Abstract
We recently showed that interleukin-1 (IL-1) is secreted by the placenta of a species of squamate reptile, the three-toed skink, Chalcides chalcides. In this study, we used immunohistochemical techniques to investigate the expression of IL-1 (in the two isoforms, IL-1α and IL-1β) and its specific membrane receptor IL-1 RtI in uterine oviduct during the peri-implantation period. We found that both IL-1 and its receptor were expressed in uterine tissues before and after ovulation (in the pre-ovulatory stage, even before the yolk had formed in the ovary). However, while IL-1α was mostly localized in the uterine mesenchyme tissue, IL-1β and IL-1RtI were present in the uterine epithelium. Our data provide a further comparison between the reproduction of mammals and squamate reptiles.
Background
Placental viviparity is present in mammals (except monotremes) and in numerous species of squamate reptiles and cartilaginous fishes. It involves the apposition of extra-embryonic membranes to the uterus and has different characteristics according to the membranes involved and the intimacy of the fetal and maternal tissues [1].
Despite the diversity of placentation mechanisms, there are functional and morphological similarities among the different types of placenta and the various classes of vertebrates. We recently demonstrated that the Ha58 gene, believed to be essential for placental development in mice, is present in the chorioallantoic placenta of a squamate reptile of the family Scincidae, the three-toed skink Chalcides chalcides [2]. We also showed that this type of placenta has many aspects in common with the mammalian placenta, like glycosylation of the feto-maternal interface [3] and endocrine secretion [4]. Moreover, immunological activity mediated by the secretion of cytokines has also been demonstrated in the chorioallantoic placenta of squamate reptiles and in the yolk sac placenta of elasmobranch fishes [5,6], suggesting that common mechanisms among different species must have developed during the evolution of placental viviparity.
Since implantation of the blastocyst in the receptive endometrium is a key event in formation of the placenta and the establishment of pregnancy [7], we investigated if cytokines that play a role in mammalian implantation have a similar function in reptiles. We focused on Interleukin-1 (IL-1) which is expressed by the murine and human endometrium during the peri-implantation period [8] and acts directly on the murine endometrium [9]. Experimental results have shown that implantation in mice is prevented by blockage of the IL-1 receptor type I (IL-1R tI) [10]. Therefore, we analyzed the expression of IL-1 and IL-1R tI in the uterus and oviduct of Chalcides chalcides at the peri-ovulation stage.
Materials and methods
Animals
In Chalcides chalcides, pregnancy lasts about 3 months, from the beginning of May to the beginning of August [11,12]. Females were captured in a shrubby area in southern Italy (Castel San Vincenzo, Isernia province, 700 m above sea level) from mid-April to mid May, at the following peri-ovulation stages: pre-vitellogenic and vitellogenic pre-ovulatory, and immediately post-ovulatory. Four females were examined for each reproductive stage which was determined by the observation of the ovarian morphology under light microscope. The experiments and animal captures were performed with the approval of institutional committees: Italian Ministry of Universities-MURST (auth. no. SCN/2D/2000/9213).
Collection and processing of tissues
Soon after their transport to the laboratory, the animals were killed by deep anaesthesia with diethyl ether. Uterine tissues at the pre-vitellogenic and vitellogenic pre-ovulatory stages, as well as incubatory chambers (uterine portion distended at the site of each ovulated egg) at the post-ovulatory stage, were excised and washed in phosphate buffered saline (PBS). Tissues for immunohistochemistry were fixed in 10% buffered neutral formalin and embedded in paraffin. Sections (5 μm) were stained with haematoxylin-eosin and processed for immunohistochemistry
Immunohistochemistry
Immunohistochemical staining of formalin-fixed 5 μm sections was performed using a mouse antiserum recognizing human IL-1 and StreptABComplex/AP (DAKO-Milan, Italy).
