −10°C to 10°C, E1; grey), or the first day of the recovery period at Ta 24°C (R1; black). The average duration of the REMS episode was calculated for each animal and for each class on the pool of single REMS episodes and REM sleep clusters and, subsequently, the values for different animals were averaged. Data relative to intervals lasting more than 23 min are shown separately. The best-fit linear regression line is shown for each condition.
On the basis of recent observations which showed that the regulatory processes which underlie the relationship between the REMS episode and the REMS interval are different for the short and long REMS interval population,32 only long REMS intervals (>3 min) have been considered in the analysis. Intervals were separated into increments of 2-min duration. For each class, the mean duration of the REMS episode was calculated by pooling the duration of single REMS episodes and REMS clusters for each animal and, subsequently, values from different animals were averaged. Since short-term REMS regulatory processes were most evident during the L period of the LD cycle,33 only data relative to the L period are shown. Due to the scarce number of intervals lasting more than 23 min,20,21 data relative to very long REMS intervals (> 23 min) were pooled and separately shown in the plot.
The results show that the duration of the Preceding REMS episode is positively related to that of the duration of the REMS interval in BL (linear regression analysis: y = 3.658x + 71.352, r = 0.877, P < 0.01). A significant relationship is also present in R1 (y = 5.906x + 86.721, r = 0.966, P < 0.01), where a steeper slope is observed, but not during exposure to low Ta (y = 0.339x + 65.586, r = 0.125, n.s.). However, the slope apparently becomes a plateau when very long REMS intervals (> 23 min), which approximately constitute 9% and 6% of the total number of long intervals in either BL or R1, respectively, are taken into account. No relationship is present between the duration of the REMS interval and that of the Subsequent REMS episode for any experimental condition (BL: y = −0.009x + 117.020, r = 0.007, n.s.; E1: y = −0.969x + 80.606, r = 0.702, n.s.; R1: y = −0.358x + 154.050, r = 0.159, n.s.).
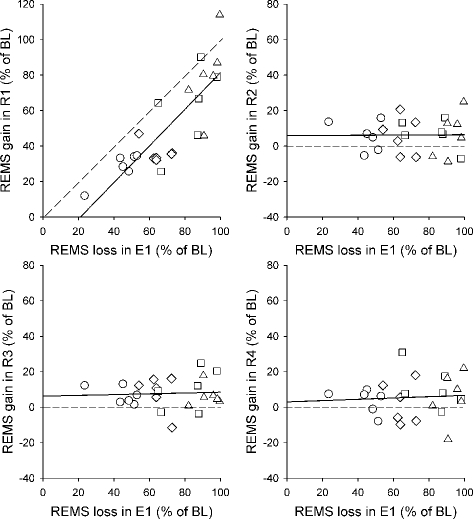
The relationship between the REMS loss during the 24-h exposure to different low Tas (E1, Ta range: −10°C to 10°C) and the REMS gain during each of the subsequent four days of recovery at Ta 24°C (R1–R4) is shown in Figure 2. REMS loss is the difference between the average daily amount of REMS of the two BL recordings and the actual experimental values, while REMS gain is the difference between the actual experimental levels and those of BL. Both loss and gain are calculated as a percentage of BL. Each point represents data from an individual rat (n=24). The different Tas of exposure are shown using different symbols. The analysis shows that during the first day of the recovery period, REMS gain appears to be proportional to the previous REMS loss. The best-fit regression line runs parallel to that which would indicate the presence of a 100% payment of the debt and intercepts the x-axis at a value which corresponds about to a 22% loss of the normal daily REMS amount (R1: y = 1.032x – 21.756, r = 0.825, P < 0.001). In each of the following three days of the recovery period the best-fit regression line runs parallel and above the x-axis, showing the presence of a tonic REMS gain that was apparently unrelated to the degree of the previous loss (R2: y = 0.005x + 5.926, r = 0.010, n.s.; R3: y = 0.022x + 6.287, r = 0.055, n.s.; R4: y = 0.038x + 2.966, r = 0.071, n.s.).
Figure 2.
