Abstract
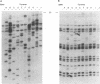
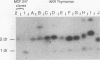
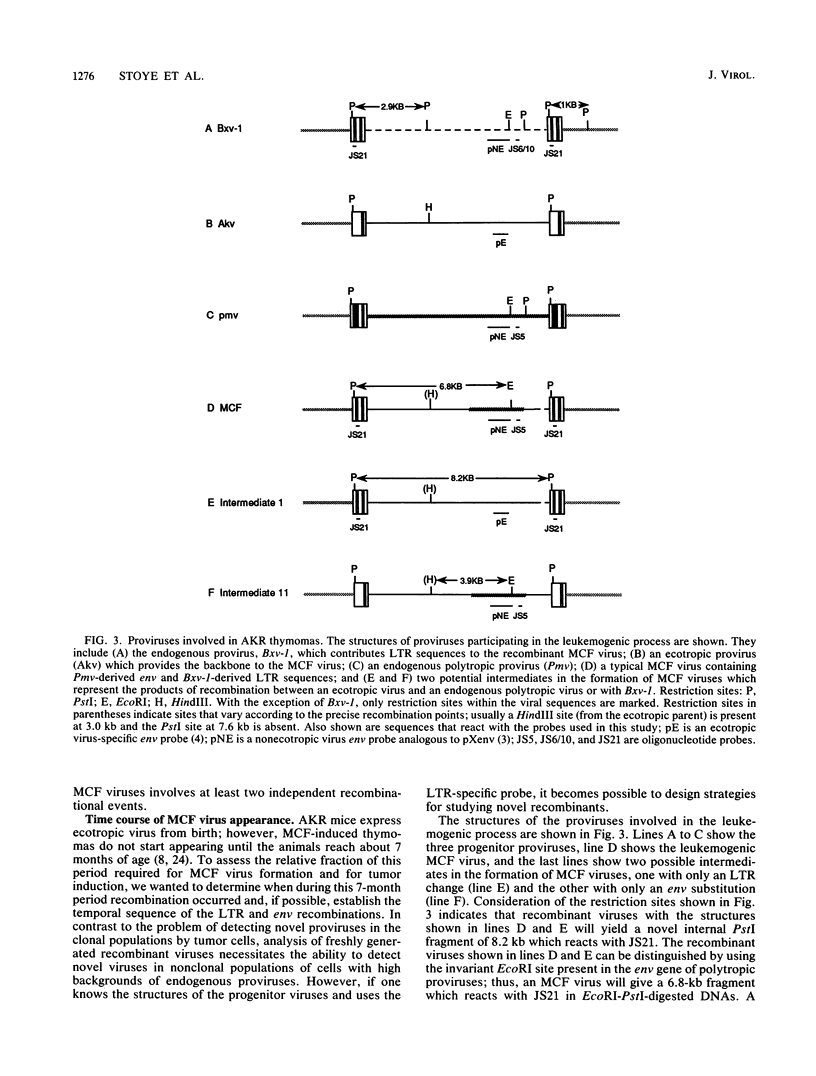
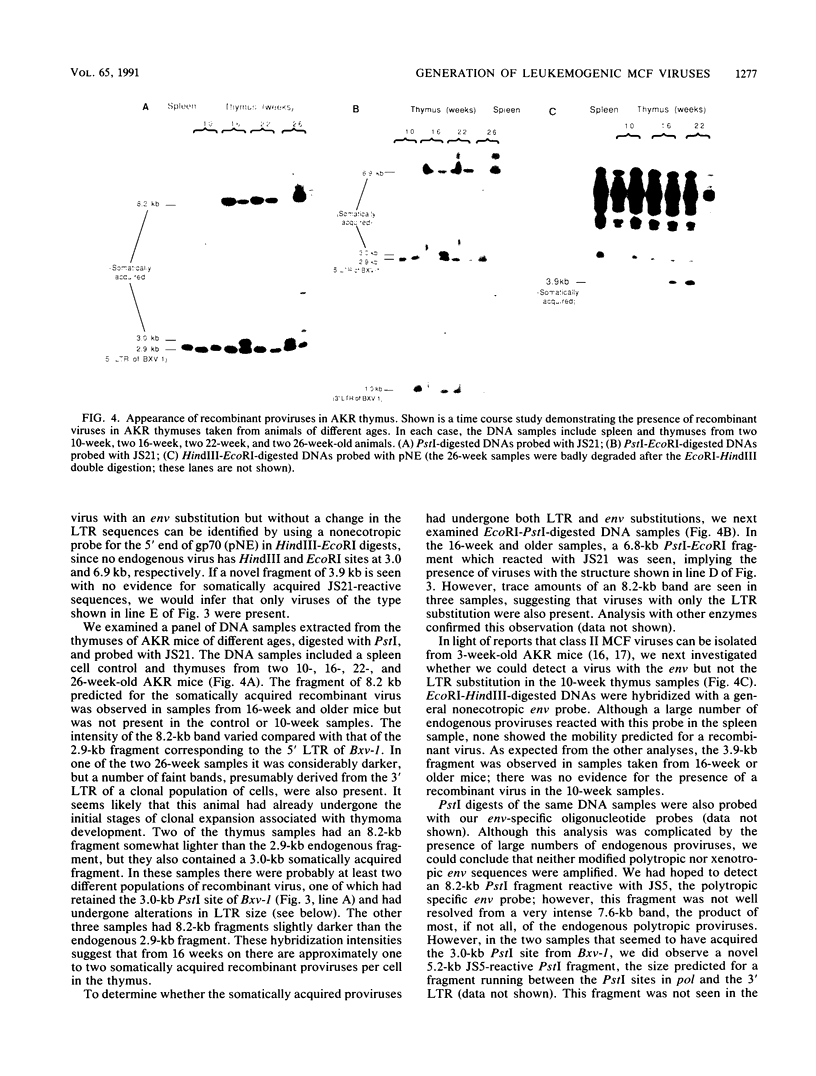
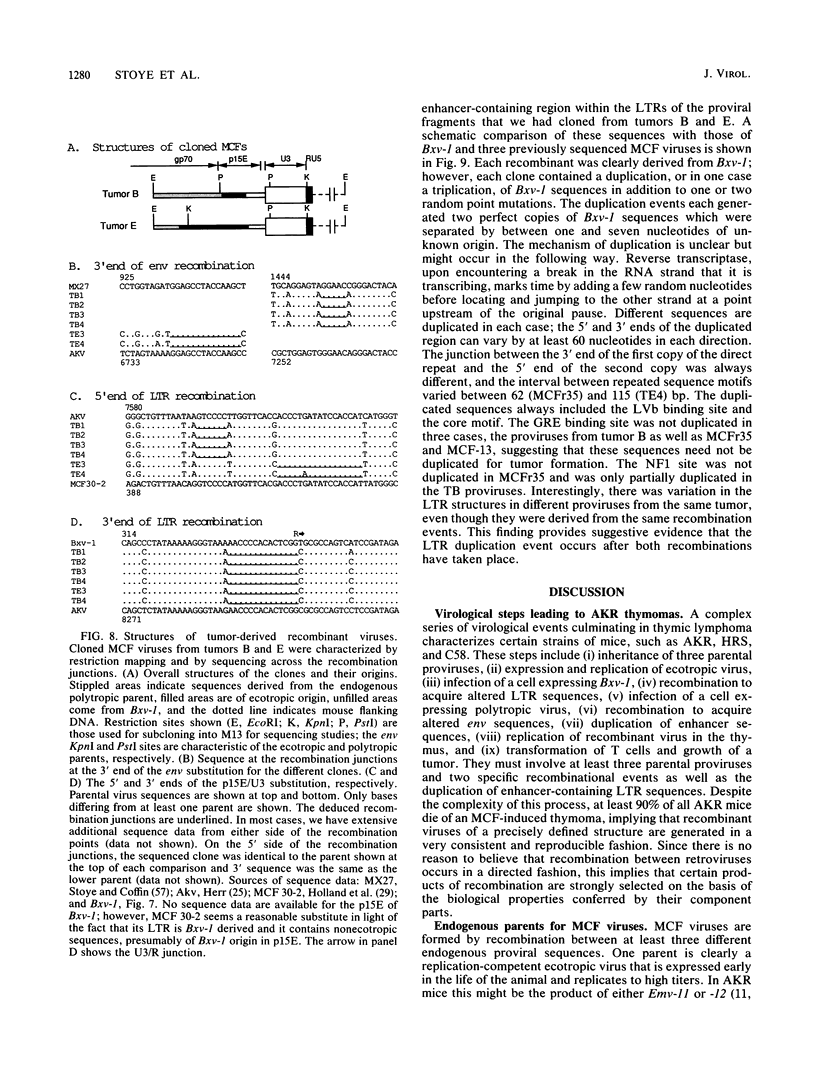
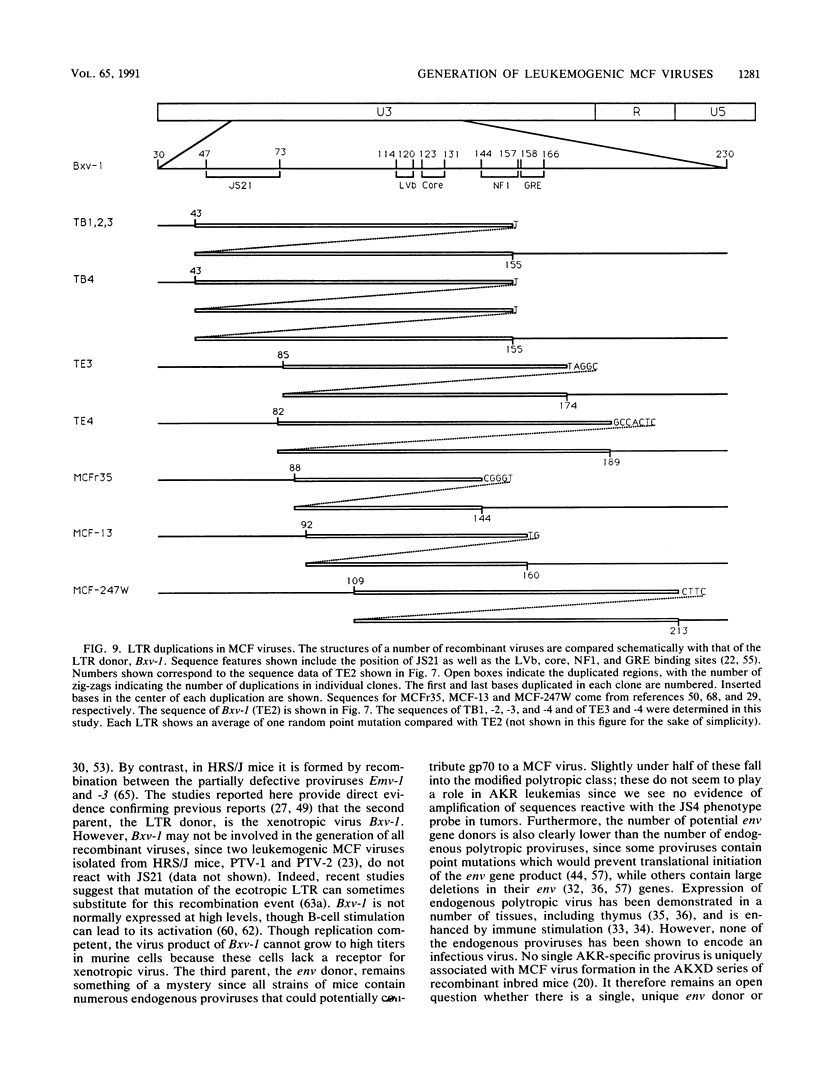
The spontaneous leukemias of AKR mice are caused by mink cell focus-forming (MCF) viruses. These viruses are generated by recombination between several endogenous murine retroviruses. The virological events leading to the generation of the leukemogenic agent were investigated by using an oligonucleotide specific for the U3 region of the leukemogenic virus and env-reactive oligonucleotide probes specific for the different classes of endogenous murine leukemia virus. It was shown that (i) the leukemogenic MCF virus is formed by recombination between at least three different endogenous sequences; (ii) the U3 donor for the leukemogenic virus is the inducible xenotropic virus Bxv-1; (iii) all spontaneous tumors contain viruses with duplicated enhancer regions in their long terminal repeats; (iv) enhancer duplication is a somatic event, since Bxv-1 contains only one copy; (v) the first recombinant virus detectable in mass populations of thymocytes by Southern hybridization analysis contains all structural features of the ultimate leukemogenic virus; and (vi) the multiple novel viruses in a given tumor represent progeny of the same unique recombination events. On the basis of these results, an analysis of the virological events leading to AKR thymomas is presented.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. W., Blais B. P., Robinson H. L. Long terminal repeat (LTR) sequences, env, and a region near the 5' LTR influence the pathogenic potential of recombinants between Rous-associated virus types 0 and 1. J Virol. 1988 Sep;62(9):3431–3437. doi: 10.1128/jvi.62.9.3431-3437.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Cloyd M. W., Linemeyer D. L., Lander M. R., Rands E., Lowy D. R. Cellular origin and role of mink cell focus-forming viruses in murine thymic lymphomas. Nature. 1982 Jan 7;295(5844):25–31. doi: 10.1038/295025a0. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Rands E., Lowy D. R. Structure of endogenous murine leukemia virus DNA in mouse genomes. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5774–5778. doi: 10.1073/pnas.77.10.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloyd M. W. Characterization of target cells for MCF viruses in AKR mice. Cell. 1983 Jan;32(1):217–225. doi: 10.1016/0092-8674(83)90512-3. [DOI] [PubMed] [Google Scholar]
