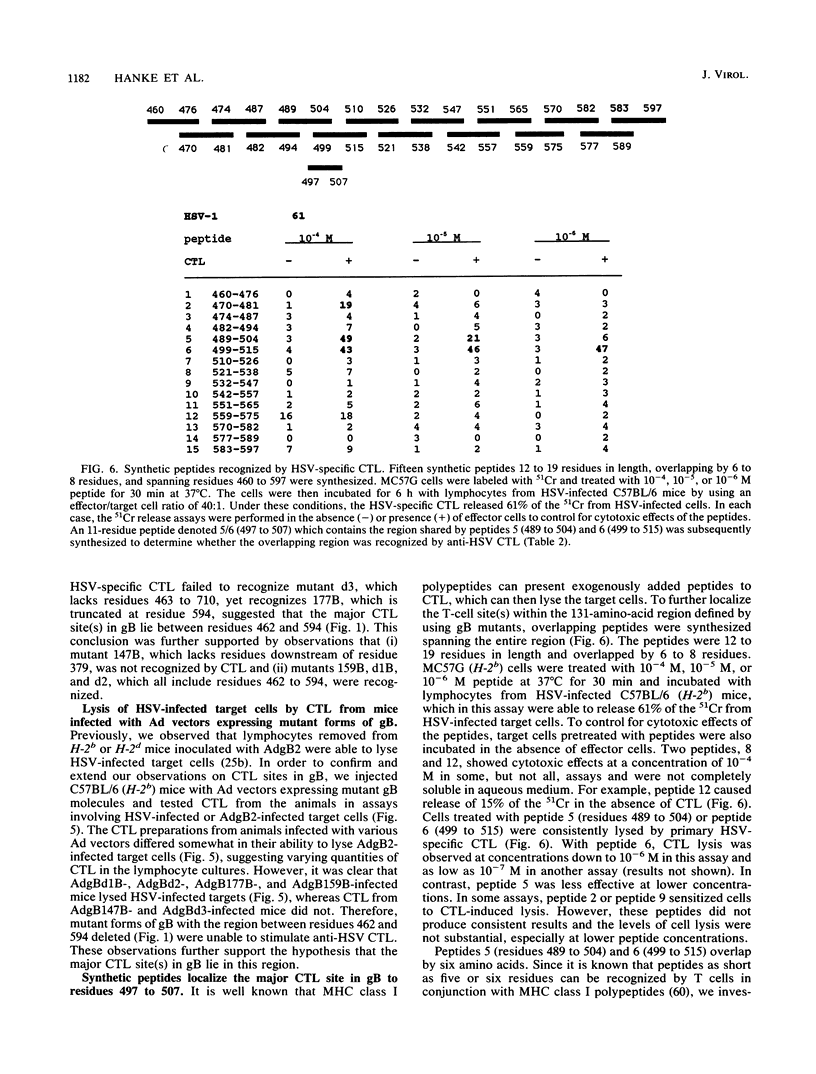
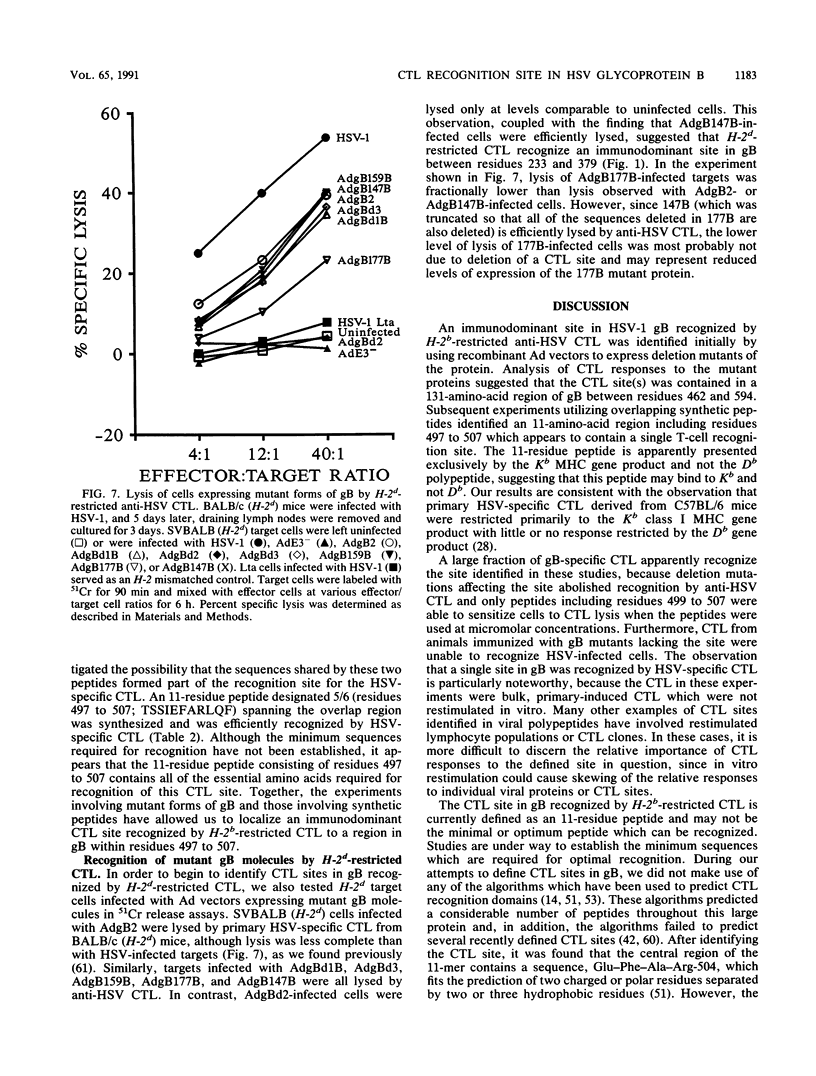
Abstract
Cytotoxic T-lymphocyte (CTL) responses to herpes simplex virus (HSV) polypeptides play an important role in recovery from infection and in preventing latency. We have previously shown that glycoprotein B (gB) is a major target recognized by HSV-specific CTLs in C57BL/6 (H-2b) and BALB/c (H-2d) mice but not in CBA/J (H-2k) mice (L. A. Witmer, K. L. Rosenthal, F. L. Graham, H. M. Friedman, A. Yee, and D. C. Johnson, J. Gen. Virol. 71:387-396, 1990). In this report, we utilize adenovirus vectors expressing gB with various deletions to localize an immunodominant site in gB, recognized by H-2b-restricted anti-HSV CTLs, to a region between residues 462 and 594. Overlapping peptides spanning this region were synthesized and used to further localize the immunodominant site to residues 489 to 515, a region highly conserved in HSV type 1 (HSV-1) and HSV-2 strains. The 11-amino-acid peptide was apparently associated exclusively with the Kb major histocompatibility complex gene product and not the Db gene product. In contrast, H-2d-restricted CTLs recognized an immunodominant site between residues 233 and 379.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babbitt B. P., Allen P. M., Matsueda G., Haber E., Unanue E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985 Sep 26-Oct 2Nature. 317(6035):359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Berzofsky J. A. Immunodominance in T lymphocyte recognition. Immunol Lett. 1988 Jun;18(2):83–92. doi: 10.1016/0165-2478(88)90046-6. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Blacklaws B. A., Nash A. A., Darby G. Specificity of the immune response of mice to herpes simplex virus glycoproteins B and D constitutively expressed on L cell lines. J Gen Virol. 1987 Apr;68(Pt 4):1103–1114. doi: 10.1099/0022-1317-68-4-1103. [DOI] [PubMed] [Google Scholar]
- Blacklaws B. A., Nash A. A. Immunological memory to herpes simplex virus type 1 glycoproteins B and D in mice. J Gen Virol. 1990 Apr;71(Pt 4):863–871. doi: 10.1099/0022-1317-71-4-863. [DOI] [PubMed] [Google Scholar]