After deparaffination in Bioclear (BioOptica – Milan, Italy) and rehydration in serial dilutions of ethanol, the histological sections were washed in Tris buffer saline (TBS) pH 7.6 and pre-incubated with normal swine serum to prevent non-specific binding. The slides were incubated first with anti-human IL-1α (R&D Systems, Abingdon, UK), IL-1β [5)] and IL-1R tI (R&D Systems) monoclonal antibodies, second with rabbit anti-mouse immunoglobulins (DAKO) diluted 1:500 in TBS, and finally with Streptavidin complex (DAKO) diluted 1:300. Each incubation was performed for 30 min at room temperature and followed by three washes in TBS. The alkaline phosphatase reaction was revealed using naphthol and new fuchsin as substrate. Endogenous alkaline phosphatase was blocked by adding 1 mM levamisole to the substrate solution. Sections were then washed for 5 min in running tap water and mounted with aqueous mounting medium. Negative controls were performed for each tissue by substituting the primary antibody with the pre-immune serum or TBS. The specificity of the staining reaction for IL-1α and IL-1β was confirmed by inhibiting the mAb through incubation (overnight at 4°C) with the specific antigen (at a molar ratio of 1:1) before using it for tissue staining.
Results
The uterine wall of Chalcides chalcides consists of several distinct layers: an outer thin serous membrane, a middle myometrium formed by an outer longitudinal and an inner circular smooth muscle layer, and an inner endometrial layer formed by luminal epithelium with cuboidal or low columnar cells and a lamina propria of connective tissue with blood vessels and alveolar glands. During the pre-vitellogenic and vitellogenic phases (Fig. 1), the endometrial layer is organized in folds separated by deep furrows. Immediately after ovulation (Fig. 2), the uterus distends around each developing egg to form an incubatory chamber. The folds decrease in length and in correspondence to the equatorial region of the egg, the folds become larger, adhering to the shell membrane.
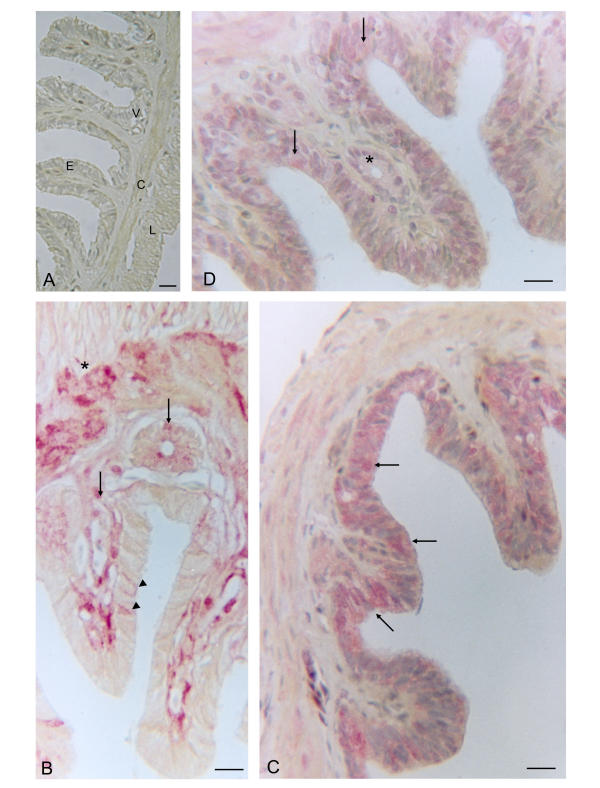
Figure 1.
Uterus of Chalcides chalcides at the pre-ovulatory vitellogenic stage. A (negative control: without primary antibody). The uterine mucosa is thrown up into many long folds. Basal to the luminal epithelium (E) of low columnar cells, a lamina propria with numerous blood vessels (V) and the myometrium with outer longitudinal (L) and inner circular (C) layers are evident. B A strong immunoreactivity for IL-1α in the connective tissue and glandular cells of the lamina propria (arrows), and a weak reaction in the myometrium (*), where only a few epithelial cells are positive (arrowheads). C. Widespread immunoreactivity for IL-1β is seen in the luminal epithelium and in the myometrium. D. Immunoreactivity for IL-1R tI in the luminal epithelium (arrows), myometrium and cytoplasm of glandular cells (*). Bars = 25 μm.