- Cloyd M. W., Chattopadhyay S. K. A new class of retrovirus present in many murine leukemia systems. Virology. 1986 May;151(1):31–40. doi: 10.1016/0042-6822(86)90101-7. [DOI] [PubMed] [Google Scholar]
- Cloyd M. W., Hartley J. W., Rowe W. P. Lymphomagenicity of recombinant mink cell focus-inducing murine leukemia viruses. J Exp Med. 1980 Mar 1;151(3):542–552. doi: 10.1084/jem.151.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M. Structure, replication, and recombination of retrovirus genomes: some unifying hypotheses. J Gen Virol. 1979 Jan;42(1):1–26. doi: 10.1099/0022-1317-42-1-1. [DOI] [PubMed] [Google Scholar]
- Copeland N. G., Bedigian H. G., Thomas C. Y., Jenkins N. A. DNAs of two molecularly cloned endogenous ecotropic proviruses are poorly infectious in DNA transfection assays. J Virol. 1984 Feb;49(2):437–444. doi: 10.1128/jvi.49.2.437-444.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S. K., Thomas C. Y., Nicklas J. A., Coffin J. M. Thymic epithelial genotype influences the production of recombinant leukemogenic retroviruses in mice. J Virol. 1983 Jul;47(1):33–45. doi: 10.1128/jvi.47.1.33-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Jolicoeur P. The tandem direct repeats within the long terminal repeat of murine leukemia viruses are the primary determinant of their leukemogenic potential. J Virol. 1984 Dec;52(3):945–952. doi: 10.1128/jvi.52.3.945-952.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Rassart E., Jolicoeur P. Thymotropism of murine leukemia virus is conferred by its long terminal repeat. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4203–4207. doi: 10.1073/pnas.80.14.4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Gautsch J. W., Jensen F. C., Lerner R. A., Hartley J. W., Rowe W. P. Biochemical evidence that MCF murine leukemia viruses are envelope (env) gene recombinants. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4676–4680. doi: 10.1073/pnas.74.10.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. H. Characterization of polytropic MuLVs from three-week-old AKR/J mice. Virology. 1986 Aug;153(1):122–136. doi: 10.1016/0042-6822(86)90013-9. [DOI] [PubMed] [Google Scholar]
- Evans L. H., Cloyd M. W. Friend and Moloney murine leukemia viruses specifically recombine with different endogenous retroviral sequences to generate mink cell focus-forming viruses. Proc Natl Acad Sci U S A. 1985 Jan;82(2):459–463. doi: 10.1073/pnas.82.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. H., Malik F. G. Class II polytropic murine leukemia viruses (MuLVs) of AKR/J mice: possible role in the generation of class I oncogenic polytropic MuLVs. J Virol. 