- Bonneau R. H., Jennings S. R. Modulation of acute and latent herpes simplex virus infection in C57BL/6 mice by adoptive transfer of immune lymphocytes with cytolytic activity. J Virol. 1989 Mar;63(3):1480–1484. doi: 10.1128/jvi.63.3.1480-1484.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burrows S. R., Sculley T. B., Misko I. S., Schmidt C., Moss D. J. An Epstein-Barr virus-specific cytotoxic T cell epitope in EBV nuclear antigen 3 (EBNA 3). J Exp Med. 1990 Jan 1;171(1):345–349. doi: 10.1084/jem.171.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Miles C., Grey H. M. The relation between major histocompatibility complex (MHC) restriction and the capacity of Ia to bind immunogenic peptides. Science. 1987 Mar 13;235(4794):1353–1358. doi: 10.1126/science.2435001. [DOI] [PubMed] [Google Scholar]
- Bzik D. J., Debroy C., Fox B. A., Pederson N. E., Person S. The nucleotide sequence of the gB glycoprotein gene of HSV-2 and comparison with the corresponding gene of HSV-1. Virology. 1986 Dec;155(2):322–333. doi: 10.1016/0042-6822(86)90196-0. [DOI] [PubMed] [Google Scholar]
- Bzik D. J., Fox B. A., DeLuca N. A., Person S. Nucleotide sequence specifying the glycoprotein gene, gB, of herpes simplex virus type 1. Virology. 1984 Mar;133(2):301–314. doi: 10.1016/0042-6822(84)90397-0. [DOI] [PubMed] [Google Scholar]
- Cai W. Z., Person S., DebRoy C., Gu B. H. Functional regions and structural features of the gB glycoprotein of herpes simplex virus type 1. An analysis of linker insertion mutants. J Mol Biol. 1988 Jun 5;201(3):575–588. doi: 10.1016/0022-2836(88)90639-0. [DOI] [PubMed] [Google Scholar]
- Claesson-Welsh L., Spear P. G. Amino-terminal sequence, synthesis, and membrane insertion of glycoprotein B of herpes simplex virus type 1. J Virol. 1987 Jan;61(1):1–7. doi: 10.1128/jvi.61.1.1-7.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi C., Berzofsky J. A. T-cell antigenic sites tend to be amphipathic structures. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7048–7052. doi: 10.1073/pnas.82.20.7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Val M., Münch K., Reddehase M. J., Koszinowski U. H. Presentation of CMV immediate-early antigen to cytolytic T lymphocytes is selectively prevented by viral genes expressed in the early phase. Cell. 1989 Jul 28;58(2):305–315. doi: 10.1016/0092-8674(89)90845-3. [DOI] [PubMed] [Google Scholar]
