Abstract
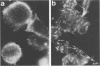
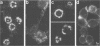
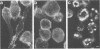
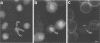
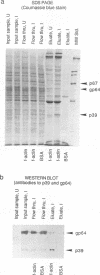
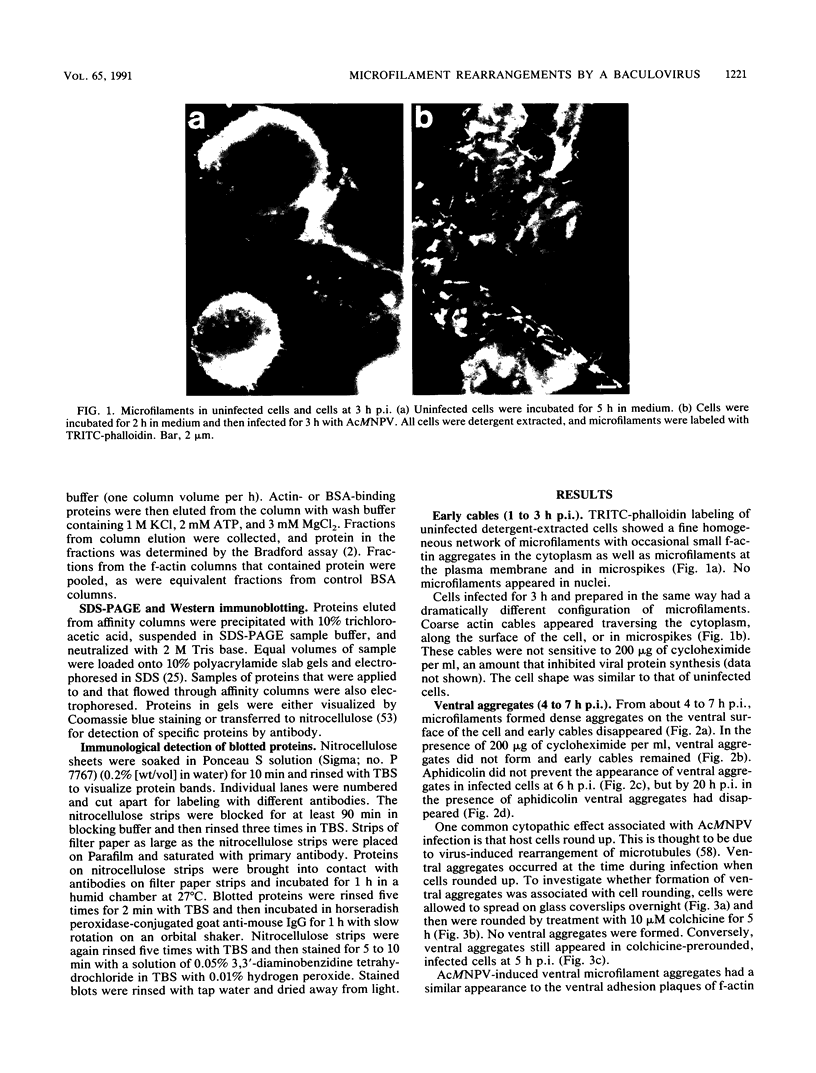
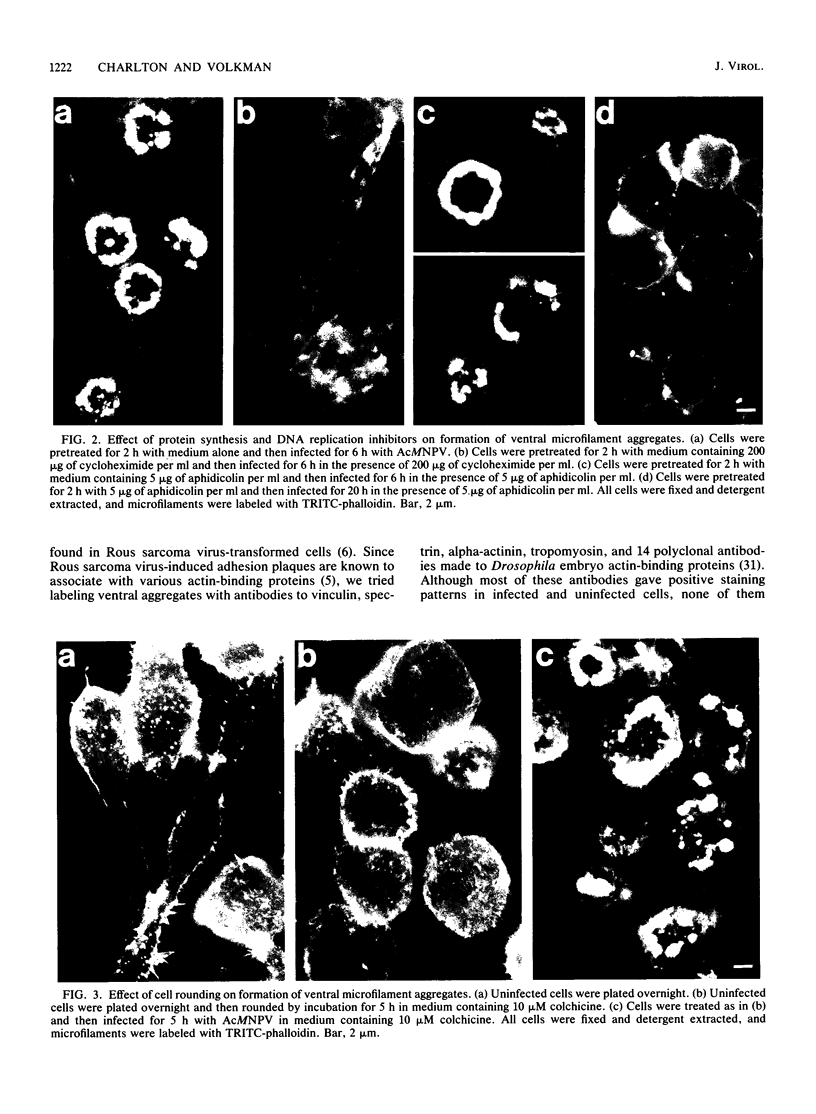
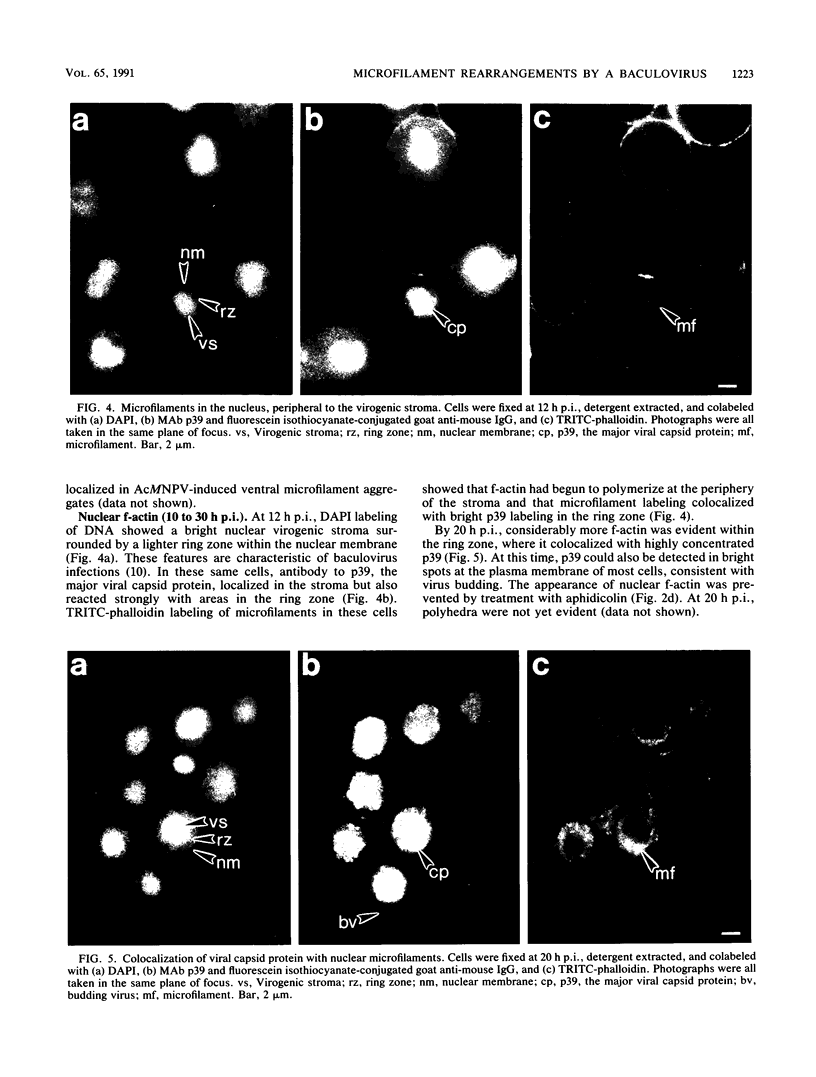
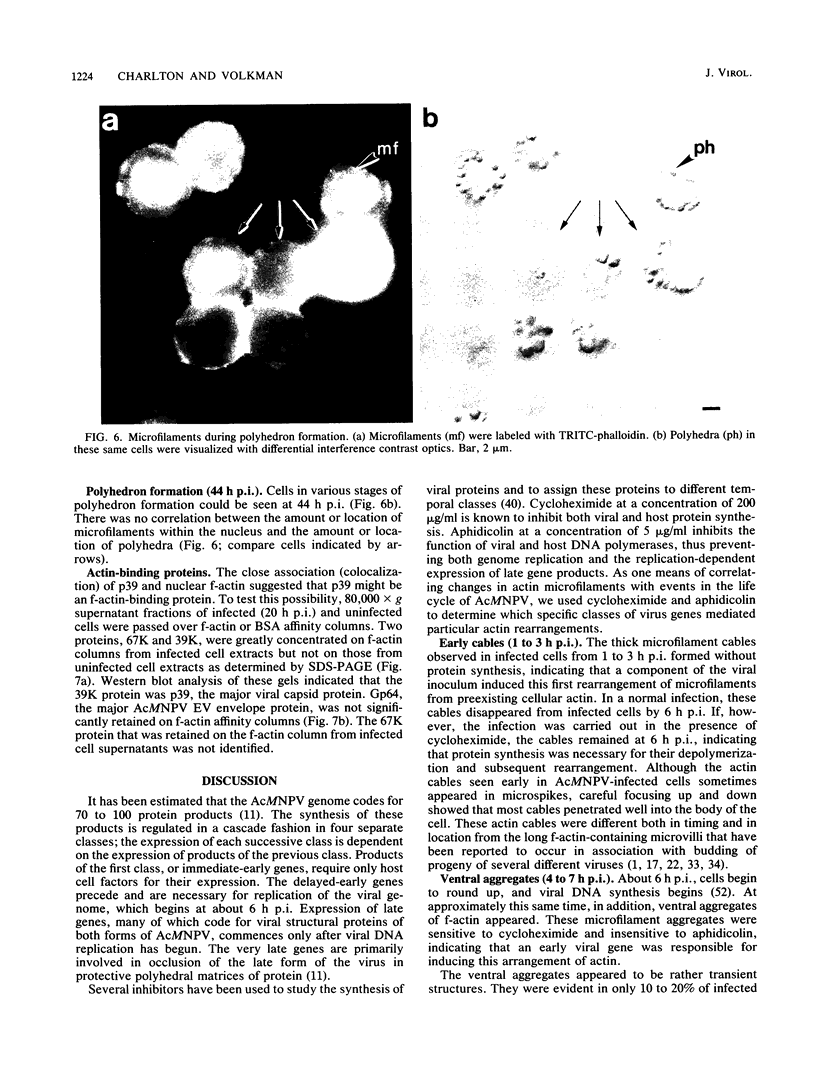
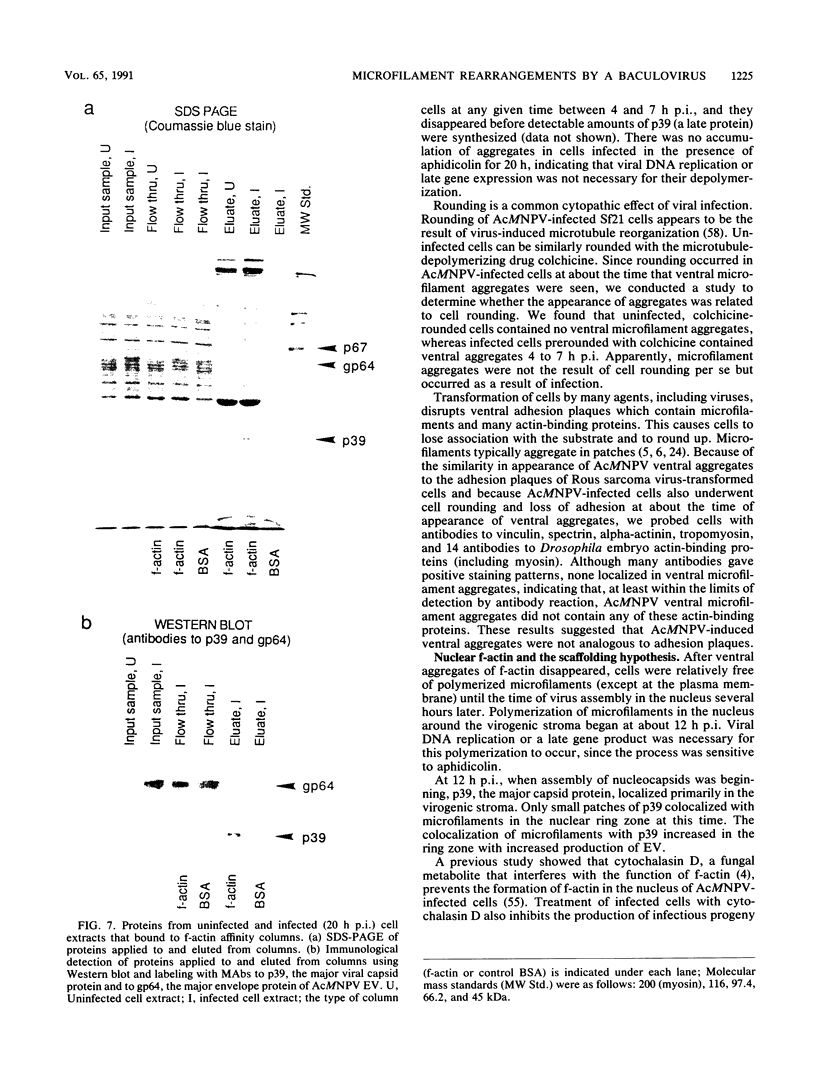
Proper assembly of nucleocapsids of the baculovirus Autographa californica nuclear polyhedrosis virus is prevented by cytochalasin D, a drug that interferes with actin microfilament function. To investigate the involvement of microfilaments in A. californica nuclear polyhedrosis virus replication, a fluorescence microscopy study was conducted that correlated changes in distribution of microfilaments with events in the life cycle of the virus. Tetramethylrhodamine isothiocyanate-labeled phalloidin was used to label microfilaments, and monoclonal antibody was used to label p39, the major viral capsid protein. Three microfilament arrangements were found in infected cells. During uptake of virus, thick cables were formed. These were insensitive to cycloheximide, indicating that this configuration was a rearrangement of preexisting cellular actin mediated by a component of the viral inoculum. At the time of cell rounding and before viral DNA replication, ventral aggregates of actin were observed. These were sensitive to cycloheximide but not to aphidicolin, indicating that an early viral gene mediated this actin rearrangement. Ventral aggregates did not result from the rounding process itself. Uninfected cells prerounded with colchicine did not form ventral aggregates. Cells prerounded with colchicine and then infected did form aggregates. At the time of exponential production of progency virus, microfilaments were found in the nucleus surrounding the virogenic stroma. In this area (where nucleocapsid assembly is known to take place) microfilaments colocalized with p39. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western immunoblot analysis identified p39 among proteins retained on an f-actin affinity column. We postulate that microfilaments in the nucleus provide a scaffold to position capsids for proper assembly and filling with DNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohn W., Rutter G., Hohenberg H., Mannweiler K., Nobis P. Involvement of actin filaments in budding of measles virus: studies on cytoskeletons of infected cells. Virology. 1986 Feb;149(1):91–106. doi: 10.1016/0042-6822(86)90090-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown S. S., Spudich J. A. Mechanism of action of cytochalasin: evidence that it binds to actin filament ends. J Cell Biol. 1981 Mar;88(3):487–491. doi: 10.1083/jcb.88.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Carley W. W., Barak L. S., Webb W. W. F-actin aggregates in transformed cells. J Cell Biol. 1981 Sep;90(3):797–802. doi: 10.1083/jcb.90.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciampor F. The role of cytoskeleton and nuclear matrix in virus replication. Acta Virol. 1988 Mar;32(2):168–189. [PubMed] [Google Scholar]
- Egly J. M., Miyamoto N. G., Moncollin V., Chambon P. Is actin a transcription initiation factor for RNA polymerase B? EMBO J. 1984 Oct;3(10):2363–2371. doi: 10.1002/j.1460-2075.1984.tb02141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner P., Carstens E. B. An overview of the structure and replication of baculoviruses. Curr Top Microbiol Immunol. 1986;131:1–19. doi: 10.1007/978-3-642-71589-1_1. [DOI] [PubMed] [Google Scholar]
- Friesen P. D., Miller L. K. The regulation of baculovirus gene expression. Curr Top Microbiol Immunol. 1986;131:31–49. doi: 10.1007/978-3-642-71589-1_3. [DOI] [PubMed] [Google Scholar]