Relationship between REMS loss during the 24-h exposure to different low Tas (10°C, circle; 5°C, rhombus; 0°C, square; −10°C, triangle) and subsequent REMS gain during each of the following four days of recovery (R1-R4) at Ta 24°C. Each point represents an individual rat (n=24). Both REMS loss and REMS gain are expressed as the percentage of the daily REMS amount in the baseline (BL) condition. The solid line is the best-fit linear regression line. In the plot relative to R1, the dotted line indicates the hypothetical best-fit regression line if REMS gain fully compensates for the previous REMS loss. In the plots relative to R2–R4, the dotted line indicates the hypothetical best-fit regression line in the case of absence of any REMS gain.
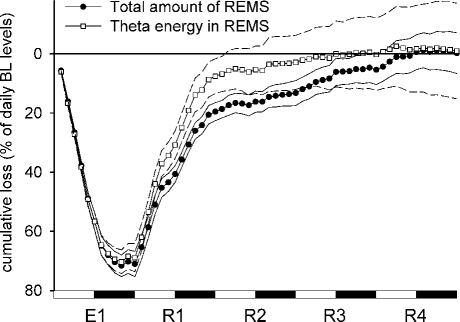
In Figure 3, the time course of the cumulative loss of REMS and that of the EEG energy within the theta band in REMS are indicated with a 2-h resolution. Calculations were made as specified for Figure 2, but the accumulation of a gain is here indicated as a reduction of loss. Thus, a loss is shown to increase by a downward course and to be reduced by an upward course of the line. For each 2-h interval, energy was calculated as the average relative power density in the theta band multiplied by the time spent in REMS. Data from the different experimental groups were pooled. The results show that the large depression in either REMS amount or theta energy which occurred during the 24-h exposure was practically compensated for during the following 4 days of recovery.
Figure 3.
Time course of the loss of REMS related parameters during exposure to different low Tas (from 10°C to −10°C, E1) and the following recovery period at Ta 24°C (R1–R4). Relative cumulative loss (% of daily baseline levels ± S.E.M.) of the total amount of REMS (filled circles) and theta energy in REMS (empty squares) is shown. Each point represents the average 2-h value for 24 animals; either the upper or the lower limit of S.E.M. is indicated by a thin line (solid for the amount, dashed for the energy).
DISCUSSION
The results of our study show that in the rat REMS occurrence is substantially regulated through changes in its duration, and it is under a precise quantitative control on both a short-term (minutes/hours) and a long-term (hours/days) scale. These two aspects will be discussed separately and the possibility of a common basis for short and long-term REMS homeostasis which relates REMS homeostasis in different species to either body or brain size, will be addressed.
Short-Term REM Sleep Homeostasis
Our analysis confirms the presence, under normal laboratory conditions, of a positive relationship between the duration of the REMS interval and that of the REMS episode which precedes the interval, while the duration of the interval does not apparently influence that of the following episode.14–17 The proportion is lost for intervals lasting more than 23 minutes, which represent 5%–10% of the population. Since the average time spent in NREMS within intervals lasting less than 23 minutes was 76.4% of the total time of each interval, but only 30.4% in those lasting more than 23 minutes, these data support previous evidence, which indicates that such a short-term relationship may somehow be overcome by the intrusion of very long periods of Wake into the interval.16,33 The positive relationship appears to be at its strongest and steepest during the first day of the recovery period, when a strong drive for sleep occurrence is present, and disappears during exposure to low Ta.