- Del Val M., Volkmer H., Rothbard J. B., Jonjić S., Messerle M., Schickedanz J., Reddehase M. J., Koszinowski U. H. Molecular basis for cytolytic T-lymphocyte recognition of the murine cytomegalovirus immediate-early protein pp89. J Virol. 1988 Nov;62(11):3965–3972. doi: 10.1128/jvi.62.11.3965-3972.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feenstra V., Hodaie M., Johnson D. C. Deletions in herpes simplex virus glycoprotein D define nonessential and essential domains. J Virol. 1990 May;64(5):2096–2102. doi: 10.1128/jvi.64.5.2096-2102.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain R. N. Antigen processing and CD4+ T cell depletion in AIDS. Cell. 1988 Aug 12;54(4):441–444. doi: 10.1016/0092-8674(88)90062-1. [DOI] [PubMed] [Google Scholar]
- Glorioso J., Schröder C. H., Kumel G., Szczesiul M., Levine M. Immunogenicity of herpes simplex virus glycoproteins gC and gB and their role in protective immunity. J Virol. 1984 Jun;50(3):805–812. doi: 10.1128/jvi.50.3.805-812.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding L. R. Specificities of killing by T lymphocytes generated against syngeneic SV40 transformants: studies employing recombinants within the H-2 complex. J Immunol. 1979 Mar;122(3):1002–1008. [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Haj-Ahmad Y., Graham F. L. Development of a helper-independent human adenovirus vector and its use in the transfer of the herpes simplex virus thymidine kinase gene. J Virol. 1986 Jan;57(1):267–274. doi: 10.1128/jvi.57.1.267-274.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hany M., Oehen S., Schulz M., Hengartner H., Mackett M., Bishop D. H., Overton H., Zinkernagel R. M. Anti-viral protection and prevention of lymphocytic choriomeningitis or of the local footpad swelling reaction in mice by immunization with vaccinia-recombinant virus expressing LCMV-WE nucleoprotein or glycoprotein. Eur J Immunol. 1989 Mar;19(3):417–424. doi: 10.1002/eji.1830190302. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings S. R., Rice P. L., Pan S., Knowles B. B., Tevethia S. S. Recognition of herpes simplex virus antigens on the surface of mouse cells of the H-2b haplotype by virus-specific cytotoxic T lymphocytes. J Immunol. 1984 Jan;132(1):475–481. [PubMed] [Google Scholar]