- Fulton A. B. Assembly associated with the cytomatrix. J Cell Biol. 1984 Jul;99(1 Pt 2):209s–211s. doi: 10.1083/jcb.99.1.209s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffre R. M., Tovell D. R., Kay C. M., Tyrrell D. L. Evidence for an interaction between the membrane protein of a paramyxovirus and actin. J Virol. 1982 Jun;42(3):963–968. doi: 10.1128/jvi.42.3.963-968.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeg U., Dienes H. P., Müller S., Falke D. Involvement of actin-containing microfilaments in HSV-induced cytopathology and the influence of inhibitors of glycosylation. Arch Virol. 1986;91(3-4):257–270. doi: 10.1007/BF01314285. [DOI] [PubMed] [Google Scholar]
- Hiller G., Weber K., Schneider L., Parajsz C., Jungwirth C. Interaction of assembled progeny pox viruses with the cellular cytoskeleton. Virology. 1979 Oct 15;98(1):142–153. doi: 10.1016/0042-6822(79)90533-6. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., McCready S. J., Cook P. R. Replication and transcription depend on attachment of DNA to the nuclear cage. J Cell Sci Suppl. 1984;1:59–79. doi: 10.1242/jcs.1984.supplement_1.5. [DOI] [PubMed] [Google Scholar]
- Jackson P., Bellett A. J. Relationship between organization of the actin cytoskeleton and the cell cycle in normal and adenovirus-infected rat cells. J Virol. 1989 Jan;63(1):311–318. doi: 10.1128/jvi.63.1.311-318.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachar B., Reese T. S. The mechanism of cytoplasmic streaming in characean algal cells: sliding of endoplasmic reticulum along actin filaments. J Cell Biol. 1988 May;106(5):1545–1552. doi: 10.1083/jcb.106.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamatsu H., Lin W., Edens J., Revel J. P. Visualization of antigens attached to cytoskeletal framework in animal cells: colocalization of simian virus 40 Vp1 polypeptide and actin in TC7 cells. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4339–4343. doi: 10.1073/pnas.80.14.