1987 Jun;61(6):1882–1892. doi: 10.1128/jvi.61.6.1882-1892.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel W. N., Stoye J. P., Taylor B. A., Coffin J. M. A linkage map of endogenous murine leukemia proviruses. Genetics. 1990 Feb;124(2):221–236. doi: 10.1093/genetics/124.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel W. N., Stoye J. P., Taylor B. A., Coffin J. M. Genetic analysis of endogenous xenotropic murine leukemia viruses: association with two common mouse mutations and the viral restriction locus Fv-1. J Virol. 1989 Apr;63(4):1763–1774. doi: 10.1128/jvi.63.4.1763-1774.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel W. N., Stoye J. P., Taylor B. A., Coffin J. M. Genetic identification of endogenous polytropic proviruses by using recombinant inbred mice. J Virol. 1989 Sep;63(9):3810–3821. doi: 10.1128/jvi.63.9.3810-3821.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golemis E. A., Speck N. A., Hopkins N. Alignment of U3 region sequences of mammalian type C viruses: identification of highly conserved motifs and implications for enhancer design. J Virol. 1990 Feb;64(2):534–542. doi: 10.1128/jvi.64.2.534-542.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N., Hiai H., Elder J. H., Schwartz R. S., Khiroya R. H., Thomas C. Y., Tsichlis P. N., Coffin J. M. Expression of leukemogenic recombinant viruses associated with a recessive gene in HRS/J mice. J Exp Med. 1980 Aug 1;152(2):249–264. doi: 10.1084/jem.152.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W., Gilbert W. Somatically acquired recombinant murine leukemia proviruses in thymic leukemias of AKR/J mice. J Virol. 1983 Apr;46(1):70–82. doi: 10.1128/jvi.46.1.70-82.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W. Nucleotide sequence of AKV murine leukemia virus. J Virol. 1984 Feb;49(2):471–478. doi: 10.1128/jvi.49.2.471-478.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggan M. D., O'Neill R. R., Kozak C. A. Nonecotropic murine leukemia viruses in BALB/c and NFS/N mice: characterization of the BALB/c Bxv-1 provirus and the single NFS endogenous xenotrope. J Virol. 1986 Dec;60(3):980–986. doi: 10.1128/jvi.60.3.980-986.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland C. A., Hartley J. W., Rowe W. P., Hopkins N. At least four viral genes contribute to the leukemogenicity of murine retrovirus MCF 247 in AKR mice. J Virol. 1985 Jan;53(1):158–165. doi: 10.1128/jvi.53.1.158-165.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland C. A., Thomas C. Y., Chattopadhyay S. K., Koehne C., O'Donnell P. V. Influence of enhancer sequences on thymotropism and leukemogenicity of mink cell focus-forming viruses. J Virol. 