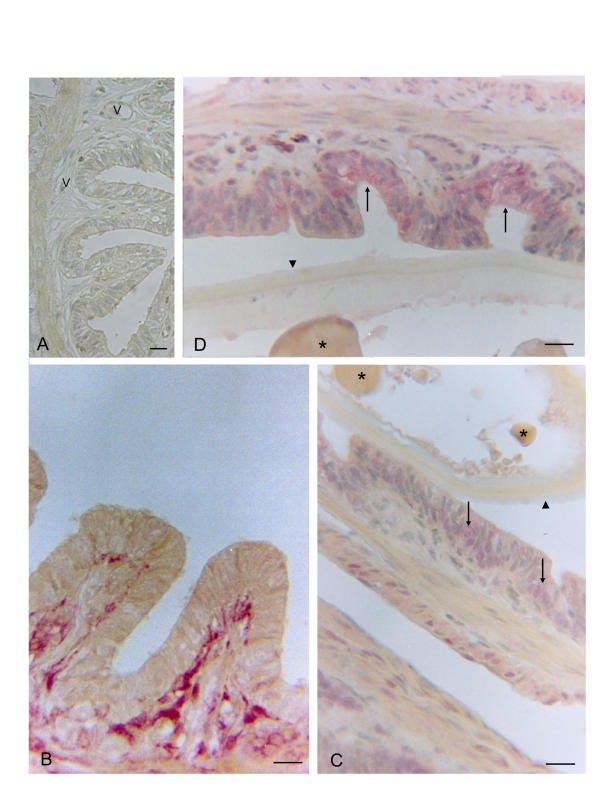
Figure 2.
Uterus of Chalcides chalcides at the post-ovulatory stage. A (negative control: without primary antibody). The endometrial folds are reduced around the developing egg and the myometrium appears distended and thinner; numerous vessels (V) are present in the lamina propria. B. Strong immunoreactivity for IL-1α can be seen in the connective tissue of the lamina propria. C. Intense immunoreactivity for IL-1β is seen in the luminal epithelium (arrows) whereas the egg envelope (arrowhead) and the yolk droplets (*) are negative. D. A strong immunoreactivity in IL-R tI: can be seen in the luminal epithelium (arrows) and a weak reaction in the myometrium. The egg envelope (arrowhead) and yolk droplets (*) are negative. Bars = 25 μm.
Immunoreactivity for IL-1α, IL-1β and IL-1R tI is present in all sections of uterine wall at each peri-ovulation stage. Specificity of the staining was revealed by the absence of immunoreactivity in the negative controls at the pre- (Fig. 1A) and post- (Fig. 2A) ovulatory stage.
In the pre-ovulatory stage there is strong immunoreactivity for IL-1α in the myometrium, the connective tissue and glandular cells of the lamina propria while only a few cells of the luminal epithelium are stained (Fig. 1B). In contrast, there is widespread positivity for IL-1β in the uterine epithelial cells, whereas only a few cells in the lamina propria are positive (Fig. 1C). Immunoreactivity for IL-1R tI is present in the luminal epithelium, myometrium and in the lamina propria, mainly in the glandular cells (Fig. 1D). No differences in immunoreactivity were observed during the pre-vitellogenic and vitellogenic stages of the pre-ovulatory period.
In the post-ovulatory stage, the immunoreactivity for both IL-1 isoforms and ILR tI shows a similar distribution to that in the pre-ovulatory stage. In fact, while IL-1α was mostly distributed in the connective tissue of the lamina propria (Fig. 2B), immunoreactivity for IL-1β and ILR tI was mainly localized in the luminal epithelium (Fig. 2C,2D). In all cases, the egg envelope and the yolk droplets were negative.
