Abstract
Sepsis remains a serious problem in critically ill patients with the mortality increasing to over half when there is attendant acute kidney injury. α-Melanocyte-stimulating hormone is a potent anti-inflammatory cytokine that inhibits many forms of inflammation including that with acute kidney injury. We tested whether a new α-melanocyte-stimulating hormone analogue (AP214), which has increased binding affinity to melanocortin receptors, improves sepsis-induced kidney injury and mortality using a cecal ligation and puncture mouse model. In the lethal cecal ligation-puncture model of sepsis, severe hypotension and bradycardia resulted and AP214 attenuated acute kidney injury of the lethal model with a bell-shaped dose-response curve. An optimum AP214 dose reduced acute kidney injury even when it was administered 6 hr after surgery and it significantly improved blood pressure and heart rate. AP214 reduced serum TNF-α and IL-10 levels with a bell-shaped dose-response curve. Additionally; NF-κB activation in the kidney and spleen, and splenocyte apoptosis were decreased by the treatment. AP214 significantly improved survival in both lethal and sublethal models. We have shown that AP214 improves hemodynamic failure, acute kidney injury, mortality and splenocyte apoptosis attenuating pro- and anti-inflammatory actions due to sepsis.
Sepsis is one of the major causes of mortality, and the incidence of sepsis in the United States is still increasing.1 Acute kidney injury (AKI) is also associated with a high mortality rate2 and AKI and sepsis increase mortality synergistically; septic AKI patients show worse mortality compared with non-septic AKI patients,3 whereas renal dysfunction in septic patients increases mortality.4 However therapies to treat or prevent sepsis-induced AKI are largely ineffective, and novel drugs available at clinical settings are urgently required.
Several pre-clinical animal models have been developed to evaluate the possible efficacy of drugs on sepsis-induced AKI.5 Among them, the cecal ligation and puncture (CLP) model is widely used because the resulting polymicrobial sepsis mimics many features of human sepsis.6 We have already demonstrated the efficacy of several drugs by a clinically relevant sepsis-induced AKI model based on CLP.7-9 In this model, animals were treated with fluid resuscitation and antibiotics similar to septic patients in an intensive care unit. AKI can be detected within 6 hr after surgery by a sensitive MRI technique9 and within 12 hr after CLP by measurement of serum creatinine.7 Moreover, our model shows not only kidney injury but multiple organ failure such as liver damage and splenocyte apoptosis.7, 10 In the present study, we further developed our CLP model with some modifications in terms of mouse strain, length of ligated cecum, and volume of fluid resuscitation.
α-Melanocyte stimulating hormone (α-MSH) darkens skin pigmentation in amphibians, rodents, and humans; however it is also a potent anti-inflammatory cytokine that decreases inflammatory cytokines, nitric oxide production, and expression of a neutrophil adhesion molecule.11, 12 α-MSH inhibits multiple forms of inflammation and shows protective effects on AKI induced by ischemia/reperfusion and cisplatin injection.13-18 α-MSH also showed protective effects in other experimental animal models such as brain ischemic injury,19 mesenteric ischemia-reperfusion injury,20 endotoxin-induced hepatitis,21 cutaneous vasculitis,22 and inflammatory bowel disease.23 Lipton et al. showed a protective effect of native α-MSH on survival of CLP animals although they did not measure sepsis-induced AKI or other organ failure.24 The purpose of the present study was to examine the effect of the α-MSH analogue AP214 that has an additional six lysine residues at the amino terminus on sepsis-induced AKI and mortality in a newly modified mouse sepsis model of CLP.
Results
Effects of AP214 treatment on AKI induced by lethal CLP in young CD-1 mice
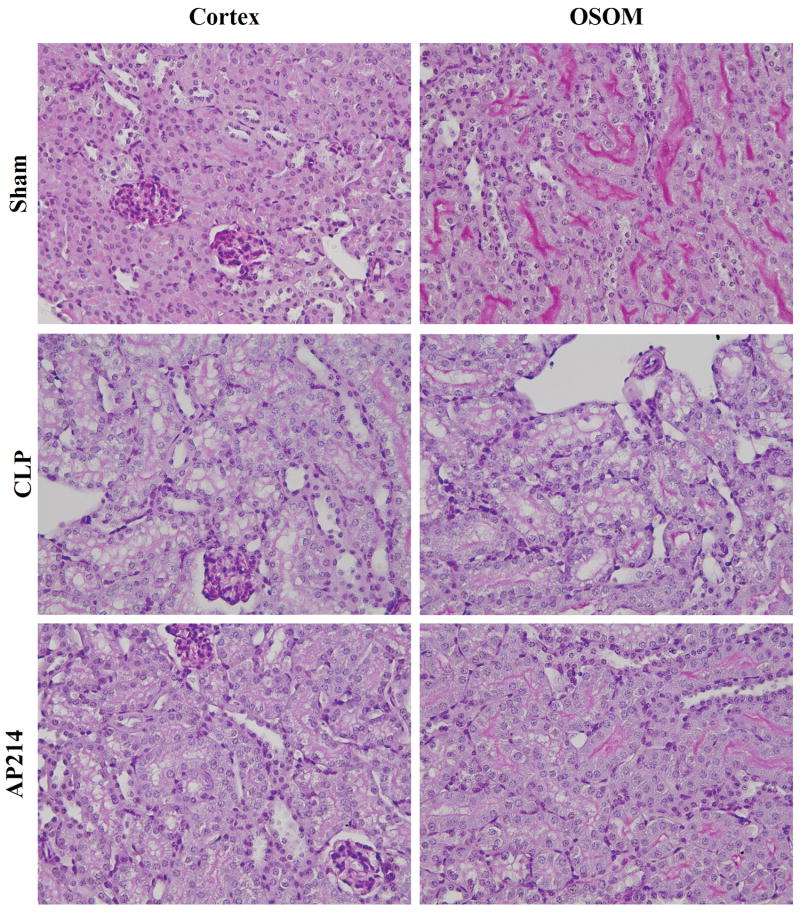
Lethal polymicrobial sepsis was induced in 8 wk old outbred CD-1 mice by placing a ligature at 15 mm from the tip of cecum and puncturing it twice with a 21-G needle. Serum creatinine increased 18 hr after surgery demonstrating the occurrence of acute kidney injury. Renal morphologic evaluation performed 18 hr after surgery showed mild tubular damage with vacuolization but no thrombosis, tubular necrosis, infiltrating inflammatory cells or cast formation both in cortex and the outer stripe of outer medulla (OSOM) (Figure 1). These histological damages were similar to our previous studies in aged C57BL/6 mice.7-10 Body weight did not increase in CD-1 mice, in contrast to our previous report that showed 7% increase,7 because the volume of fluid therapy that started at 6 hr after surgery and every 12 hr thereafter was reduced from 1.5 ml to 1.0 ml (data not shown).
Figure 1. Renal pathology.
Animals were sacrificed 18 hr after lethal CLP surgery. Representative renal histology of the cortex and the outer stripe of the outer medulla (OSOM) in each group were shown. Original magnification: X400.