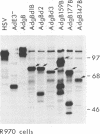
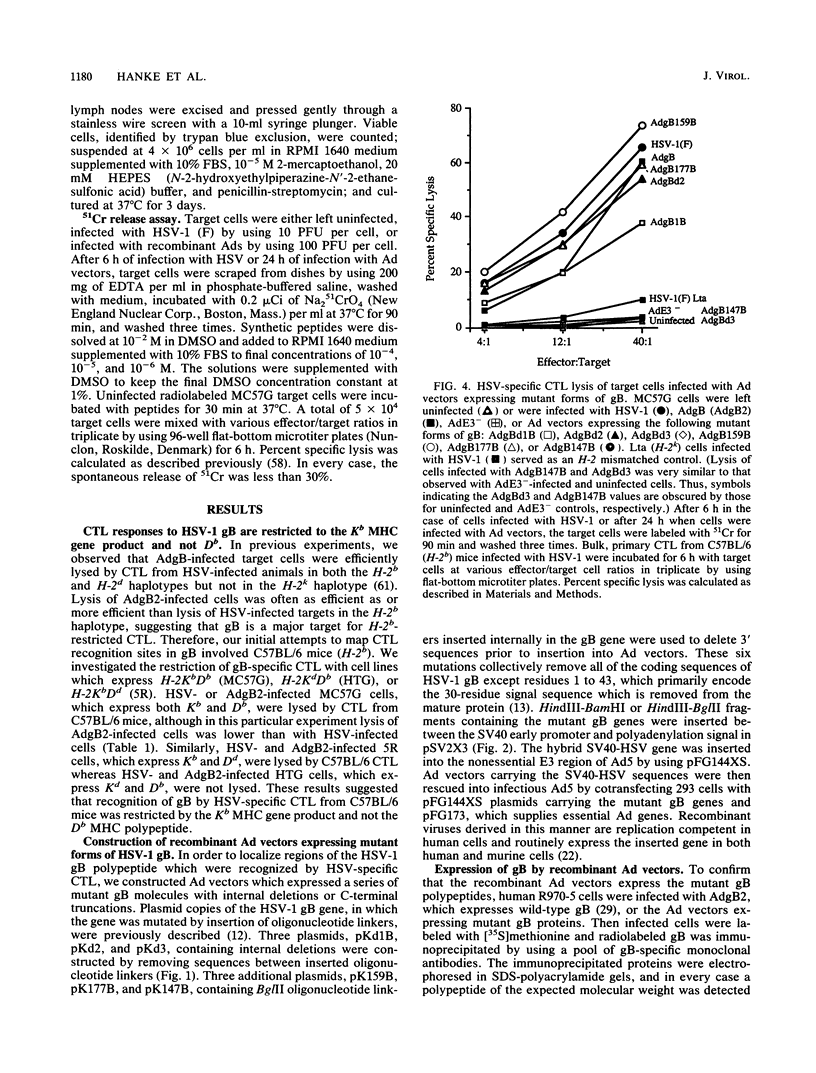
- Johnson D. C., Ghosh-Choudhury G., Smiley J. R., Fallis L., Graham F. L. Abundant expression of herpes simplex virus glycoprotein gB using an adenovirus vector. Virology. 1988 May;164(1):1–14. doi: 10.1016/0042-6822(88)90613-7. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Smiley J. R. Intracellular transport of herpes simplex virus gD occurs more rapidly in uninfected cells than in infected cells. J Virol. 1985 Jun;54(3):682–689. doi: 10.1128/jvi.54.3.682-689.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
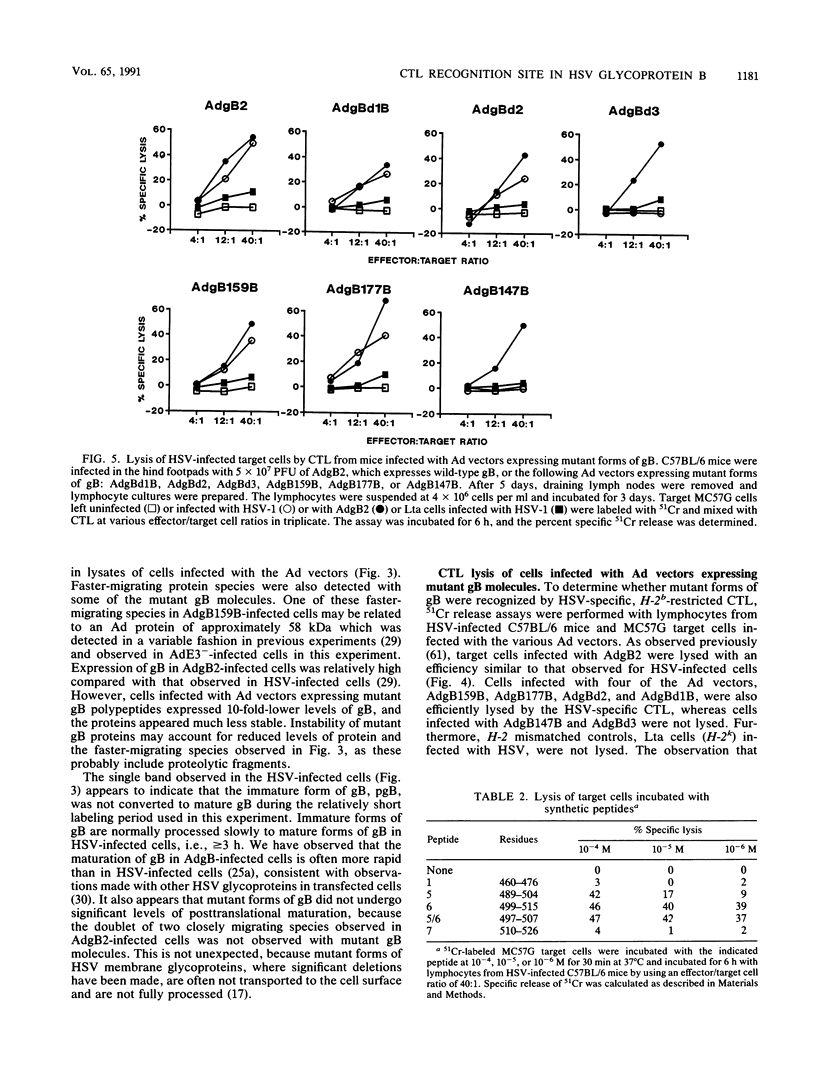
- Johnson R. M., Lancki D. W., Fitch F. W., Spear P. G. Herpes simplex virus glycoprotein D is recognized as antigen by CD4+ and CD8+ T lymphocytes from infected mice. Characterization of T cell clones. J Immunol. 1990 Jul 15;145(2):702–710. [PubMed] [Google Scholar]
- Martin S., Courtney R. J., Fowler G., Rouse B. T. Herpes simplex virus type 1-specific cytotoxic T lymphocytes recognize virus nonstructural proteins. J Virol. 1988 Jul;62(7):2265–2273. doi: 10.1128/jvi.62.7.2265-2273.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S., Moss B., Berman P. W., Laskey L. A., Rouse B. T. Mechanisms of antiviral immunity induced by a vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D: cytotoxic T cells. J Virol. 1987 Mar;61(3):726–734. doi: 10.1128/jvi.61.3.726-734.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott M. R., Graham F. L., Hanke T., Johnson D. C. Protection of mice against lethal challenge with herpes simplex virus by vaccination with an adenovirus vector expressing HSV glycoprotein B. Virology. 1989 Mar;169(1):244–247. doi: 10.1016/0042-6822(89)90064-0. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- McLaughlin-Taylor E., Willey D. E., Cantin E. M., Eberle R., Moss B., Openshaw H. A recombinant vaccinia virus expressing herpes simplex virus type 1 glycoprotein B induces cytotoxic T lymphocytes in mice. J Gen Virol. 1988 Jul;69(Pt 7):1731–1734. doi: 10.1099/0022-1317-69-7-1731. [DOI] [PubMed] [Google Scholar]