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumoto T., Hirano A., Kurimura T., Takagi A. In situ electron microscopical observation of cells infected with herpes simplex virus. J Gen Virol. 1981 Feb;52(Pt 2):267–278. doi: 10.1099/0022-1317-52-2-267. [DOI] [PubMed] [Google Scholar]
- Keddie B. A., Aponte G. W., Volkman L. E. The pathway of infection of Autographa californica nuclear polyhedrosis virus in an insect host. Science. 1989 Mar 31;243(4899):1728–1730. doi: 10.1126/science.2648574. [DOI] [PubMed] [Google Scholar]
- Kellie S. Cellular transformation, tyrosine kinase oncogenes, and the cellular adhesion plaque. Bioessays. 1988 Jan;8(1):25–30. doi: 10.1002/bies.950080107. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luftig R. B. Does the cytoskeleton play a significant role in animal virus replication? J Theor Biol. 1982 Nov 7;99(1):173–191. doi: 10.1016/0022-5193(82)90397-6. [DOI] [PubMed] [Google Scholar]
- Meyer R. K., Burger M. M., Tschannen R., Schäfer R. Actin filament bundles in vaccinia virus infected fibroblasts. Arch Virol. 1981;67(1):11–18. doi: 10.1007/BF01314597. [DOI] [PubMed] [Google Scholar]
- Miller K. G., Alberts B. M. F-actin affinity chromatography: technique for isolating previously unidentified actin-binding proteins. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4808–4812. doi: 10.1073/pnas.86.13.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. G., Field C. M., Alberts B. M. Actin-binding proteins from Drosophila embryos: a complex network of interacting proteins detected by F-actin affinity chromatography. J Cell Biol. 1989 Dec;109(6 Pt 1):2963–2975. doi: 10.1083/jcb.109.6.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltenburger H. G., Krieg A. Bioinsecticides: II. Baculoviridae. Adv Biotechnol Processes. 1984;3:291–313. [PubMed] [Google Scholar]