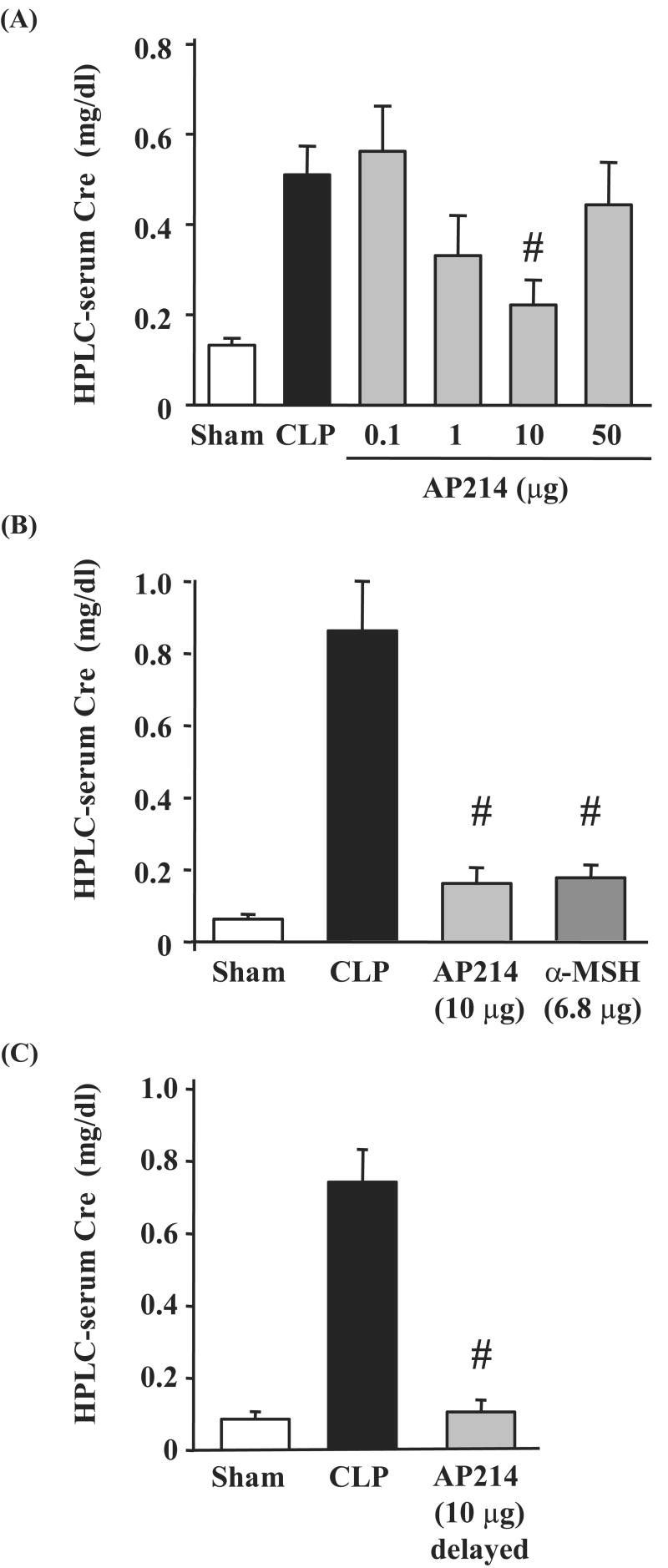
Treatment with AP214 attenuated AKI with a bell-shaped dose-response curve; 10 μg treatment had a maximum effect, 1 μg showed a partial improvement, whereas, neither 0.1 μg nor 50 μg treatment showed any effect against AKI (Figure 2A). Native α-MSH treatment with 6.8 μg (an amount equimolar to 10 μg AP214) also showed a protective effect on AKI (Figure 2B). AP214 treatment at the dose of 10 μg significantly ameliorated tubular damage in both the cortex and the OSOM (Figure 1, 3A,B). Treatment with the maximally efficacious dose of 10 μg reduced AKI and histological damage even when started 6 hrs after surgery (Figure 2C, 3C,D and Supplementary Figure 10). Treatment of sham-operated mice with 10 μg of AP214 did not cause any change in serum creatinine (data not shown).
Figure 2. Effect of AP214 on AKI induced by CLP.
Mice were sacrificed 18 hr after lethal CLP surgery for measurement of serum creatinine by HPLC method. AP214 was injected at 0, 6 hr (A, B) or only at 6 hr (delayed-treatment) (C). Equimolar native α-MSH (6.8 μg) was injected at 0 and 6 hr (B). Values are mean ± SEM (n = 4∼5 in sham-operated group, n = 10∼14 in CLP group, n = 7∼10 in each AP214 treatment group). #, P < 0.05 vs. CLP.
Figure 3. Pathological score.
The tubular damage score (see Methods section) was measured in the cortex and the OSOM. Values are mean ± SEM (n = 4 in sham-operated group, n = 8 in CLP group, n = 6 in each AP214 group). #, P < 0.05 vs. CLP.
Severe neutropenia was improved by AP214
The present lethal CLP model showed severe neutropenia at 18 hr after surgery in accordance with previous reports.25, 26. AP214 treatment at the dose of 10 μg significantly improved neutropenia in peripheral blood in lethal CLP, although there was no significant difference in the total white blood cell count between the CLP and AP214 treatment groups. AP214 did not cause any change in sham-operated mice (Table 1).
Table 1.
| WBC (/μl) | Neutrophil (/μl) | Lymphocyte + Monocyte (/μl) | |
|---|---|---|---|
| Sham + NS (n = 4) | 5673 ± 130 | 1247 ± 92 | 4318 ± 163 |
| Sham + AP214 (n = 4) | 5125 ± 550 | 1307 ± 130 | 3758 ± 429 |
| CLP + NS (n = 7) | 1520 ± 335 | 88 ± 69 | 1413 ± 353 |
| CLP + AP214 (n = 7) | 1049 ± 247 | 679 ± 131# | 347 ± 116# |
P < 0.05 vs. CLP + NS
Effects of AP214 treatment on liver damage in lethal CLP
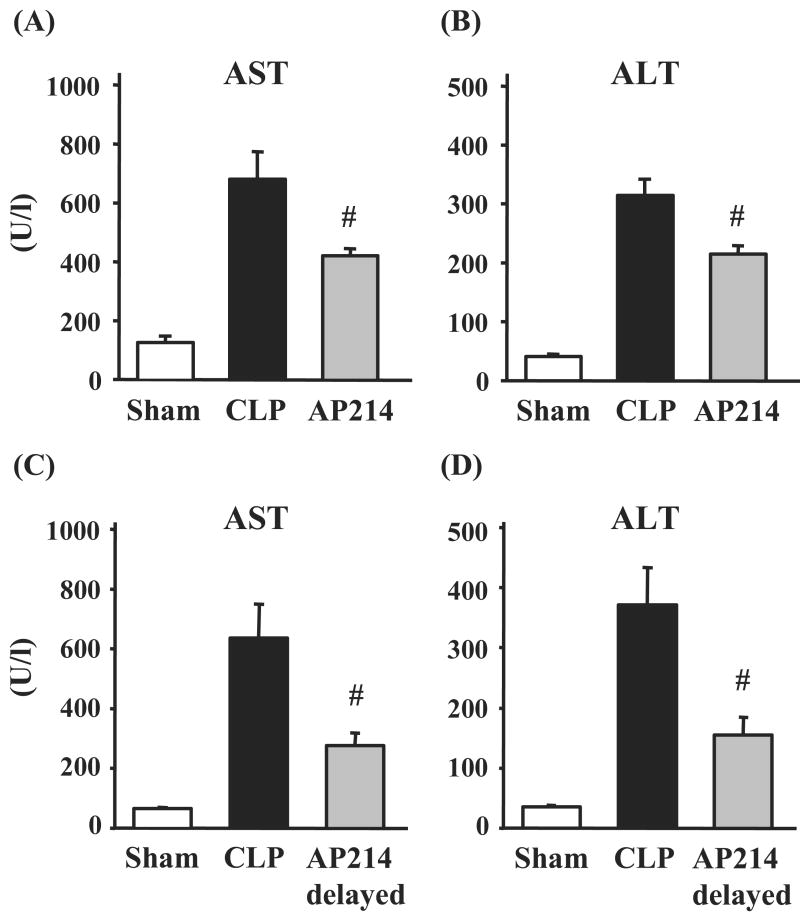
We previously reported liver damage in mouse CLP model.10 The lethal CLP model showed significant increases of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) at 18 hr after surgery compared with sham-operated animals [AST: vehicle 680.9 ± 92.5 U/l (n = 14), sham 126.4 ± 22.3 U/l (n = 5), p<0.05; ALT: vehicle 314.3 ± 30.1 U/l (n = 14), sham 40.6 ± 3.8 U/l (n = 5), p<0.05]. AP214 treatment at the dose of 10 μg significantly attenuated liver damage in lethal CLP (Figure 4A,B) and did not cause any change in sham-operated mice (data not shown). Delayed 10 μg AP214 treatment that was started at 6 hr after surgery also showed a protective effect against liver damage by lethal CLP (Figure 4C,D).
Figure 4. Effect of AP214 on liver damage induced by CLP.
Serum aspartate transaminase (AST) and alanine transaminase (ALT) were measured 18 hr after lethal CLP surgery. AP214 was injected at 0 and 6 hr (A, B) or only at 6 hr (delayed-treatment) (C). Values are mean ± SEM (n = 5 in sham-operated group, n = 14 in CLP group, n = 7 in AP214 group). #, P < 0.05 vs. CLP.