- Murray R. J., Kurilla M. G., Griffin H. M., Brooks J. M., Mackett M., Arrand J. R., Rowe M., Burrows S. R., Moss D. J., Kieff E. Human cytotoxic T-cell responses against Epstein-Barr virus nuclear antigens demonstrated by using recombinant vaccinia viruses. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2906–2910. doi: 10.1073/pnas.87.8.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash A. A., Jayasuriya A., Phelan J., Cobbold S. P., Waldmann H., Prospero T. Different roles for L3T4+ and Lyt 2+ T cell subsets in the control of an acute herpes simplex virus infection of the skin and nervous system. J Gen Virol. 1987 Mar;68(Pt 3):825–833. doi: 10.1099/0022-1317-68-3-825. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Whitton J. L., Lewicki H., Tishon A. Fine dissection of a nine amino acid glycoprotein epitope, a major determinant recognized by lymphocytic choriomeningitis virus-specific class I-restricted H-2Db cytotoxic T lymphocytes. J Exp Med. 1988 Aug 1;168(2):559–570. doi: 10.1084/jem.168.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Para M. F., Parish M. L., Noble A. G., Spear P. G. Potent neutralizing activity associated with anti-glycoprotein D specificity among monoclonal antibodies selected for binding to herpes simplex virions. J Virol. 1985 Aug;55(2):483–488. doi: 10.1128/jvi.55.2.483-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellett P. E., Kousoulas K. G., Pereira L., Roizman B. Anatomy of the herpes simplex virus 1 strain F glycoprotein B gene: primary sequence and predicted protein structure of the wild type and of monoclonal antibody-resistant mutants. J Virol. 1985 Jan;53(1):243–253. doi: 10.1128/jvi.53.1.243-253.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfizenmaier K., Jung H., Starzinski-Powitz A., Röllinghoff M., Wagner H. The role of T cells in anti-herpes simplex virus immunity. I. Induction of antigen-specific cytotoxic T lymphocytes. J Immunol. 1977 Sep;119(3):939–944. [PubMed] [Google Scholar]