1989 Mar;63(3):1284–1292. doi: 10.1128/jvi.63.3.1284-1292.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Organization, distribution, and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus musculus. J Virol. 1982 Jul;43(1):26–36. doi: 10.1128/jvi.43.1.26-36.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M., Holland C. A., Lung M. L., Chattopadhyay S. K., Lowy D. R., Hopkins N. H. Nucleotide sequence of the 3' end of MCF 247 murine leukemia virus. J Virol. 1983 Jan;45(1):291–298. doi: 10.1128/jvi.45.1.291-298.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S., Rowe W. P., Martin M. A. Cloning of endogenous murine leukemia virus-related sequences from chromosomal DNA of BALB/c and AKR/J mice: identification of an env progenitor of AKR-247 mink cell focus-forming proviral DNA. J Virol. 1982 Nov;44(2):625–636. doi: 10.1128/jvi.44.2.625-636.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg A. M., Khan A. S., Steinberg A. D. Multiple endogenous xenotropic and mink cell focus-forming murine leukemia virus-related transcripts are induced by polyclonal immune activators. J Virol. 1988 Oct;62(10):3545–3550. doi: 10.1128/jvi.62.10.3545-3550.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg A. M., Steinberg A. D., Khan A. S. Increased expression of novel full-length endogenous mink cell focus-forming-related transcripts in autoimmune mouse strains. Virology. 1988 Jan;162(1):274–276. doi: 10.1016/0042-6822(88)90422-9. [DOI] [PubMed] [Google Scholar]
- Laigret F., Repaske R., Boulukos K., Rabson A. B., Khan A. S. Potential progenitor sequences of mink cell focus-forming (MCF) murine leukemia viruses: ecotropic, xenotropic, and MCF-related viral RNAs are detected concurrently in thymus tissues of AKR mice. J Virol. 1988 Feb;62(2):376–386. doi: 10.1128/jvi.62.2.376-386.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. E., Lerner R. A., Wilson M. C. Normal expression of polymorphic endogenous retroviral RNA containing segments identical to mink cell focus-forming virus. J Virol. 1985 Dec;56(3):691–700. doi: 10.1128/jvi.56.3.691-700.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. P., D'Andrea A. D., Lodish H. F., Baltimore D. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature. 1990 Feb 22;343(6260):762–764. doi: 10.1038/343762a0. [DOI] [PubMed] [Google Scholar]