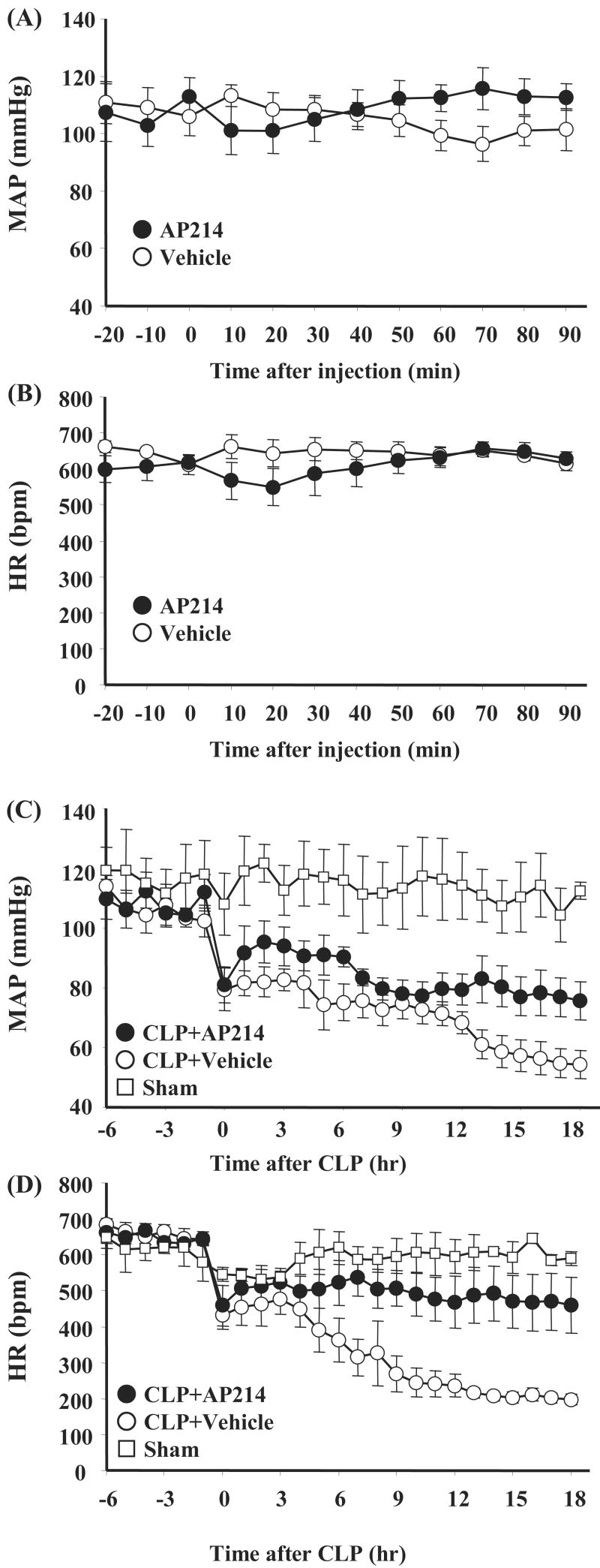
AP214 treatment improved hypotension in lethal CLP
Blood pressure and heart rate measurement was performed in conscious animals by radiotelemetery. Injection of AP214 on normal CD-1 mice at the dose of 10 μg did not alter blood pressure and heart rate compared with vehicle injection (Figure 5A,B). Sepsis induced by lethal CLP surgery caused severe hypotension and decreases of heart rate. AP214 treatment prevented the progression of severe hypotension and bradycardia [blood pressure at 18 hr: sham 112.4 ± 2.8 mmHg (n = 3), CLP + vehicle 54.4 ± 4.7 mmHg (n = 5), CLP + AP214 75.7 ± 6.4 mmHg (n = 5), p<0.05 (vs CLP + vehicle); heart rate: sham 459.4 ± 77.0 bpm (n = 3), CLP + vehicle 197.6 ± 14.2 bpm (n = 5), CLP + AP214 459.4 ± 77.0 bpm (n = 5), p<0.05 (vs CLP + vehicle)]. (Figure 5C,D).
Figure 5. AP214 prevented hypotension and bradycardia by CLP.
Telemetric recordings of mean arterial pressure (MAP) and heart rate (HR) after injection of AP214 (closed circle) or vehicle (open circle) to normal CD-1 mice are shown in (A) and (B). Values are mean ± SEM (n = 5 in vehicle group, n = 5 in AP214 group). MAP and HR in CLP mice with AP214 treatment (closed circle) or vehicle injection (open circle), and sham-operated group (open square) are shown in (C) and (D). Values are mean ± SEM (n = 3 in sham-operated group, n = 5 in CLP group, n = 5 in AP214 group).
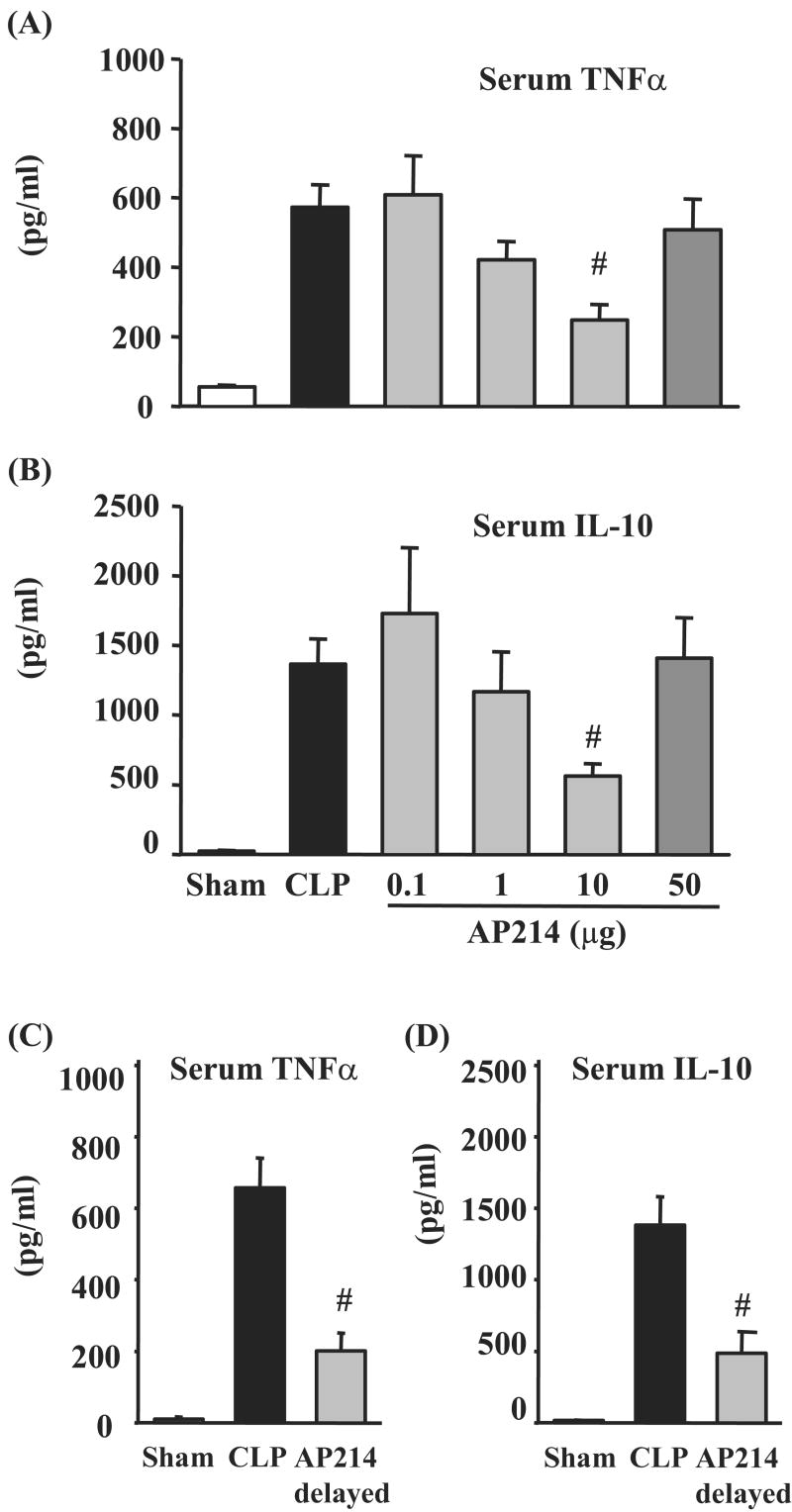
Serum TNF-α and IL-10 levels in lethal CLP were suppressed by AP214
We evaluated serum TNF-α and IL-10 levels by ELISA 18 hr after surgery in lethal CLP animals. Both serum TNF-α and IL-10 levels increased in CLP animals [TNF-α: Vehicle 572.5 ± 65.5 pg/ml (n = 14), sham 55.3 ± 5.3 pg/ml (n = 5), p<0.05, IL-10: Vehicle 1365.2 ± 182.4 pg/ml (n = 14), sham 20.2 ± 9.3 pg/ml (n = 5), p<0.05]. Administration of AP214 reduced serum TNF-α and IL-10 levels with bell-shaped dose-response curves (Figure 6A,B). AP214 had a maximum effect on sepsis-induced AKI at the dose of 10 μg and the same dose of 10 μg also had a maximum effect on reducing serum TNF-α and IL-10. Delayed AP214 treatment (10 μg) also reduced serum TNF-α and IL-10 (Figure 6C,D).