- Prevec L., Campbell J. B., Christie B. S., Belbeck L., Graham F. L. A recombinant human adenovirus vaccine against rabies. J Infect Dis. 1990 Jan;161(1):27–30. doi: 10.1093/infdis/161.1.27. [DOI] [PubMed] [Google Scholar]
- Reddehase M. J., Koszinowski U. H. Significance of herpesvirus immediate early gene expression in cellular immunity to cytomegalovirus infection. Nature. 1984 Nov 22;312(5992):369–371. doi: 10.1038/312369a0. [DOI] [PubMed] [Google Scholar]
- Rhim J. S., Cho H. Y., Huebner R. J. Non-producer human cells induced by murine sarcoma virus. Int J Cancer. 1975 Jan 15;15(1):23–29. doi: 10.1002/ijc.2910150104. [DOI] [PubMed] [Google Scholar]
- Rosenthal K. L., Smiley J. R., South S., Johnson D. C. Cells expressing herpes simplex virus glycoprotein gC but not gB, gD, or gE are recognized by murine virus-specific cytotoxic T lymphocytes. J Virol. 1987 Aug;61(8):2438–2447. doi: 10.1128/jvi.61.8.2438-2447.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal K. L., Zinkernagel R. M. Cross-reactive cytotoxic T cells to serologically distinct vesicular stomatitis virus. J Immunol. 1980 May;124(5):2301–2308. [PubMed] [Google Scholar]
- Rothbard J. B., Taylor W. R. A sequence pattern common to T cell epitopes. EMBO J. 1988 Jan;7(1):93–100. doi: 10.1002/j.1460-2075.1988.tb02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz M., Aichele P., Vollenweider M., Bobe F. W., Cardinaux F., Hengartner H., Zinkernagel R. M. Major histocompatibility complex--dependent T cell epitopes of lymphocytic choriomeningitis virus nucleoprotein and their protective capacity against viral disease. Eur J Immunol. 1989 Sep;19(9):1657–1667. doi: 10.1002/eji.1830190921. [DOI] [PubMed] [Google Scholar]
- Spouge J. L., Guy H. R., Cornette J. L., Margalit H., Cease K., Berzofsky J. A., DeLisi C. Strong conformational propensities enhance T cell antigenicity. J Immunol. 1987 Jan 1;138(1):204–212. [PubMed] [Google Scholar]