- Li Y., Golemis E., Hartley J. W., Hopkins N. Disease specificity of nondefective Friend and Moloney murine leukemia viruses is controlled by a small number of nucleotides. J Virol. 1987 Mar;61(3):693–700. doi: 10.1128/jvi.61.3.693-700.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly F., Duran-Reynals M. L., Rowe W. P. Correlation of early murine leukemia virus titer and H-2 type with spontaneous leukemia in mice of the BALB/c times AKR cross: a genetic analysis. J Exp Med. 1975 Apr 1;141(4):882–889. [PMC free article] [PubMed] [Google Scholar]
- Lung M. L., Hartley J. W., Rowe W. P., Hopkins N. H. Large RNase T1-resistant oligonucleotides encoding p15E and the U3 region of the long terminal repeat distinguish two biological classes of mink cell focus-forming type C viruses of inbred mice. J Virol. 1983 Jan;45(1):275–290. doi: 10.1128/jvi.45.1.275-290.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey A. C., Coppola M. A., Thomas C. Y. Origin of pathogenic determinants of recombinant murine leukemia viruses: analysis of Bxv-1-related xenotropic viruses from CWD mice. J Virol. 1990 Nov;64(11):5491–5499. doi: 10.1128/jvi.64.11.5491-5499.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath M. S., Weissman I. L. AKR leukemogenesis: identification and biological significance of thymic lymphoma receptors for AKR retroviruses. Cell. 1979 May;17(1):65–75. doi: 10.1016/0092-8674(79)90295-2. [DOI] [PubMed] [Google Scholar]
- Moroni C., Stoye J. P., DeLamarter J. F., Erb P., Jay F. A., Jongstra J., Martin D., Schumann G. Normal B-cell activation involves endogenous retroviral antigen expression: implications for leukemogenesis. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1205–1210. doi: 10.1101/sqb.1980.044.01.130. [DOI] [PubMed] [Google Scholar]
- Nikbakht K. N., Boone L. R., Glover P. L., Myer F. E., Yang W. K. Characterization of a molecular clone of RFM/Un mouse chromosomal DNA that contains a full-length endogenous murine leukaemia virus-related proviral genome. J Gen Virol. 1987 Mar;68(Pt 3):683–693. doi: 10.1099/0022-1317-68-3-683. [DOI] [PubMed] [Google Scholar]
- O'Donnell P. V., Stockert E., Obata Y., Old L. J. Leukemogenic properties of AKR dualtropic (MCF) viruses: amplification of murine leukemia virus-related antigens on thymocytes and acceleration of leukemia development in AKR mice. Virology. 1981 Jul 30;112(2):548–563. doi: 10.1016/0042-6822(81)90301-9. [DOI] [PubMed] [Google Scholar]
- O'Neill R. R., Buckler C. E., Theodore T. S., Martin M. A., Repaske R. Envelope and long terminal repeat sequences of a cloned infectious NZB xenotropic murine leukemia virus. J Virol. 1985 Jan;53(1):100–106. doi: 10.1128/jvi.53.1.100-106.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panganiban A. T., Fiore D. Ordered interstrand and intrastrand DNA transfer during reverse transcription. Science. 1988 Aug 26;241(4869):1064–1069. doi: 10.1126/science.2457948. [DOI] [PubMed] [Google Scholar]