Discussion
Acceptance of the semi-allogenic fetus by the maternal tissues has been a big dilemma for scientists throughout the last two decades. Great evidence indicates that the secretion of immunoregulatory peptides, namely cytokines, at the materno-fetal interface plays an important role in preventing the attack and destruction of the fetal tissues by the maternal immune system [13]. Many cytokines and their specific receptors have been found in the mammalian placenta independently of the animal species or its type of placenta [14]. Cytokines are also present in the chorioallantoic placenta of the three-toed skink Chalcides chalcides and in the yolk sac placenta of Mustelus canis [5,6,15].
The present study demonstrates for the first time that the α and β isoforms of IL-1 and its functional membrane receptor (IL-1R tI) are expressed in uterine tissues at the peri-ovulation stage in the placental squamate reptile, Chalcides chalcides. Both, in the pre- and in the post-ovulatory stages, IL-1α was found mainly in the uterine lamina propria and myometrium, whereas IL-1β and IL-1R tI were mainly distributed in the luminal epithelium. Moreover, the two IL-1 isoforms and the receptor were expressed in the pre-ovulatory stage, even before the yolk had formed in the ovary.
Our findings suggest a role for IL-1 system throughout the phase preceding implantation and pregnancy.
In Chalcides chalcides, implantation occurs in the incubatory chambers, expanded areas of the oviduct that contain the egg and subsequently the embryo. In these areas, the extraembryonic membranes take contact with the uterine epithelium forming placental structures that, although closely interdigitated with the uterine epithelium, never penetrate it [16]. Moreover, as in elasmobranch fishes, the egg shell persists in the placenta of squamate reptiles for most of gestation [17,18]. While it is clear that fetal antigens and maternal uterine tissues are not in intimate contact in the placenta of reptiles, the presence of cytokines, IL-1 and TGF β, in the chorioallantoic placenta of Chalcides chalcides suggests that maternal immune response can occur during gestation [15].
The present findings on the expression of IL-1 system in the uterine wall since the pre-ovulatory stage, suggest that the IL-1-mediated response occurs in anticipation of the presence of fetal antigens into the maternal tissues.
The contribution of cytokines to uterine receptivity has been reported in humans [19] and several cytokines and their specific membrane receptors have a potential biological action during the secretory phase, at the time of blastocyst implantation [20]. In particular, IL-1 and its functional receptor (IL-1R tI) are expressed by human endometrial tissue during the late secretory phase whereas IL-1 R tII, a decoy non-signalling receptor, is down-regulated in the mid-secretory phase, especially during the implantation window [21].
IL-1 cytokine and IL-1 signalling system are evolutionarily conserved. In fact high homology in the sequence of IL-1β gene has been shown in different animal species including cartilaginous fishes suggesting that IL-1 is part of the immune response since the most primitive classes of vertebrates [22]. Moreover, IL-1 receptors show significant sequence similarities in their cytoplasmic domain to Toll-like receptors that mediate dorsoventral polarity in Drosophila and the immune response to microbial products in insects and mammals [23,24].
In lizards, secretion of IL-1 has been reported in splenic phagocytes exposed to temperature changes or sex steroids [25,26]. A reduced daily activity cycle has also been reported in the lizard Sceloporus occidentalis after injection of IL-1β [27].
Our study, while confirming IL-1 as a mediator of the materno-fetal relationships in different species of vertebrates [5,6], indicates IL-1 system as a regulator of uterine receptivity in viviparous placental squamate reptiles. This finding confirms that common immunological mechanisms have evolved in viviparous animals to allow blastocyst implantation and embryonic development in the maternal tissues.
Acknowledgments
Acknowledgments
This work was supported by research grants from the University of Siena.