Figure 6. AP214 reduced serum TNF-α and IL-10.
Serum TNF-α and IL-10 levels were measured by ELISA. AP214 was injected at 0 and 6 hr (A, B) or only at 6 hr (delayed-treatment) (C, D). Animals were sacrificed 18 hr after lethal CLP surgery. Values are mean ± SEM (n = 5 in sham-operated group, n = 14 in CLP group, n = 7 in each AP214 group). #, P < 0.05 vs. CLP.
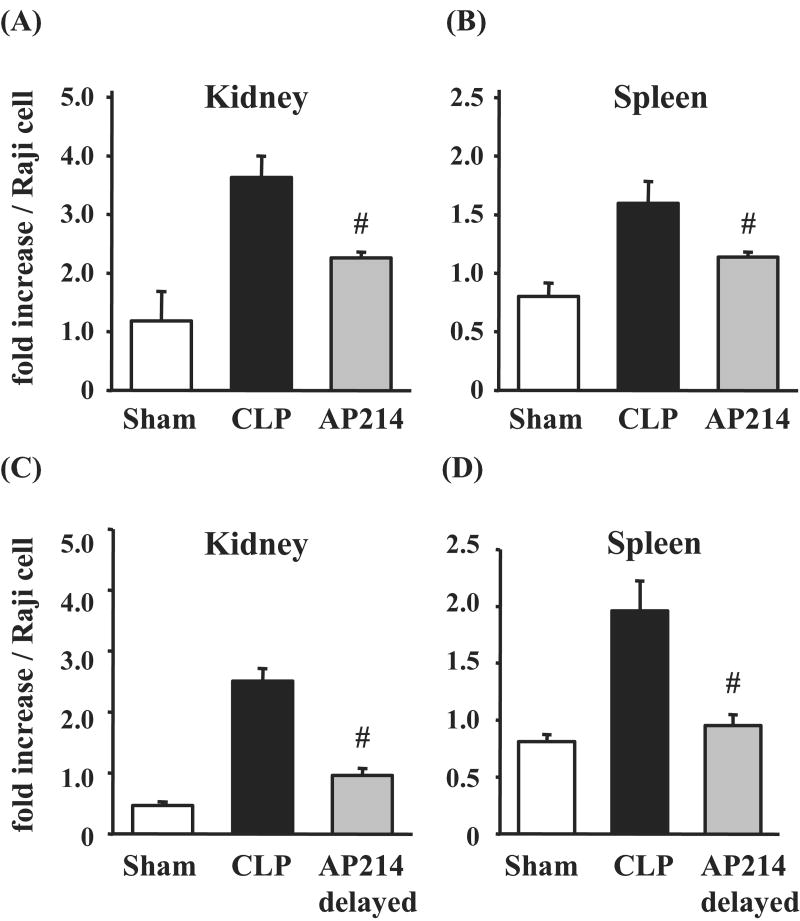
Nuclear factor-κB activation in kidney and spleen was inhibited by AP214
We examined nuclear factor-κB (NF-κB) activation in kidney and spleen because α-MSH has been reported to inhibit NF-κB activation induced by several inflammatory signals.27 NF-κB activation in kidney and spleen at 18 hr after lethal CLP surgery was significantly increased compared with sham-operated animals. AP214 treatment at the dose of 10 μg significantly inhibited the increase of NF-κB activation both in kidney and spleen (Figure 7A,B). Delayed AP214 treatment (10 μg) also decreased NF-κB activation (Figure 7C,D).
Figure 7. NF-κB activation in kidney and spleen.
NF-κB activation in kidney and spleen was evaluated by ELISA. AP214 was injected at 0 and 6 hr (A, B) or only at 6 hr (delayed-treatment) (C, D). OD value of each sample was normalized with that of Raji cell nuclear extract. Values are mean ± SEM (n = 4 in sham-operated group, n = 6-7 in CLP group, n = 6-7 in AP214 group). #, P < 0.05 vs. CLP.
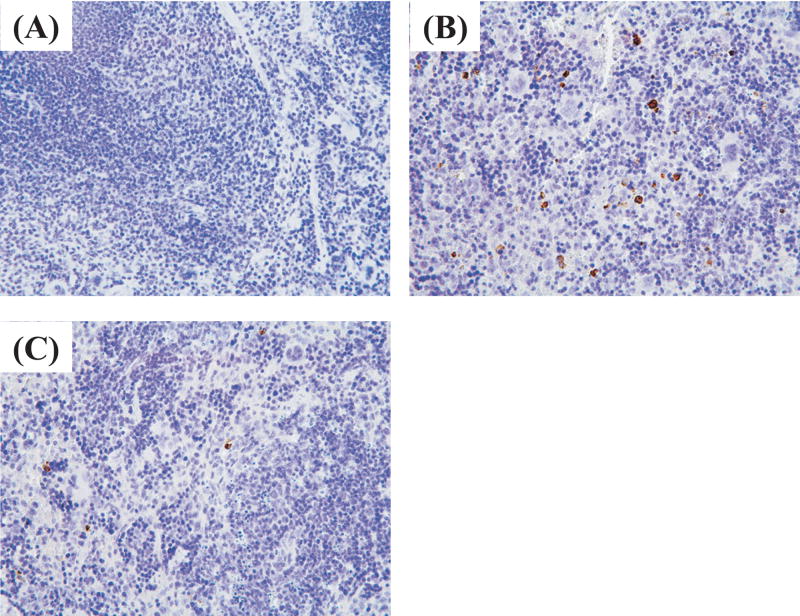
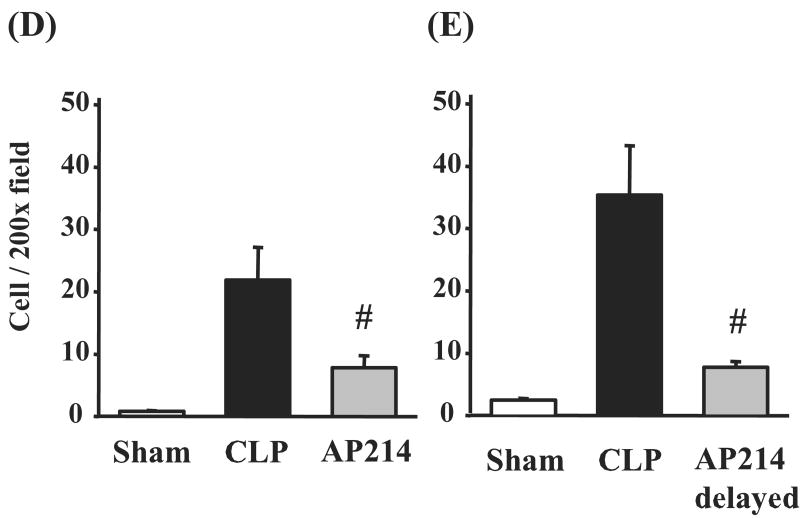
AP214 reduced splenocyte apoptosis in lethal CLP
Lymphocyte apoptosis is reported to contribute to sepsis severity.28 Therefore, we investigated the effect of AP214 on splenocyte apoptosis. Splenocyte apoptosis was evaluated by immunohistochemical analysis of activated caspase-3. In sham operated animals, activated caspase-3 positive stained cells were rarely found in spleen (Figure 8A). CLP increased activated caspase-3 positive stained cells profoundly in the white pulp of spleen at 18 hr after surgery and AP214 treatment at the dose of 10 μg significantly reduced splenocyte apoptosis (Figure 8B,C,D). Delayed AP214 treatment (10 μg) also reduced splenocyte apoptosis (Figure 8E). No apoptotic cell was detectable in the kidney as previously reported 10.