- Mortara R. A., Koch G. L. An association between actin and nucleocapsid polypeptides in isolated murine retroviral particles. J Submicrosc Cytol Pathol. 1989 Apr;21(2):295–306. [PubMed] [Google Scholar]
- Murti K. G., Chen M., Goorha R. Interaction of frog virus 3 with the cytomatrix. III. Role of microfilaments in virus release. Virology. 1985 Apr 30;142(2):317–325. doi: 10.1016/0042-6822(85)90340-x. [DOI] [PubMed] [Google Scholar]
- Nakayasu H., Ueda K. Association of rapidly-labelled RNAs with actin in nuclear matrix from mouse L5178Y cells. Exp Cell Res. 1985 Oct;160(2):319–330. doi: 10.1016/0014-4827(85)90179-x. [DOI] [PubMed] [Google Scholar]
- Nakayasu H., Ueda K. Small nuclear RNA-protein complex anchors on the actin filaments in bovine lymphocyte nuclear matrix. Cell Struct Funct. 1984 Dec;9(4):317–325. doi: 10.1247/csf.9.317. [DOI] [PubMed] [Google Scholar]
- Nelson W. G., Pienta K. J., Barrack E. R., Coffey D. S. The role of the nuclear matrix in the organization and function of DNA. Annu Rev Biophys Biophys Chem. 1986;15:457–475. doi: 10.1146/annurev.bb.15.060186.002325. [DOI] [PubMed] [Google Scholar]
- Razin S. V. DNA interactions with the nuclear matrix and spatial organization of replication and transcription. Bioessays. 1987 Jan;6(1):19–23. doi: 10.1002/bies.950060106. [DOI] [PubMed] [Google Scholar]
- Rice W. C., Miller L. K. Baculovirus transcription in the presence of inhibitors and in nonpermissive Drosophila cells. Virus Res. 1986 Nov;6(2):155–172. doi: 10.1016/0168-1702(86)90047-x. [DOI] [PubMed] [Google Scholar]
- Scheer U., Hinssen H., Franke W. W., Jockusch B. M. Microinjection of actin-binding proteins and actin antibodies demonstrates involvement of nuclear actin in transcription of lampbrush chromosomes. Cell. 1984 Nov;39(1):111–122. doi: 10.1016/0092-8674(84)90196-x. [DOI] [PubMed] [Google Scholar]