Contributor Information
Roberta Romagnoli, Email: romagnolir@unisi.it.
Chiara Cateni, Email: chcate@unisi.it.
Fabio M Guarino, Email: fabio.guarino@unina.it.
Elisa Bigliardi, Email: bigliardi@unisi.it.
Luana Ricci Paulesu, Email: paulesu@unisi.it.
References
- Amoroso EC. Placentation. In: Parkes AS, editor. Marshall's Physiology of Reproduction. London: Longmans Green and Co; 1952. pp. 127–311. [Google Scholar]
- Paulesu L, Cateni C, Romagnoli R, Chellini F, Angelini F, Guarino FM, Rider V, Imakawa K, Bigliardi E. Evidence of H beta 58, a gene involved in mammalian placental development, in the three-toed skink, Chalcides chalcides (Squamata: Scincidae), a viviparous placentotrophic reptile. Placenta. 2001;22:735–41. doi: 10.1053/plac.2001.0714. [DOI] [PubMed] [Google Scholar]
- Jones CJP, Cateni C, Paulesu LR. Glycosylation of the materno-foetal interface in the pregnant viviparous placentotrophic lizard Chalcides chalcides : a lectin histochemical study. Placenta. 2002;24:489–500. doi: 10.1053/plac.2002.0950. [DOI] [PubMed] [Google Scholar]
- Guarino FM, Paulesu L, Cardone A, Bellini L, Ghiara G, Angelini F. Endocrine activity of the corpus luteum and placenta during pregnancy in Chalcides chalcides (Reptilia, Squamata) Gen Comp Endocrinol. 1998;111:261–70. doi: 10.1006/gcen.1998.7098. [DOI] [PubMed] [Google Scholar]
- Paulesu L, Romagnoli R, Marchetti M, Cintorino M, Ghiara P, Guarino FM, Ghiara G. Cytokines in the viviparous reproduction of squamate reptiles: interleukin-1 alpha (IL-1 alpha) and IL-1 beta in placental structures of a skink. Placenta. 1995;16:193–205. doi: 10.1016/0143-4004(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Cateni C, Paulesu L, Bigliardi E, Hamlett WC. The interleukin 1 (IL-1. system in the uteroplacental complex of a cartilaginous fish, the smoothhound shark, Mustelus canis. Reprod Biol Endocrinol. 2003;1:25. doi: 10.1186/1477-7827-1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266:1508–18. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- De M, Sanford TR, Wood GW. Expression of interleukin 1, interleukin 6 and tumour necrosis factor alpha in mouse uterus during the peri-implantation period of pregnancy. J Reprod Fertil. 1993;97:83–9. doi: 10.1530/jrf.0.0970083. [DOI] [PubMed] [Google Scholar]
- Simon C, Valbuena D, Krussel J, Bernal A, Murphy CR, Shaw T, Pellicer A, Polan ML. Interleukin-1 receptor antagonist prevents embryonic implantation by a direct effect on the endometrial epithelium. Fertil Steril. 1998;70:896–906. doi: 10.1016/S0015-0282(98)00275-1. [DOI] [PubMed] [Google Scholar]
- Simon C, Frances A, Piquette GN, Danasouri IE, Zurawski G, Dang W, Polan ML. Embryonic implantation in mice is blocked by interleukin-1 receptor antagonist. Endocrinology. 1994;134:521–8. doi: 10.1210/en.134.2.521. [DOI] [PubMed] [Google Scholar]
- Giacomini E. Materiaux pour l'etude developpement de Seps chalcides. Archivio Italiano di Biologia. 1891;16:332–59. [Google Scholar]
- Angelini F, Ghiara G. Viviparity in squamates. In: Ghiara G, editor. Symposium on the evolution of terrestrial vertebrates, Selected Symposia and Monographs U.Z.I. Vol. 4. Modena: Mucchi Editore; 1991. pp. 305–34. [Google Scholar]