Figure 8. Immunohistochemical analysis of activated caspase-3 in spleen.
Immunohistochemistry with anti-activated caspase-3 antibody in sham-operated group (A), CLP group (B), AP214-treated group (C); Original magnification: X400. Counts of positive stained cells were shown. AP214 was injected at 0 and 6 hr (D) or only at 6 hr (delayed-treatment) (E). Values are mean ± SEM (n = 3 in sham-operated group, n = 5 in CLP group, n = 5 in AP214 group). #, P < 0.05 vs. CLP.
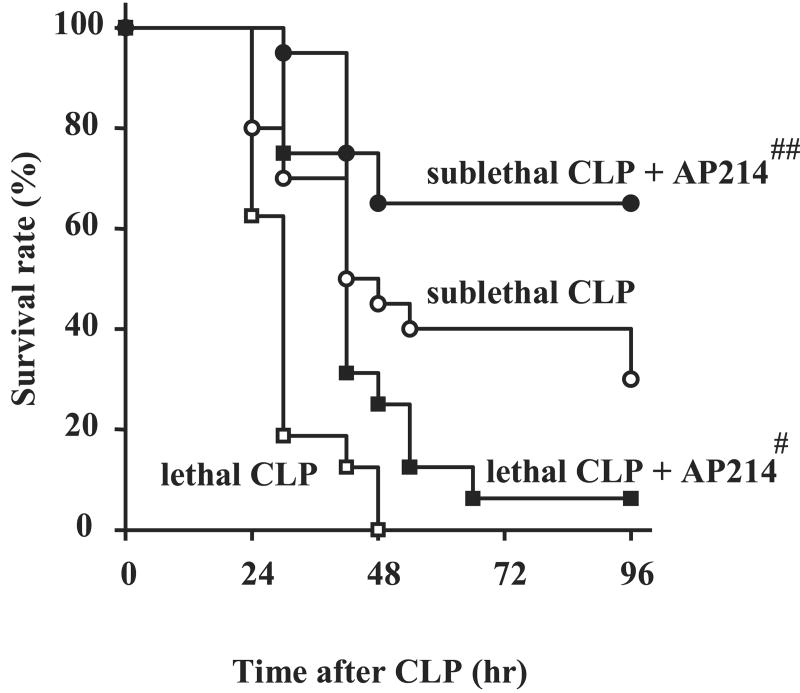
AP214 improved survival both in lethal and sublethal CLP
We evaluated the effects of 10 μg AP214 treatment on survival after lethal CLP, in which 15 mm of the cecum was ligated. Drug or vehicle was administrated just after surgery, at 6 hr post surgery and every 12 hr thereafter. All vehicle-treated animals in the lethal CLP model died within 48 hrs after surgery; treatment with AP214 improved mortality (Figure 9). We also evaluated the effect of AP214 on sublethal CLP model, in which only 8 mm of the cecum was ligated. After sublethal CLP, 30-40% of control animals survived at 96 hr and treatment with AP214 increased the survival rate to 65% (Figure 4). AKI after sublethal CLP was evaluated at 18 hr after surgery. Compared with our lethal CLP model, the control animals in sublethal CLP showed lower serum creatinine levels and treatment with AP214 at the dose of 10 μg showed a trend towards decreased serum Cr [vehicle 0.29 ± 0.06 mg/dl (n = 15) vs AP214 0.19 ± 0.04 mg/dl (n = 15), P= 0.089].
Figure 9. AP214 improved survival of CLP.
AP214 treatment at the dose of 10 μg improved the survival rate both in lethal and sublethal CLP model. Open squares indicate lethal CLP group (n = 16), closed squares AP214 treatment group in lethal CLP (n = 16), open circles sublethal CLP group (n = 20), and closed circles AP214 treatment group in sublethal CLP (n =20). #, P < 0.05 vs. lethal CLP. ##, P < 0.05 vs. sublethal CLP.
Discussion
We have evaluated the effects of the α-MSH analogue AP214 on sepsis-induced AKI and mortality by using a modified rodent CLP sepsis model in outbred mice. AP214 showed a protective effect on sepsis-induced AKI and improved survival in lethal and sublethal CLP models. We also demonstrated potential mechanisms of AP214 action on several pro- and anti-inflammatory pathways and splenocyte apoptosis.
We developed a new CLP model as follows; we changed the mouse strain from inbred C57BL/6 to outbred CD-1 mice that are more representative of a genetically heterogeneous human population. In our previous reports, we used aged C57BL/6 mice because CLP surgery in young C57BL/6 mice produced a wide range of creatinine values without much histologic evidence of renal damage.7 In contrast, 8 week old CD-1 mice showed more pathological renal damage compared with young C57BL/6 mice. Since the length of the ligated cecum determines the severity in other CLP models,29, 30 we developed two different models: A lethal CLP model (ligation 15 mm from the end of cecum) and a sublethal CLP model (8 mm) models (Figure 9). Finally, we reduced the volume of fluid resuscitation compared to our previous studies to avoid volume overload and maintain constant body weight.
Mice subjected to lethal CLP were severely hypotensive and bradycardic (Figure 5). The radiotelemetry data in the present study indicate that systemic circulation failure induced by CLP may contribute to organ hypoperfusion, including hypoperfusion of kidney that has been previously shown in CLP animals.8, 31 Severe systemic circulation failure might contribute to sepsis-induced AKI in this model. AP214 significantly improved blood pressure and heart rate. Recent studies revealed that melanocyte stimulating hormones including α-MSH have important roles in cardiovascular regulation.32 It has been reported that α-MSH increases blood pressure and heart rate by activation of MCR4 that is primarily expressed in the central nervous system.33 Hence, AP214 might improve sepsis via a direct hemodynamic effect. However, exogenous α-MSH increases blood pressure only when it is administered by intracerebroventricular injection; intravenous injection does not alter blood pressure in rodents.33, 34 Similarly, intravenous AP214 did not increase blood pressure and heart rate in normal mice. However, the present data does not clarify the precise mechanism by which intravenously injected AP214 exerts its hemodynamic effects in the septic CLP mice, as it is not possible to differentiate between a direct hemodynamic effect from an initial anti-inflammatory effect with subsequent improvement in hemodynamics.
α-MSH has broad anti-inflammatory properties and inhibits organ injury in various inflammatory animal models.35 We have already demonstrated that the native α-MSH ameliorated AKI caused by ischemia reperfusion injury.13-15, 17, 18 Although one report showed that native α-MSH improved survival of sepsis induced by CLP, its effect on sepsis-induced AKI and multiple organ failure were not determined.24 In the current study, we found that a α-MSH analogue AP214 improved sepsis-induced multiple organ failure including AKI and mortality. Moreover, we found that AP214 had a bell-shaped dose-response curve for sepsis-induced AKI. There are several reports that demonstrated biphasic dose-response curves in the anti-inflammatory influence of α-MSH. Both the large and small doses were ineffective for in vivo animal models of ear swelling induced by picryl chloride36, 37 and crystal-induced peritonitis38, and in vitro cell culture models such as TNF-α production in microglia39, cyclic AMP production in mast cells40 and glutathione peroxidase activity in melanocytes and keratinocytes41. The cause of this phenomenon is unknown, but might be explained in part by receptor downregulation to avoid excess effects. Equimolar native α-MSH also showed a protective effect on sepsis-induced AKI, although binding affinity of AP214 for melanocortin receptors in vitro were higher than native α-MSH. These data suggest that effects of AP214 in vivo may not be straightforward and confounded by pharmacokinetic parameters and/or indirect regulatory influences. Further evaluations would be required to clarify the mechanisms of these biphasic responses of AP214 both in physiological and pathological in vivo conditions.