The presence of a positive correlation between the duration of an event and that of the interval which separates such an event from the next one suggests that a preparatory process (either cumulative or dissipative) occurring during the event is one determinant of the duration of the following interval. The propensity for the event to occur is thought to build up during the interval until a threshold level is reached and the event is forced to occur. In particular, it has been proposed by some authors that the short-term REMS need builds up during the whole interval,33 and by others that it builds up during NREMS.15 Since such a propensity dissipates during the event, less time will be required for the build-up in the subsequent interval in the case of the event not being sufficiently long. This kind of regulation characterizes different behaviors involved in the maintenance of body homeostasis. For example, in the rat, the duration of the interval between two consecutive meals has been shown to depend on the size of the preceding meal, but does not influence that of the meal that follows.34
The short-term component of REMS regulation is expressed as a function of the ultradian wake-sleep cycle and varies between species with body size.35 The duration of the wake-sleep cycle can be defined as the time between the onsets of subsequent REMS episodes. On average, the duration of the wake-sleep cycle and that of the REMS episode are short for small animals such as mice and rats and longer for larger animals such as cats and humans.35 In different species, the normal ultradian rhythmicity of REMS occurrence under baseline conditions is guaranteed by the fact that a new REMS episode starts as soon as a given threshold in the levels of REMS need (“ultradian” threshold) is reached during the REMS interval. Since BL values can be taken as the amount satisfying the 100% daily need for the animal, such a threshold can be calculated for each species as the ratio between the REMS daily need and the number of REMS episodes within the 24h-period in BL (rat, No. = 58, dur = 1.9 min30; cat, No. = 27, dur = 6.3 min36; humans, No = 4.2, dur = 23.9 min37). Thus, the rat has to start a new REMS episode each time 1.7% (i.e., 100%/58) of the REMS daily amount is required, while the cat and the human can wait until 3.7% (i.e., 100%/27) and 23.8% (i.e., 100%/4.2) is needed, respectively. It has to be taken into account that this modality for the calculation of the ultradian threshold should be considered a precise calculation for polyphasic species, such as rats and cats, in which REMS is distributed rather homogenously across the day, and it should be considered a good approximation for a monophasic species, such as humans.
Long-Term REM Sleep Homeostasis
The results of our analysis confirm the presence of a precise quantitative control of REMS occurrence in cold-exposed animals on a 12h/24h-scale.18,20,21,28,29 In particular, a fast REMS rebound occurs during the first day of the recovery period, during which the REMS gain is proportional to the previous REMS loss and is not apparently influenced by nonspecific factors linked to Ta levels during exposure. An apparent total compensation of the REMS loss was observed at the end of the fourth day of the recovery period, due to a slow rebound occurring at a rate that is independent from the degree of the previous deprivation. With respect to this, it is important to remember that such a slow REMS rebound reaches statistical individual significance only during R3.30 A “fast rebound” threshold appears, therefore, to separate the REMS quota that needs to be quickly recovered from the quota whose recovery can be somehow postponed or missed, indicating the minimum size necessary for REMS loss to be followed by an evident REMS rebound. As shown in Figure 2, in the rat this threshold corresponds to a loss of about 22% of the normal daily REMS amount. The proportion of these quotas corresponds to that previously observed in rats that were totally deprived of sleep for 24 hours by gentle handling. In these studies, about 80%–85% of the REMS loss was shown to be recovered during the first day,19,22 while the remaining 15%–20% was apparently postponed.22
A specific increase in theta power during REMS has been observed during both the exposure to low Tas and the following recovery period,30 indicating the possibility of taking theta power as a marker of REMS intensity. Thus, REMS intensity would appear to be higher in conditions in which the drive for REMS is enhanced, either during or following cold exposure. However, the analysis of the cumulative changes in theta energy which has been carried out in the present study would suggest that the intensity parameter is not too critical for REMS regulation, since, as shown in Figure 3, the time course of the cumulative loss in the total amount of REMS is not substantially different from that of theta energy.
The concept that a precise proportional relationship between the REMS rebound and the REMS debt appears only if the REMS debt goes beyond a threshold level was introduced by Parmeggiani and collaborators in a series of studies on the effects of cold exposure on sleep regulation in the cat.18,27 On the basis of these studies, the daily REMS amount was separated into two quotas; “obligate” (OQ, corresponding to about 64% of daily REMS amount) and “facultative” (FQ, the remaining 36%).18 The latter was not apparently recovered after REMS deprivation. However, in this experiment the analysis was stopped soon after the apparent return to baseline levels of REMS amount. Thus, due to the lack of a whole 24-h analysis, the possibility that, in the cat, at least a part of the missing rebound may occur at a slow rate during the night cannot be excluded. According to this model, a REMS rebound becomes apparent during the early recovery period only if the size of the OQ loss during exposure is larger than that of the FQ. If not, the rebound is masked, since the OQ which needs to be recovered simply substitutes the FQ during the day in which recovery occurs. Thus, it is possible to calculate that the fast rebound threshold for the cat corresponds to a 72% loss of the daily REMS amount (the sum of the whole FQ, corresponding to 36% of the daily REMS amount + a portion of the OQ, corresponding to 36% of the daily REMS amount). Thus, in the cat the size of the fast rebound threshold is about 3 times as large as in the rat.