- Quint W., Boelens W., van Wezenbeek P., Cuypers T., Maandag E. R., Selten G., Berns A. Generation of AKR mink cell focus-forming viruses: a conserved single-copy xenotrope-like provirus provides recombinant long terminal repeat sequences. J Virol. 1984 May;50(2):432–438. doi: 10.1128/jvi.50.2.432-438.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quint W., Boelens W., van Wezenbeek P., Robanus Maandag E., Berns A. Generation of AKR mink cell focus-forming virus: nucleotide sequence of the 3' end of a somatically acquired AKR-MCF. Virology. 1984 Jul 30;136(2):425–434. doi: 10.1016/0042-6822(84)90178-8. [DOI] [PubMed] [Google Scholar]
- Rimm D. L., Horness D., Kucera J., Blattner F. R. Construction of coliphage lambda Charon vectors with BamHI cloning sites. Gene. 1980 Dec;12(3-4):301–309. doi: 10.1016/0378-1119(80)90113-4. [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Haseltine W. A., Lenz J., Ruprecht R., Cloyd M. W. Tissue selectivity of murine leukemia virus infection is determined by long terminal repeat sequences. J Virol. 1985 Sep;55(3):862–866. doi: 10.1128/jvi.55.3.862-866.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P. Studies of genetic transmission of murine leukemia virus by AKR mice. I. Crosses with Fv-1 n strains of mice. J Exp Med. 1972 Nov 1;136(5):1272–1285. doi: 10.1084/jem.136.5.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck N. A., Baltimore D. Six distinct nuclear factors interact with the 75-base-pair repeat of the Moloney murine leukemia virus enhancer. Mol Cell Biol. 1987 Mar;7(3):1101–1110. doi: 10.1128/mcb.7.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck N. A., Renjifo B., Hopkins N. Point mutations in the Moloney murine leukemia virus enhancer identify a lymphoid-specific viral core motif and 1,3-phorbol myristate acetate-inducible element. J Virol. 1990 Feb;64(2):543–550. doi: 10.1128/jvi.64.2.543-550.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoye J. P., Coffin J. M. Polymorphism of murine endogenous proviruses revealed by using virus class-specific oligonucleotide probes. J Virol. 1988 Jan;62(1):168–175. doi: 10.1128/jvi.62.1.168-175.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoye J. P., Coffin J. M. The four classes of endogenous murine leukemia virus: structural relationships and potential for recombination. J Virol. 1987 Sep;61(9):2659–2669. doi: 10.1128/jvi.61.9.2659-2669.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoye J. P., Fenner S., Greenoak G. E., Moran C., Coffin J. M. Role of endogenous retroviruses as mutagens: the hairless mutation of mice. Cell. 1988 Jul 29;54(3):383–391. doi: 10.1016/0092-8674(88)90201-2. [DOI] [PubMed] [Google Scholar]