- Saito S. Cytokine network at the feto-maternal interface. J Reprod Immunol. 2000;47:87–103. doi: 10.1016/S0165-0378(00)00060-7. [DOI] [PubMed] [Google Scholar]
- Paulesu L, Cateni C. Immunoregulatory peptides in placental viviparity. In: Pandalai SG, editor. Recent Res Devel Peptides. Vol. 1. 2002. pp. 107–16. [Google Scholar]
- Paulesu L. Cytokines in mammalian reproduction and speculation about their possible involvement in nonmammalian viviparity. Microsc Res Tech. 1997;38:188–94. doi: 10.1002/(SICI)1097-0029(19970701/15)38:1/2<188::AID-JEMT19>3.3.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Blackburn DG, Callard IP. Morphogenesis of placental membranes in the viviparous, placentotrophic lizard Chalcides chalcides (Squamata: Scincidae) J Morph. 1997;232:35–55. doi: 10.1002/(SICI)1097-4687(199704)232:1<35::AID-JMOR2>3.3.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Blackburn DG. Histology of the late-stage placentae in the matrotrophic skink Chalcides chalcides (Lacertilia; Scincidae) J Morph. 1993;216:179–95. doi: 10.1002/jmor.1052160206. [DOI] [PubMed] [Google Scholar]
- Hamlett WC, Wourms JP, Hudson JS. Ultrastructure of the full term shark yolk sac placenta. I. Morphology and cellular transport at the fetal attachment site. J Ultrastructure Res. 1985;91:192–206. doi: 10.1016/s0022-5320(85)80013-7. [DOI] [PubMed] [Google Scholar]
- Lessey BA. The role of the endometrium during embryo implantation. Hum Reprod. 2000;15:39–50. [PubMed] [Google Scholar]
- Lindhart CA, Bentin-Ley U, Ravn V, Islin H, Hviid T, Rex S, Bangsboll S, Sorensen S. Biochemical evaluation of endometrial function at the time of implantation. Fertil Steril. 2002;78:221–233. doi: 10.1016/S0015-0282(02)03240-5. [DOI] [PubMed] [Google Scholar]
- Boucher A, Kharfi A, Al-Akoum M, Bossù P, Akoum A. Cycle-dependent expression of interleukin-1 receptor type II in the human endometrium. Biol Reprod. 2001;65:890–898. doi: 10.1095/biolreprod65.3.890. [DOI] [PubMed] [Google Scholar]
- Bird S, Wang T, Zou J, Cunningham C, Secombes CJ. The first cytokine sequence within cartilaginous fish: IL-1 beta in the small spotted catshark (Scyliorhinus canicula) J Immunol. 2002;168:3329–3340. doi: 10.4049/jimmunol.168.7.3329. [DOI] [PubMed] [Google Scholar]
- O'Neill LAJ, Greene C. Signal transduction pathways activated by the IL-1 receptor family: ancient signaling machinery in mammals, insects, and plants. J Leukoc Biol. 1998;63:650–7. [PubMed] [Google Scholar]
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- Mondal S, Rai U. In vitro effect of temperature on phagocytic and cytotoxic activities of splenic phagocytes of the wall lizard, Hemidactylus flaviviridis. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:391–8. doi: 10.1016/S1095-6433(00)00356-1. [DOI] [PubMed] [Google Scholar]
- Mondal S, Rai U. In vitro effect of sex steroids on cytotoxic activities of splenic phagocytes of the wall lizard, Hemidactylus flaviviridis. Gen Comp Endocrinol. 2002;125:264–71. doi: 10.1006/gcen.2001.7744. [DOI] [PubMed] [Google Scholar]
- Dunlap KD, Church DR. Interleukin-1 beta reduces daily activity level in male lizards, Sceloropus occidentalis. Brain Behav Immun. 1996;10:68–73. doi: 10.1006/brbi.1996.0006. [DOI] [PubMed] [Google Scholar]