No fully comparable data can be obtained from studies on humans, since, of course, the exposure to different very low Tas cannot be used as a tool for systematic dose-response studies on sleep deprivation in our species. However, in humans, a relevant rebound of REMS was only elicited following more than 2 days of total sleep deprivation.38–40 A REMS rebound was also observed under short-term protocols in which total sleep deprivation was limited to a part of the night,5,6 but this procedure may introduce some circadian confounds.
The fact that cold exposure does not interfere with NREMS occurrence to such a great extent as with REMS occurrence allows us to consider data coming from selective REMS deprivation studies on humans to be rather more comparable to those obtained from cold exposure studies on animals. On this basis, the absence of a very large REMS rebound following 2 or 3 days of selective REMS deprivation has led to the conclusion that the homeostatic drive for REMS might be weaker for humans than for rats and cats2,4,41 or that a REMS loss of one day in the human may be functionally comparable to a loss of only a few hours in the rat.9 The possible biological reasons underlying these differences have not been extensively discussed. However, it has been proposed that the strong rebound observed in rodents is only a nonspecific response depending on the stress caused by sleep deprivation.10
We have tried to calculate the levels of the fast rebound threshold in humans using data from the comprehensive study by Endo et al.,4 in which a rather large REMS rebound (39% of the baseline) was observed following a 3-day selective REMS deprivation. Since the REMS loss during the 3 days of deprivation and the REMS gain during the first day of the recovery period were 273% and 39% of the normal daily amount, respectively, the fast rebound threshold was calculated as the difference between the 2 values (i.e., 234%). This value is more than 10 times as large as that observed in the rat and 3 times as large as that in the cat. Interestingly, in this study, the daily REMS amount was shown to be maintained, on average, 8% above the baseline during the following days of the recovery period, suggesting the possibility of a slow rebound.
The concept that REMS amount is under a homeostatic control has been challenged by different observations showing that in the rat an excess of REMS may be induced in the absence of any previous deprivation by means of manipulation of the environment.29,42 Moreover, a REMS rebound of disproportionate size was observed following immobilisation stress.43 Whatever the meaning of such relatively short-lasting modifications, which are considered to depend on the high sensitivity of REMS to the phasic and tonic changes in the activity of the autonomic nervous system,44 they appear to be relatively small in size. Similarly, a precise quantitative regulation apparently disappears following an extended period of either sleep deprivation or sleep restriction in the rat, although a clear REMS rebound is always present in the first day of recovery.9,13,23 However, it has been proposed that prolonged sleep deprivation or sleep restriction processes may induce allostatic changes which could influence the daily REMS need in an adaptive way.13
A Common Basis for Short and Long-Term REM Sleep Homeostasis
In the 3 species that we have considered, the fast rebound threshold appears to increase proportionally with the increase in the ultradian threshold. This suggests that REMS ultradian rhythmicity and the size of the fast REMS rebound share a common regulatory process that appears to be related to body size. In our analysis we used data from 3 species, in which both short and long-term aspects of REMS regulation have been more extensively studied and consolidated. However, we are aware that additional data from other species will help to test this hypothesis.
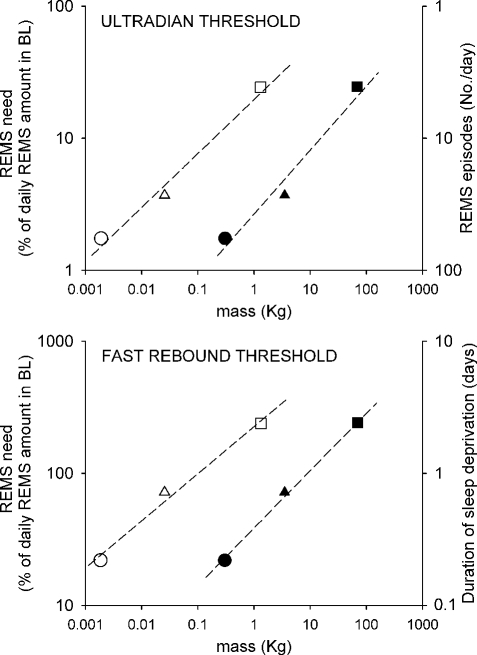
In Figure 4, the relationship between either the ultradian or the fast rebound threshold (upper and lower diagram, respectively) and either body or brain mass is shown on a log scale using data from rat, cat, and human studies. A body mass of 0.3 kg, 3.5 kg, and 70 kg has been arbitrarily assumed for rat, cat, and human, respectively. Brain masses are taken from Savage and West.45 The ultradian threshold is shown in terms of the percentage of REMS daily need on the left axis and in terms of number of REMS episodes/day on the right axis. The fast rebound threshold is shown in terms of the loss of the daily need of REMS on the left axis and in terms of days of REMS deprivation which are necessary for such a threshold to be reached on the right axis.