Failure to regulate hyperactivation of the inflammatory system plays an important role in pathophysiology of sepsis and blocking inflammatory mediators has been often proposed as a treatment strategy for sepsis. However, many clinical trials with drugs that inhibit specific inflammatory mediators have not been successful. α-MSH has a breadth of anti-inflammatory activities and can improve inflammatory disorders through suppressing multiple pathways. Moreover, α-MSH modulates inflammatory responses thorough inhibition of the nuclear transcription factor NF-κB activation, which plays the central role in immune and inflammation systems.27 In the present study, we demonstrated the inhibition of systemic TNF-α production (Figure 6) and renal and splenic NF-κB activation (Figure 7). AP214 reduced serum TNF-α levels with a bell-shaped dose-response curve similar to its effects on serum creatinine levels. This indicates that modulation of the exacerbated inflammatory response is beneficial for AKI induced by lethal CLP. AP214 can be an agonist of several melanocortin receptors (MCR1, MCR3, MCR4, and MCR5). MCR1 and MCR3 are known to mediate anti-inflammatory effects and MCR4 regulates hemodynamics in the central nerve system as described above, whereas MCR5 has been implicated in multiple exocrine functions.42 Further investigation on the contribution of each melanocortin receptor pathway will be necessary to further understand the mechanisms of action of α-MSH in sepsis and to minimize unwanted effects from other melanocortin receptors.
The action of α-MSH is reminiscent of corticosteroids because both of them have multiple anti-inflammatory effects through several mechanisms including inhibiting NF-κB pathway.43 It has been recently suggested that ‘physiologic’ but not high doses of corticosteroids might be beneficial to septic patients.44 Our data indicates that AP214 similarly has a relatively narrow therapeutic window since 10 μg treatment but not 50 μg showed protective effects in CLP. Therefore, translation of AP214 to studies in human sepsis will likely require precise evaluation of the pharmacokinetics and dose responses of AP214 in humans before large-scale clinical trials can be initiated.
Serum IL-10 levels were increased in our mouse sepsis model and suppressed after AP214 treatment. Although the role of IL-10 as an anti-inflammatory cytokine is apparent in renal ischemia/reperfusion and cisplatin-induced renal injury,16 the role of IL-10 and its relationship to α-MSH in sepsis is less clear. IL-10 knockout mice showed higher mortality in CLP than wild type mice and anti-IL-10 monoclonal antibody treatment worsened the survival after CLP when injected at 0 or 2 hr before surgery.45, 46 On the other hand, anti-IL-10 monoclonal antibody treatment was protective when it was injected 10 hr after CLP.46 Therefore, IL-10 appears to have dual effects on sepsis that depends on the timing. The finding that AP214 decreased serum IL-10 levels at 18 hr after surgery in the present study indicates that excessive IL-10 at the late phase might worsen sepsis-induced AKI.
The relationship between α-MSH and IL-10 is also unclear. In vitro, α-MSH modestly stimulates IL-10 production in human peripheral blood mononuclear cells.47, 48 There is one report in vivo showing no effect of [Nle4, D-Phe7] α-MSH on lipopolysaccaride (LPS)-stimulated plasma IL-10 levels in monkeys.49 Our results clearly showed that the increase of serum IL-10 by CLP was attenuated by α-MSH. One possible explanation for this discrepancy is that LPS, especially at the subclinical dose used in the primate study,49 causes an increase in IL-10 that is more resistant to α-MSH. The observation that LPS signaling via Toll-like receptor 4 does not contribute to AKI in polymicrobial sepsis by CLP supports this view.10 The mechanisms of α-MSH to decrease IL-10 production in vivo during sepsis need further investigation. Because the effect of IL-10 disruption on sepsis outcomes depends on timing, the success of AP214 as a clinical therapeutic may require optimization of the timing of administration.
Apoptosis was detected predominantly in lymphocytes in human sepsis,50 and several strategies to decrease immune cell apoptosis have been reported to improve the survival rate of CLP animals.51-53 CLP animals in the present study showed splenocyte apoptosis, which was reduced after AP214 treatment. α-MSH has been reported to activate anti-apoptotic pathways in cultured melanocytes54, 55 and reduce renal tubular cell apoptosis in a bilateral ureteral obstruction model.56 It might be possible that AP214 acts on splenocytes and suppresses apoptotic pathways.
Conclusion
The α-MSH analogue AP214 ameliorated severe septic shock and sepsis-induced AKI and mortality in outbred CD-1 mouse models of polymicrobial sepsis. We found the biphasic dose-response curves of AP214 against AKI and cytokine productions, indicating that strict regulation of α-MSH dosing might be important in sepsis. We also demonstrated that AP214 improved systemic hemodynamics, pro- and anti-inflammatory actions, renal and splenic NF-κB activation, and splenocyte apoptosis. These results suggested that AP214 might be useful for the prevention or treatment of sepsis in humans.
Materials and Methods
Reagents
AP214 is an analogue of α-MSH modified with six lysine residues at N-terminus of native α-MSH (Ac-KKKKKKSYSMEHFRWGKPV-NH2). AP214 was provided by Action Pharma A/S (Aarhus and Copenhagen, Denmark). AP214 has a higher binding affinity for melanocortin receptors compared with the native α-MSH [Inhibition constants (AP214 vs MSH): MCR1: 2.9 vs 5.7 nM; MCR3: 1.9 vs 22 nM; MCR4: 3.7 vs 24 nM and MCR5: 110 vs 1300 nM (Jonassen et al. J Am Soc Nephrol S17: S326A, 2006)]. Native α-MSH was purchased from Phoenix Pharmaceuticals (Mountain View, CA).
Cecal ligation and puncture (CLP) model
All animal experiments were conducted in accordance with an animal study protocol approved by NIDDK animal care and use committee. 8 week old male CD-1 mice (Charles River Laboratories, Wilmington, MA) were allowed food and water ad libitum. CLP was performed as previously described with some modifications.7 Under isoflurane anesthesia, a 1.5 cm midline incision was made and the cecum was exposed. A 4-0 silk ligature was placed 15 mm from the cecal tip in lethal CLP and 8 mm in sublethal CLP. The cecum was punctured twice with a 21-gauge needle and gently squeezed to confirm leakage of cecal contents. In sham-operated animals, the cecum was located, but neither ligated nor punctured. The abdominal incision was closed in two layers with 4-0 silk sutures, and 1 ml of prewarmed normal saline (NS) was injected intraperitoneally. Treatment with fluid and antibiotic was started at 6 hr after surgery with subcutaneous injection of imipenem/cilastatin (14 mg/kg) in 1 mL of NS, treatment in the survival study was continued every 12 hr with imipenem/cilastatin (7 mg/kg) in 1 mL of NS. AP214 (0.1, 1, 10, and 50 μg) or native α-MSH (6.8 μg) dissolved in NS 0.15 ml was administered intravenously as a bolus just after surgery, at 6 hr post-surgery and then every 12 hr thereafter in the survival study. In the vehicle-treated CLP group, animals received 0.15 ml of NS intravenously at the indicated times. In another experiment, 10 μg of AP214 dissolved in 0.15 ml of NS or only vehicle was given intravenously 6 hr after surgery (delayed-treatment group). Blood, kidneys and spleen were collected under isoflurane anesthesia at 18 hr after surgery except for survival study.
Morphologic evaluation of kidneys
Kidney specimens fixed in 10% formalin and embedded in paraffin were stained with periodic acid-Schiff reagent (PAS). Histologic changes in the cortex and in the outer stripe of the outer medulla (OSOM) were scored by a blinded observer.7
Measurement of blood chemistry, blood cell counts and cytokines
Serum creatinine was measured by HPLC.57 Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured by autoanalyzer (Hitachi 917, Boehringer Mannheim, Indianapolis, IN). Peripheral blood cell counts were analyzed by an automated hematology analyzer (Abott Cell-Dyn 3500, GMI, Ramsey, MN). Tumor necrosis factor-α (TNF α) and interleukin 10 (IL-10) were measured by ELISA (R&D Systems, Minneapolis, MN).
Measurement of blood pressure and heart rate
The mean blood pressure and heart rate were measured by radiotelemetry. Under anesthesia with ketamine (50 mg/kg), xylazine (5 mg/kg) and acepromazine (1 mg/kg), a telemeter catheter was implanted in the left carotid artery and advanced to reach the aortic arch. The attached telemetry transmitter (model TA11PA-C10, Data Sciences International, St. Paul. MN) was placed in a subcutaneous pocket on the left flank one week before CLP surgery or AP214 administration. The telemeter signals were processed as previously described.58 Blood pressure and heart rate data was analyzed from 6 hr before CLP surgery for 24 hrs, or 20 min before injection of AP214 or vehicle until 90 min after.