- Townsend A. R., Gotch F. M., Davey J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell. 1985 Sep;42(2):457–467. doi: 10.1016/0092-8674(85)90103-5. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., McMichael A. J., Carter N. P., Huddleston J. A., Brownlee G. G. Cytotoxic T cell recognition of the influenza nucleoprotein and hemagglutinin expressed in transfected mouse L cells. Cell. 1984 Nov;39(1):13–25. doi: 10.1016/0092-8674(84)90187-9. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Walker C. M., Paetkau V., Rawls W. E., Rosenthal K. L. Abrogation of anti-Pichinde virus cytotoxic T cell memory by cyclophosphamide and restoration by coinfection or interleukin 2. J Immunol. 1985 Aug;135(2):1401–1407. [PubMed] [Google Scholar]
- Whitton J. L., Gebhard J. R., Lewicki H., Tishon A., Oldstone M. B. Molecular definition of a major cytotoxic T-lymphocyte epitope in the glycoprotein of lymphocytic choriomeningitis virus. J Virol. 1988 Mar;62(3):687–695. doi: 10.1128/jvi.62.3.687-695.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton J. L., Tishon A., Lewicki H., Gebhard J., Cook T., Salvato M., Joly E., Oldstone M. B. Molecular analyses of a five-amino-acid cytotoxic T-lymphocyte (CTL) epitope: an immunodominant region which induces nonreciprocal CTL cross-reactivity. J Virol. 1989 Oct;63(10):4303–4310. doi: 10.1128/jvi.63.10.4303-4310.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witmer L. A., Rosenthal K. L., Graham F. L., Friedman H. M., Yee A., Johnson D. C. Cytotoxic T lymphocytes specific for herpes simplex virus (HSV) studied using adenovirus vectors expressing HSV glycoproteins. J Gen Virol. 1990 Feb;71(Pt 2):387–396. doi: 10.1099/0022-1317-71-2-387. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R., Smith G. L., Moss B. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1785–1789. doi: 10.1073/pnas.82.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R. The binary logic of antigen processing and presentation to T cells. Cell. 1990 Jul 27;62(2):203–206. doi: 10.1016/0092-8674(90)90356-j. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Adler B., Holland J. J. Cell-mediated immunity to vesicular stomatitis virus infections in mice. Exp Cell Biol. 1978;46(1-2):53–70. doi: 10.1159/000162882. [DOI] [PubMed] [Google Scholar]