Figure 4.
Relationship between REMS “ultradian” (upper diagram) and “fast rebound” (lower diagram) thresholds and either body (filled symbol) or brain (empty symbol) mass, in the rat (circle), cat (triangle), and human (square). Values are shown on a double log plot. The best power function curve estimation (broken line) is shown for each relationship.
For both thresholds the best curve estimation that has been made for the relationship between the threshold value, expressed in terms of percentage of REMS daily need and either body or brain mass, is shown on a double log plot (ultradian: y = 2,65x0.49, r2 = 0.968; fast rebound: y = 38.68x0.43, r2 = 0.997). The relationship is maintained, but with a lower exponent, if brain mass is used as the independent variable (ultradian: y = 19.77x0.41, r2 = 0.986; fast rebound: y = 226.03x0.36, r2 = 0.986).
Different sleep-related parameters, such as the duration of the wake-sleep cycle35,45,46 or that of the REMS interval 47 are known to vary allometrically with respect to either body or brain mass in different species. In particular, Savage and West have recently shown45 that the ratio between total sleep time and wake time within a 24-h period decreases with the increase in body mass. According to these authors, any possible metabolic brain damage occurring during wakefulness is larger in small animals than in large ones, due to the higher metabolic rate that occurs in the former. Consequently, small animals should need more sleep per wake time unit for brain repair.45 In our opinion, this hypothesis would account not only for the presence of a more intense REMS rebound in small animals than in large ones following a comparable time of deprivation, but also for the capacity of both humans48 and cats49 to stand REMS deprivation for a time duration that is sufficient to lead rats to death.50
In conclusion, it may be hypothesized that REMS is a behavior involved in the homeostatic control of a specific physiological variable that could be either stored or dissipated during REMS occurrence. Whatever the nature of this variable may be, it is important to take into account that its control by REMS apparently requires a functional release from the hypothalamic integrative control of body temperature. 44
ABBREVIATIONS
- BL:
Baseline
- D:
Dark
- DPW:
Power in the delta band of the EEG
- E:
Exposure
- EEG:
Electroencephalogram
- FQ:
Facultative quota
- L:
Light
- NREMS:
non-REM sleep
- MA:
Motor activity
- OQ:
Obligate quota
- R:
Recovery
- REMS:
REM sleep
- REMS
interval: Time interval between two consecutive REM sleep episodes
- SPW:
Power in the sigma band of the EEG
- Ta:
Ambient temperature
- Theta
energy: Energy in the theta band of the EEG
- Thy:
Hypothalamic temperature
- TPW:
Power in the theta band of the EEG
ACKNOWLEDGMENTS
Institution at which the work was performed: Department of Human and General Physiology, Alma Mater Studiorum-University of Bologna, Italy.
This work has been supported by grants from both the Ministero dell'Universitáa e della Ricerca, Italy and the University of Bologna. No other significant financial interest/relationship to disclose.