NF-κB activation assay
NF-κB p65 activity was quantified by using the TransAM Assay (Active Motif, Carlsbad, CA). Briefly, a NF-κB consensus oligonucleotide bound to a 96-well plate was incubated with 100 μg of whole cell extracts from kidney and spleen, followed by p65 primary antibody and secondary antibody conjugated with horseradish peroxidase. The optical density (OD) values of peroxidase reaction product from each sample were normalized with the OD values of Raji nuclear extract (Active Motif).
Immunohistochemical analysis of activated caspase-3 in spleen
Immunohistochemical staining of 4 μm paraffin sections was performed with anti-activated caspase-3 antibody (Cell Signaling Technology, Beverly, MA). The sections were deparaffinized, incubated in 10 mmol/L citrate-buffer, pH 6.0 for 10 min at 95°C and preincubated with 3% hydrogen peroxide for 10 min. After blocking with goat serum, sections were incubated for 1 hr at room temperature with primary antibodies. Two-step immunohistochemical staining with horseradish peroxidase-conjugated secondary antibody was conducted (EnVison+ System-HRP (DAB), DakoCytomation, Glostrup, Denmark) with diaminobenzidine substrate, then counterstaining with hematoxylin. Positive stained cells were counted from five randomly selected non-overlapping 200X fields.
Statistical analysis
Results of statistical analyses are expressed as means ± SEM. Differences among experimental groups were confirmed by one-way ANOVA followed by Dunnett's test for individual comparison of group means vs the control group. Survival analyses were compared by a log-rank test. These calculations were performed with SigmaStat v3.10 (Systat Software Inc, Richmond, CA). The null hypothesis was rejected when P < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Yan Qin for technical assistance.
Disclosure This research was supported by the Intramural Research Program of the NIH, NIDDK. Thomas Jonassen, Jørgen Frøkiær, and Søren Nielsen have a financial interest in Action Pharma, which supplied no research funding, other than providing AP214.
References
- 1.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 2.Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. Jama. 1996;275:1489–1494. [PubMed] [Google Scholar]
- 3.Neveu H, Kleinknecht D, Brivet F, et al. Prognostic factors in acute renal failure due to sepsis. Results of a prospective multicentre study. The French Study Group on Acute Renal Failure. Nephrol Dial Transplant. 1996;11:293–299. doi: 10.1093/oxfordjournals.ndt.a027256. [DOI] [PubMed] [Google Scholar]
- 4.Russell JA, Singer J, Bernard GR, et al. Changing pattern of organ dysfunction in early human sepsis is related to mortality. Crit Care Med. 2000;28:3405–3411. doi: 10.1097/00003246-200010000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4:854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- 6.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock--a review of laboratory models and a proposal. J Surg Res. 1980;29:189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 7.Miyaji T, Hu X, Yuen PS, et al. Ethyl pyruvate decreases sepsis-induced acute renal failure and multiple organ damage in aged mice. Kidney Int. 2003;64:1620–1631. doi: 10.1046/j.1523-1755.2003.00268.x. [DOI] [PubMed] [Google Scholar]
- 8.Yasuda H, Yuen PS, Hu X, et al. Simvastatin improves sepsis-induced mortality and acute kidney injury via renal vascular effects. Kidney Int. 2006;69:1535–1542. doi: 10.1038/sj.ki.5000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dear JW, Kobayashi H, Jo SK, et al. Dendrimer-enhanced MRI as a diagnostic and prognostic biomarker of sepsis-induced acute renal failure in aged mice. Kidney Int. 2005;67:2159–2167. doi: 10.1111/j.1523-1755.2005.00321.x. [DOI] [PubMed] [Google Scholar]
- 10.Dear JW, Yasuda H, Hu X, et al. Sepsis-induced organ failure is mediated by different pathways in the kidney and liver: Acute renal failure is dependent on MyD88 but not renal cell apoptosis. Kidney Int. 2006;69:832–836. doi: 10.1038/sj.ki.5000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Star RA, Rajora N, Huang J, et al. Evidence of autocrine modulation of macrophage nitric oxide synthase by alpha-melanocyte-stimulating hormone. Proc Natl Acad Sci U S A. 1995;92:8016–8020. doi: 10.1073/pnas.92.17.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohda Y, Chiao H, Star RA. alpha-Melanocyte-stimulating hormone and acute renal failure. Curr Opin Nephrol Hypertens. 1998;7:413–417. doi: 10.1097/00041552-199807000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Chiao H, Kohda Y, McLeroy P, et al. Alpha-melanocyte-stimulating hormone protects against renal injury after ischemia in mice and rats. J Clin Invest. 1997;99:1165–1172. doi: 10.1172/JCI119272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiao H, Kohda Y, McLeroy P, et al. Alpha-melanocyte-stimulating hormone inhibits renal injury in the absence of neutrophils. Kidney Int. 1998;54:765–774. doi: 10.1046/j.1523-1755.1998.00075.x. [DOI] [PubMed] [Google Scholar]
- 15.Kwon TH, Frokiaer J, Fernandez-Llama P, et al. Reduced abundance of aquaporins in rats with bilateral ischemia-induced acute renal failure: prevention by alpha-MSH. Am J Physiol. 1999;277:F413–427. doi: 10.1152/ajprenal.1999.277.3.F413. [DOI] [PubMed] [Google Scholar]
- 16.Deng J, Kohda Y, Chiao H, et al. Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int. 2001;60:2118–2128. doi: 10.1046/j.1523-1755.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- 17.Deng J, Hu X, Yuen PS, et al. Alpha-melanocyte-stimulating hormone inhibits lung injury after renal ischemia/reperfusion. Am J Respir Crit Care Med. 2004;169:749–756. doi: 10.1164/rccm.200303-372OC. [DOI] [PubMed] [Google Scholar]
- 18.Gong H, Wang W, Kwon TH, et al. EPO and alpha-MSH prevent ischemia/reperfusion-induced down-regulation of AQPs and sodium transporters in rat kidney. Kidney Int. 2004;66:683–695. doi: 10.1111/j.1523-1755.2004.00791.x. [DOI] [PubMed] [Google Scholar]
- 19.Huh SK, Lipton JM, Batjer HH. The protective effects of alpha-melanocyte stimulating hormone on canine brain stem ischemia. Neurosurgery. 1997;40:132–139. doi: 10.1097/00006123-199701000-00030. [DOI] [PubMed] [Google Scholar]
- 20.Hassoun HT, Zou L, Moore FA, et al. Alpha-melanocyte-stimulating hormone protects against mesenteric ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1059–1068. doi: 10.1152/ajpgi.00073.2001. [DOI] [PubMed] [Google Scholar]
- 21.Chiao H, Foster S, Thomas R, et al. Alpha-melanocyte-stimulating hormone reduces endotoxin-induced liver inflammation. J Clin Invest. 1996;97:2038–2044. doi: 10.1172/JCI118639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scholzen TE, Sunderkotter C, Kalden DH, et al. Alpha-melanocyte stimulating hormone prevents lipopolysaccharide-induced vasculitis by down-regulating endothelial cell adhesion molecule expression. Endocrinology. 2003;144:360–370. doi: 10.1210/en.2002-220651. [DOI] [PubMed] [Google Scholar]
- 23.Rajora N, Boccoli G, Catania A, et al. alpha-MSH modulates experimental inflammatory bowel disease. Peptides. 1997;18:381–385. doi: 10.1016/s0196-9781(96)00345-2. [DOI] [PubMed] [Google Scholar]
- 24.Lipton JM, Ceriani G, Macaluso A, et al. Antiinflammatory effects of the neuropeptide alpha-MSH in acute, chronic, and systemic inflammation. Ann N Y Acad Sci. 1994;741:137–148. doi: 10.1111/j.1749-6632.1994.tb39654.x. [DOI] [PubMed] [Google Scholar]