- Stoye J. P., Moroni C. Endogenous retrovirus expression in stimulated murine lymphocytes. Identification of a new locus controlling mitogen induction of a defective virus. J Exp Med. 1983 May 1;157(5):1660–1674. doi: 10.1084/jem.157.5.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoye J. P., Moroni C. Phenotypic mixing of retroviruses in mitogen-stimulated lymphocytes: analysis of xenotropic and defective endogenous mouse viruses. J Gen Virol. 1984 Feb;65(Pt 2):317–326. doi: 10.1099/0022-1317-65-2-317. [DOI] [PubMed] [Google Scholar]
- Stoye J. P., Moroni C. Proliferation and differentiation requirements for the induction of two retroviral loci during B-cell activation. J Gen Virol. 1985 Jan;66(Pt 1):109–120. doi: 10.1099/0022-1317-66-1-109. [DOI] [PubMed] [Google Scholar]
- Thomas C. Y. AKR ecotropic murine leukemia virus SL3-3 forms envelope gene recombinants in vivo. J Virol. 1986 Jul;59(1):23–30. doi: 10.1128/jvi.59.1.23-30.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. Y., Coffin J. M. Genetic alterations of RNA leukemia viruses associated with the development of spontaneous thymic leukemia in AKR/J mice. J Virol. 1982 Aug;43(2):416–426. doi: 10.1128/jvi.43.2.416-426.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. Y., Khiroya R., Schwartz R. S., Coffin J. M. Role of recombinant ecotropic and polytropic viruses in the development of spontaneous thymic lymphomas in HRS/J mice. J Virol. 1984 May;50(2):397–407. doi: 10.1128/jvi.50.2.397-407.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsichlis P. N., Coffin J. M. Recombinants between endogenous and exogenous avian tumor viruses: role of the C region and other portions of the genome in the control of replication and transformation. J Virol. 1980 Jan;33(1):238–249. doi: 10.1128/jvi.33.1.238-249.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F. K., Davison B., Chaffin K. Murine leukemia virus long terminal repeat sequences can enhance gene activity in a cell-type-specific manner. Mol Cell Biol. 1985 Oct;5(10):2832–2835. doi: 10.1128/mcb.5.10.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Putten H., Quint W., van Raaij J., Maandag E. R., Verma I. M., Berns A. M-MuLV-induced leukemogenesis: integration and structure of recombinant proviruses in tumors. Cell. 1981 Jun;24(3):729–739. doi: 10.1016/0092-8674(81)90099-4. [DOI] [PubMed] [Google Scholar]