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Cerri has received research support from the European Sleep Research Society through a sponsorship from Sanofi-Aventis. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Borbély AA. Sleep: circadian rhythm versus recovery process. In: Koukkou M, Lehmann L, Angst J, editors. Functional states of the brain: Their determinants. Amsterdam: Elsevier; 1980. pp. 151–61. [Google Scholar]
- 2.Borbély AA, Achermann P. Sleep homeostasis and models of sleep regulation. In: Kryger MH, Roth T, Dement WE, editors. Principles and practice of sleep medicine. Philadelphia: WB Saunders; 2005. pp. 405–17. [Google Scholar]
- 3.Endo T, Schwierin B, Borbély AA, Tobler I. Selective and total sleep deprivation: effect on the sleep EEG in the rat. Psychiatry Res. 1997;66:97–110. doi: 10.1016/s0165-1781(96)03029-6. [DOI] [PubMed] [Google Scholar]
- 4.Endo T, Roth C, Landolt HP, et al. Selective REM sleep deprivation in humans: effects on sleep and sleep EEG. Am J Physiol. 1998;43:R1186–94. doi: 10.1152/ajpregu.1998.274.4.R1186. [DOI] [PubMed] [Google Scholar]
- 5.Beersma DGM, Dijk DJ, Block CGH, Everhardus I. REM sleep deprivation during 5 hours leads to an immediate REM sleep rebound and to suppression of non-REM sleep intensity. Electroencephalogr Clin Neurophysiol. 1990;76:114–22. doi: 10.1016/0013-4694(90)90209-3. [DOI] [PubMed] [Google Scholar]
- 6.Brunner DP, Dijk DJ, Tobler I, Borbely AA. Effects of partial sleep deprivation on sleep stages and EEG power spectra: evidence for non-REM and REM sleep homeostasis. Electroencephal Clin Neurofisiol. 1990;75:492–9. doi: 10.1016/0013-4694(90)90136-8. [DOI] [PubMed] [Google Scholar]
- 7.Dijk DJ, Franken P. Interaction of sleep homeostasis and circadian rhythmicity: dependent or independent systems? In: Kryger MH, Roth T, Dement WE, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia: WB Saunders; 2005. pp. 418–34. [Google Scholar]
- 8.Benington JH, Heller HC. Implications of sleep deprivation experiments for our understanding of sleep homeostasis. Sleep. 1999;22:1033–7. [PubMed] [Google Scholar]
- 9.Rechtschaffen A, Bergmann BM, Gilliand MA, Bauer K. Effects of method, duration, and sleep stage on rebounds from sleep deprivation in the rat. Sleep. 1999;22:11–31. doi: 10.1093/sleep/22.1.11. [DOI] [PubMed] [Google Scholar]
- 10.Horne JA. REM sleep-by default? Neurosci Biobehav Rev. 2000;24:777–97. doi: 10.1016/s0149-7634(00)00037-3. [DOI] [PubMed] [Google Scholar]
- 11.Franken P. Long-term vs. short-term processes regulating REM sleep. J Sleep Res. 2002;11:17–28. doi: 10.1046/j.1365-2869.2002.00275.x. [DOI] [PubMed] [Google Scholar]
- 12.Borbély AA, Tobler I, Hanagasioglu M. Effect of sleep deprivation on sleep and EEG power spectra in the rat. Behav Brain Res. 1984;14:171–82. doi: 10.1016/0166-4328(84)90186-4. [DOI] [PubMed] [Google Scholar]
- 13.Kim Y, Laposky AD, Bergmann BM, Turek FW. Repeated sleep restriction in rats leads to homeostatic and allostatic responses during recovery sleep. Proc Natl Acad Sci U S A. 2007;104:10697–702. doi: 10.1073/pnas.0610351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ursin R. Sleep stage relation within the sleep cycles in the cat. Brain Res. 1970;11:91–7. doi: 10.1016/0006-8993(70)90157-5. [DOI] [PubMed] [Google Scholar]
- 15.Benington JH, Heller HC. REM sleep timing is controlled homeostatically by accumulation of REM-sleep propensity in non-REM sleep. Am J Physiol. 1994;266:R1992–R2000. doi: 10.1152/ajpregu.1994.266.6.R1992. [DOI] [PubMed] [Google Scholar]
- 16.Vivaldi EA, Ocampo A, Wyeneken U, Roncagliolo M, Zapata AM. Short-term homeostasis of active sleep and the architecture of sleep in the rat. J Neurophysiol. 1994;72:1745–55. doi: 10.1152/jn.1994.72.4.1745. [DOI] [PubMed] [Google Scholar]