- 25.Remick DG, Newcomb DE, Bolgos GL, et al. Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock. 2000;13:110–116. doi: 10.1097/00024382-200013020-00004. [DOI] [PubMed] [Google Scholar]
- 26.Kuhn JF, Godshall CJ, Scott MJ, et al. Immediate and delayed leukocyte apoptosis in two models of peritonitis. Inflammation. 2001;25:389–397. doi: 10.1023/a:1012854731259. [DOI] [PubMed] [Google Scholar]
- 27.Manna SK, Aggarwal BB. Alpha-melanocyte-stimulating hormone inhibits the nuclear transcription factor NF-kappa B activation induced by various inflammatory agents. J Immunol. 1998;161:2873–2880. [PubMed] [Google Scholar]
- 28.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 29.Torres MB, De Maio A. An exaggerated inflammatory response after CLP correlates with a negative outcome. J Surg Res. 2005;125:88–93. doi: 10.1016/j.jss.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 30.Singleton KD, Wischmeyer PE. Distance of cecum ligated influences mortality, tumor necrosis factor-alpha and interleukin-6 expression following cecal ligation and puncture in the rat. Eur Surg Res. 2003;35:486–491. doi: 10.1159/000073387. [DOI] [PubMed] [Google Scholar]
- 31.Wu L, Gokden N, Mayeux PR. Evidence for the role of reactive nitrogen species in polymicrobial sepsis-induced renal peritubular capillary dysfunction and tubular injury. J Am Soc Nephrol. 2007;18:1807–1815. doi: 10.1681/ASN.2006121402. [DOI] [PubMed] [Google Scholar]
- 32.Humphreys MH. Cardiovascular and renal actions of melanocyte-stimulating hormone peptides. Curr Opin Nephrol Hypertens. 2007;16:32–38. doi: 10.1097/MNH.0b013e3280117fb5. [DOI] [PubMed] [Google Scholar]
- 33.Matsumura K, Tsuchihashi T, Abe I, et al. Central alpha-melanocyte-stimulating hormone acts at melanocortin-4 receptor to activate sympathetic nervous system in conscious rabbits. Brain Res. 2002;948:145–148. doi: 10.1016/s0006-8993(02)03045-7. [DOI] [PubMed] [Google Scholar]
- 34.Hill C, Dunbar JC. The effects of acute and chronic alpha melanocyte stimulating hormone (alphaMSH) on cardiovascular dynamics in conscious rats. Peptides. 2002;23:1625–1630. doi: 10.1016/s0196-9781(02)00103-1. [DOI] [PubMed] [Google Scholar]
- 35.Catania A, Lipton JM. alpha-Melanocyte stimulating hormone in the modulation of host reactions. Endocr Rev. 1993;14:564–576. doi: 10.1210/edrv-14-5-564. [DOI] [PubMed] [Google Scholar]
- 36.Hiltz ME, Lipton JM. Antiinflammatory activity of a COOH-terminal fragment of the neuropeptide alpha-MSH. Faseb J. 1989;3:2282–2284. [PubMed] [Google Scholar]
- 37.Hiltz ME, Catania A, Lipton JM. Alpha-MSH peptides inhibit acute inflammation induced in mice by rIL-1 beta, rIL-6, rTNF-alpha and endogenous pyrogen but not that caused by LTB4, PAF and rIL-8. Cytokine. 1992;4:320–328. doi: 10.1016/1043-4666(92)90073-z. [DOI] [PubMed] [Google Scholar]
- 38.Getting SJ, Gibbs L, Clark AJ, et al. POMC gene-derived peptides activate melanocortin type 3 receptor on murine macrophages, suppress cytokine release, and inhibit neutrophil migration in acute experimental inflammation. J Immunol. 1999;162:7446–7453. [PubMed] [Google Scholar]
- 39.Delgado R, Carlin A, Airaghi L, et al. Melanocortin peptides inhibit production of proinflammatory cytokines and nitric oxide by activated microglia. J Leukoc Biol. 1998;63:740–745. doi: 10.1002/jlb.63.6.740. [DOI] [PubMed] [Google Scholar]
- 40.Adachi S, Nakano T, Vliagoftis H, et al. Receptor-mediated modulation of murine mast cell function by alpha-melanocyte stimulating hormone. J Immunol. 1999;163:3363–3368. [PubMed] [Google Scholar]
- 41.Haycock JW, Rowe SJ, Cartledge S, et al. Alpha-melanocyte-stimulating hormone reduces impact of proinflammatory cytokine and peroxide-generated oxidative stress on keratinocyte and melanoma cell lines. J Biol Chem. 2000;275:15629–15636. doi: 10.1074/jbc.275.21.15629. [DOI] [PubMed] [Google Scholar]
- 42.Chen W, Kelly MA, Opitz-Araya X, et al. Exocrine gland dysfunction in MC5-R-deficient mice: evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell. 1997;91:789–798. doi: 10.1016/s0092-8674(00)80467-5. [DOI] [PubMed] [Google Scholar]
- 43.McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr Rev. 1999;20:435–459. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- 44.Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. Jama. 2002;288:862–871. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 45.van der Poll T, Marchant A, Buurman WA, et al. Endogenous IL-10 protects mice from death during septic peritonitis. J Immunol. 1995;155:5397–5401. [PubMed] [Google Scholar]
- 46.Song GY, Chung CS, Chaudry IH, et al. What is the role of interleukin 10 in polymicrobial sepsis: anti-inflammatory agent or immunosuppressant? Surgery. 1999;126:378–383. [PubMed] [Google Scholar]
- 47.Bhardwaj RS, Schwarz A, Becher E, et al. Pro-opiomelanocortin-derived peptides induce IL-10 production in human monocytes. J Immunol. 1996;156:2517–2521. [PubMed] [Google Scholar]
- 48.Yamaoka-Tojo M, Tojo T, Shioi T, et al. Central neurotranspeptide, alpha-melanocyte-stimulating hormone (alpha-MSH) is upregulated in patients with congestive heart failure. Intern Med. 2006;45:429–434. doi: 10.2169/internalmedicine.45.1546. [DOI] [PubMed] [Google Scholar]
- 49.Vulliemoz NR, Xiao E, Xia-Zhang L, et al. Melanocortin modulation of inflammatory cytokine and neuroendocrine responses to endotoxin in the monkey. Endocrinology. 2006;147:1878–1883. doi: 10.1210/en.2005-1430. [DOI] [PubMed] [Google Scholar]
- 50.Hotchkiss RS, Swanson PE, Freeman BD, et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Oberholzer C, Tschoeke SK, Moldawer LL, et al. Local thymic caspase-9 inhibition improves survival during polymicrobial sepsis in mice. J Mol Med. 2006;84:389–395. doi: 10.1007/s00109-005-0017-1. [DOI] [PubMed] [Google Scholar]
- 52.Weaver JG, Rouse MS, Steckelberg JM, et al. Improved survival in experimental sepsis with an orally administered inhibitor of apoptosis. Faseb J. 2004;18:1185–1191. doi: 10.1096/fj.03-1230com. [DOI] [PubMed] [Google Scholar]
- 53.Wesche-Soldato DE, Chung CS, Lomas-Neira J, et al. In vivo delivery of caspase-8 or Fas siRNA improves the survival of septic mice. Blood. 2005;106:2295–2301. doi: 10.1182/blood-2004-10-4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bohm M, Wolff I, Scholzen TE, et al. alpha-Melanocyte-stimulating hormone protects from ultraviolet radiation-induced apoptosis and DNA damage. J Biol Chem. 2005;280:5795–5802. doi: 10.1074/jbc.M406334200. [DOI] [PubMed] [Google Scholar]
- 55.Kadekaro AL, Kavanagh R, Kanto H, et al. alpha-Melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer Res. 2005;65:4292–4299. doi: 10.1158/0008-5472.CAN-04-4535. [DOI] [PubMed] [Google Scholar]
- 56.Li C, Shi Y, Wang W, et al. alpha-MSH prevents impairment in renal function and dysregulation of AQPs and Na-K-ATPase in rats with bilateral ureteral obstruction. Am J Physiol Renal Physiol. 2006;290:F384–396. doi: 10.1152/ajprenal.00282.2004. [DOI] [PubMed] [Google Scholar]
- 57.Yuen PS, Dunn SR, Miyaji T, et al. A simplified method for HPLC determination of creatinine in mouse serum. Am J Physiol Renal Physiol. 2004;286:F1116–1119. doi: 10.1152/ajprenal.00366.2003. [DOI] [PubMed] [Google Scholar]
- 58.Kim SM, Chen L, Mizel D, et al. Low plasma renin and reduced renin secretory responses to acute stimuli in conscious COX-2-deficient mice. Am J Physiol Renal Physiol. 2007;292:F415–422. doi: 10.1152/ajprenal.00317.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.