- Schliwa M., van Blerkom J. Structural interaction of cytoskeletal components. J Cell Biol. 1981 Jul;90(1):222–235. doi: 10.1083/jcb.90.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrom M., Bablanian R. Altered cellular morphology resulting from cytocidal virus infection. Arch Virol. 1981;70(3):173–187. doi: 10.1007/BF01315124. [DOI] [PubMed] [Google Scholar]
- Simard R., Bibor-Hardy V., Dagenais A., Bernard M., Pinard M. F. Role of the nuclear matrix during viral replication. Methods Achiev Exp Pathol. 1986;12:172–199. [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. Analysis of baculovirus genomes with restriction endonucleases. Virology. 1978 Sep;89(2):517–527. doi: 10.1016/0042-6822(78)90193-9. [DOI] [PubMed] [Google Scholar]
- Staufenbiel M., Epple P., Deppert W. Progressive reorganization of the host cell cytoskeleton during adenovirus infection. J Virol. 1986 Dec;60(3):1186–1191. doi: 10.1128/jvi.60.3.1186-1191.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockem W., Hoffmann H. U., Gruber B. Dynamics of the cytoskeleton in Amoeba proteus. I. Redistribution of microinjected fluorescein-labeled actin during locomotion, immobilization and phagocytosis. Cell Tissue Res. 1983;232(1):79–96. doi: 10.1007/BF00222375. [DOI] [PubMed] [Google Scholar]
- Stokes G. V. High-voltage electron microscope study of the release of vaccinia virus from whole cells. J Virol. 1976 May;18(2):636–643. doi: 10.1128/jvi.18.2.636-643.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P. Contribution of actin to the structure of the cytoplasmic matrix. J Cell Biol. 1984 Jul;99(1 Pt 2):15s–21s. doi: 10.1083/jcb.99.1.15s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaka K., Akagi M., Miyoshi K., Mio M. Role of microfilaments in the exocytosis of rat peritoneal mast cells. Int Arch Allergy Appl Immunol. 1988;87(2):213–221. doi: 10.1159/000234675. [DOI] [PubMed] [Google Scholar]