- 17.Barbato G, Wehr TA. Homeostatic regulation of REM sleep in humans during extended sleep. Sleep. 1998;21:267–76. doi: 10.1093/sleep/21.3.267. [DOI] [PubMed] [Google Scholar]
- 18.Parmeggiani PL, Cianci T, Calasso M, Zamboni G, Perez E. Quantitative analysis of short term deprivation and recovery of desynchronized sleep in cats. Electroencephal Clin Neurophysiol. 1980;50:293–302. doi: 10.1016/0013-4694(80)90157-1. [DOI] [PubMed] [Google Scholar]
- 19.Franken P, Dijk DJ, Tobler I, Borbély AA. Sleep deprivation in rats: effects on EEG power spectra, vigilance states, and cortical temperature. Am J Physiol. 1991;261:R198–R208. doi: 10.1152/ajpregu.1991.261.1.R198. [DOI] [PubMed] [Google Scholar]
- 20.Amici R, Zamboni G, Perez E, et al. Pattern of desynchronized sleep during deprivation and recovery induced in the rat by changes in ambient temperature. J Sleep Res. 1994;3:250–6. doi: 10.1111/j.1365-2869.1994.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 21.Amici R, Zamboni G, Perez E, Jones CA, Parmeggiani PL. The influence of a heavy thermal load on REM sleep in the rat. Brain Res. 1998;781:252–8. doi: 10.1016/s0006-8993(97)01242-0. [DOI] [PubMed] [Google Scholar]
- 22.Schwierin B, Borbély AA, Tobler I. Prolonged effects of 24-h total sleep deprivation on sleep and sleep EEG in the rat. Neurosci Lett. 1999;261:61–4. doi: 10.1016/s0304-3940(98)01006-4. [DOI] [PubMed] [Google Scholar]
- 23.Borges Machado R, Hipólide DC, Amélia Benedito-Silva A, Tufik S. Sleep deprivation induced by the modified multiple platform technique quantification of sleep loss and recovery. Brain Res. 2004;1004:45–51. doi: 10.1016/j.brainres.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Tobler I, Borbély AA. Sleep EEG in the rat as a function of prior waking. Electroencephal Clin Neurophysiol. 1986;64:74–6. doi: 10.1016/0013-4694(86)90044-1. [DOI] [PubMed] [Google Scholar]
- 25.Parmeggiani PL. Thermoregulation and sleep. Front Biosci. 2003;8:s557–67. doi: 10.2741/1054. [DOI] [PubMed] [Google Scholar]
- 26.Heller HC. Temperature, thermoregulation, and sleep. In: Kryger MH, Roth T, Dement WE, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia: WB Saunders; 2005. pp. 292–304. [Google Scholar]
- 27.Parmeggiani PL, Rabini C. Sleep and environmental temperature. Arch Ital Biol. 1970;108:369–87. [PubMed] [Google Scholar]
- 28.Franken P, Tobler I, Borbély AA. Effects of 12-h sleep deprivation and of 12-h cold exposure on sleep regulation and cortical temperature in the rat. Physiol Behav. 1993;54:885–94. doi: 10.1016/0031-9384(93)90297-s. [DOI] [PubMed] [Google Scholar]
- 29.Zamboni G, Amici R, Perez E, Jones CA, Parmeggiani PL. Pattern of REM sleep occurrence in continuous darkness following the exposure to low ambient temperature in the rat. Behav Brain Res. 2001;122:25–32. doi: 10.1016/s0166-4328(01)00175-9. [DOI] [PubMed] [Google Scholar]
- 30.Cerri M, Ocampo-Garcés A, Amici R, et al. Cold exposure and sleep in the rat: effects on sleep architecture and EEG. Sleep. 2005;28:694–705. doi: 10.1093/sleep/28.6.694. [DOI] [PubMed] [Google Scholar]
- 31.Amici R, Baracchi F, Cerri M, et al. REM sleep homeostasis: a matter of size? Sleep Biol Rhythms. 2007;5(Suppl.1):A86. [Google Scholar]
- 32.Amici R, Jones CA, Perez E, Zamboni G. A physiologic view of REM sleep structure. In: Parmeggiani PL, Velluti R, editors. The physiologic nature of sleep. London: Imperial College Press; 2005. pp. 161–85. [Google Scholar]
- 33.Vivaldi E, Ocampo-Garces A, Villegas R. Short-term homeostasis of REM sleep throughout a 12:12 Light:Dark schedule in the rat. Sleep. 2005;28:931–43. doi: 10.1093/sleep/28.8.931. [DOI] [PubMed] [Google Scholar]
- 34.Thomas DW, Mayer J. Meal taking and regulation of food intake by normal and hypothalamic hyperfagic rats. J Comp Physiol Psychol. 1968;66:642–53. doi: 10.1037/h0026520. [DOI] [PubMed] [Google Scholar]
- 35.Zepelin H, Siegel JM, Tobler I. Mammalian sleep. In: Kryger MH, Roth T, Dement WE, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia: WB Saunders; 2005. pp. 91–100. [Google Scholar]
- 36.Bowersox SS, Sterman MB. Changes in circadian sleep and waking patterns after somatosensory deafferentation in the cat. Electroencephal Clin Neurophysiol. 1983;56:623–7. doi: 10.1016/0013-4694(83)90029-9. [DOI] [PubMed] [Google Scholar]
- 37.Le Bon O, Staner L, Rivelli SK, Hoffman G, Pelc I, Linkowski P. Correlations using the NREM-REM cycle frequency support distinct regulation mechanisms for REM and NREM sleep. J Appl Physiol. 2002;93:141–46. doi: 10.1152/japplphysiol.00917.2001. [DOI] [PubMed] [Google Scholar]
- 38.Berger RJ, Oswald I. Effects of sleep deprivation on behavior subsequent sleep and dreaming. J Ment Sci. 1962;108:457–65. doi: 10.1192/bjp.108.455.457. [DOI] [PubMed] [Google Scholar]
- 39.Williams HL, Hammack JT, Daily RL, Dement WC, Lubin A. Responses to auditory stimulation, sleep loss and the EEG stages of sleep. Electroencephal Clin Neurophysiol. 1964;16:269–79. doi: 10.1016/0013-4694(64)90109-9. [DOI] [PubMed] [Google Scholar]
- 40.Kales A, Tan TL, Kollar EJ, Naitoh P, Preston TA, Malmstrom EJ. Sleep patterns following 205 hours of sleep deprivation. Psychosom Med. 1970;32:189–200. doi: 10.1097/00006842-197003000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Werth E, Cote KA, Gallmann E, Borbély AA, Achermann P. Selective REM sleep deprivation during daytime. 1. Time course of interventions and recovery sleep. Am J Physiol. 2002;283:R521–6. doi: 10.1152/ajpregu.00462.2001. [DOI] [PubMed] [Google Scholar]
- 42.Fishman R, Roffwarg HP. REMS inhibition by light in the albino rat. Exp Neurol. 1972;36:166–78. doi: 10.1016/0014-4886(72)90144-6. [DOI] [PubMed] [Google Scholar]
- 43.Rampin C, Cespuglio R, Chastrette N, Jouvet M. Immobilisation stress induces a paradoxical sleep rebound in the rat. Neurosci Lett. 1991;126:113–8. doi: 10.1016/0304-3940(91)90532-x. [DOI] [PubMed] [Google Scholar]
- 44.Parmeggiani PL. Physiologic regulation in sleep. In: Kryger MH, Roth T, Dement WE, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia: WB Saunders; 2005. pp. 185–91. [Google Scholar]
- 45.Savage WM, West GB. A quantitative, theoretical framework for understanding mammalian sleep. Proc Natl Acad Sci U S A. 2007;104:1051–6. doi: 10.1073/pnas.0610080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dallaire A, Toutain PL, Ruckebusch Y. Sur la périodicité du sommeil paradoxal: faits et hypotheses. Physiol Behav. 1974;13:395–400. doi: 10.1016/0031-9384(74)90094-8. [DOI] [PubMed] [Google Scholar]
- 47.Zamboni G, Perez E, Amici R, Jones CA, Parmeggiani PL. Control of REMS: an aspect of the regulation of physiological homeostasis. Arch Ital Biol. 1999;137:249–62. [PubMed] [Google Scholar]
- 48.Landolt HH, Posthuma de Boer L. Effect of chronic phenelzine treatment on REM sleep: report of three patients. Neuropsychopharmacology. 2001;25:S63–7. doi: 10.1016/S0893-133X(01)00321-9. [DOI] [PubMed] [Google Scholar]
- 49.Dement WC. The paradox of sleep: the early years. Arch Ital Biol. 2004;142:333–45. [PubMed] [Google Scholar]
- 50.Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat: an update of the 1989 paper. Sleep. 2002;25:18–24. doi: 10.1093/sleep/25.1.18. [DOI] [PubMed] [Google Scholar]