- Tjia S. T., Carstens E. B., Doerfler W. Infection of Spodoptera frugiperda cells with Autographa californica nuclear polyhedrosis virus II. The viral DNA and the kinetics of its replication. Virology. 1979 Dec;99(2):399–409. doi: 10.1016/0042-6822(79)90018-7. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkov N. I., Ivanova M. I., Uscheva A. A., Krachmarov C. P. Association of actin with DNA and nuclear matrix from Guerin ascites tumour cells. Mol Cell Biochem. 1989 May 4;87(1):47–56. doi: 10.1007/BF00421082. [DOI] [PubMed] [Google Scholar]
- Volkman L. E. Autographa californica MNPV nucleocapsid assembly: inhibition by cytochalasin D. Virology. 1988 Apr;163(2):547–553. doi: 10.1016/0042-6822(88)90295-4. [DOI] [PubMed] [Google Scholar]
- Volkman L. E., Goldsmith P. A., Hess R. T. Evidence for microfilament involvement in budded Autographa californica nuclear polyhedrosis virus production. Virology. 1987 Jan;156(1):32–39. doi: 10.1016/0042-6822(87)90433-8. [DOI] [PubMed] [Google Scholar]
- Volkman L. E., Zaal K. J. Autographa californica M nuclear polyhedrosis virus: microtubules and replication. Virology. 1990 Mar;175(1):292–302. doi: 10.1016/0042-6822(90)90211-9. [DOI] [PubMed] [Google Scholar]
- Wang E., Goldberg A. R. Changes in microfilament organization and surface topogrophy upon transformation of chick embryo fibroblasts with Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4065–4069. doi: 10.1073/pnas.73.11.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitt M. A., Manning J. S. A phosphorylated 34-kDa protein and a subpopulation of polyhedrin are thiol linked to the carbohydrate layer surrounding a baculovirus occlusion body. Virology. 1988 Mar;163(1):33–42. doi: 10.1016/0042-6822(88)90231-0. [DOI] [PubMed] [Google Scholar]
- Wilson M. E., Price K. H. Association of Autographa californica nuclear polyhedrosis virus (AcMNPV) with the nuclear matrix. Virology. 1988 Nov;167(1):233–241. doi: 10.1016/0042-6822(88)90073-6. [DOI] [PubMed] [Google Scholar]