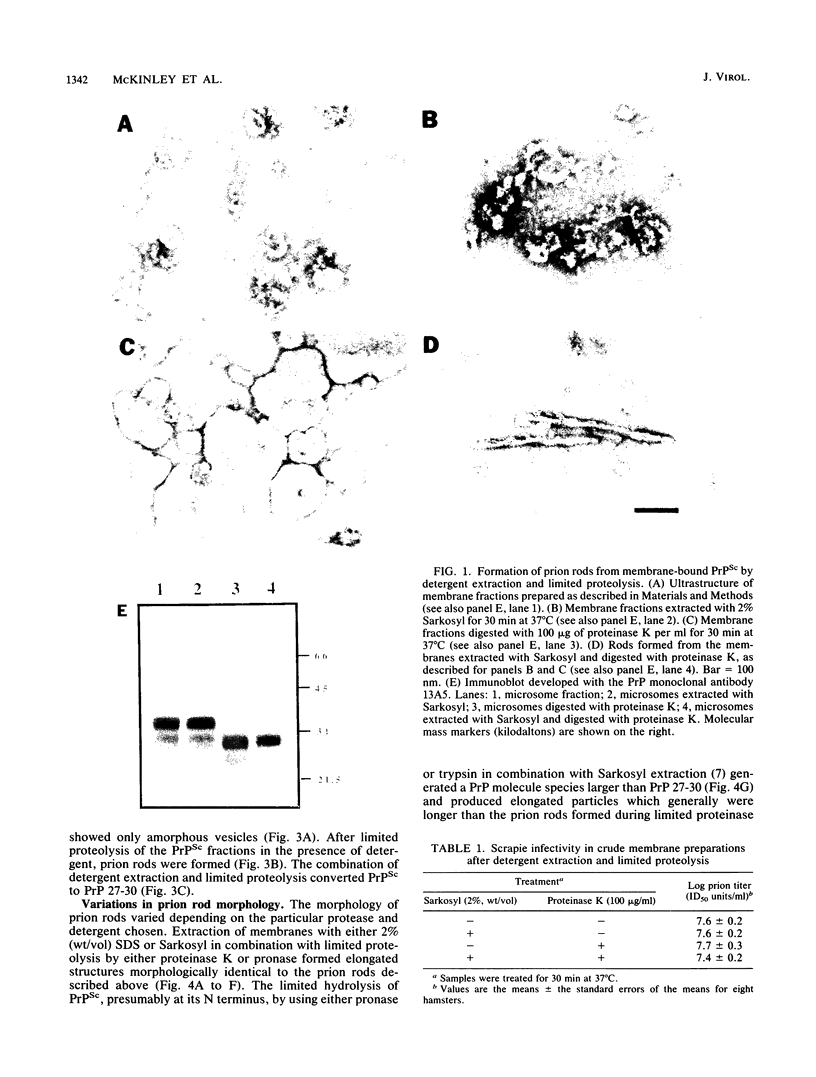
Abstract
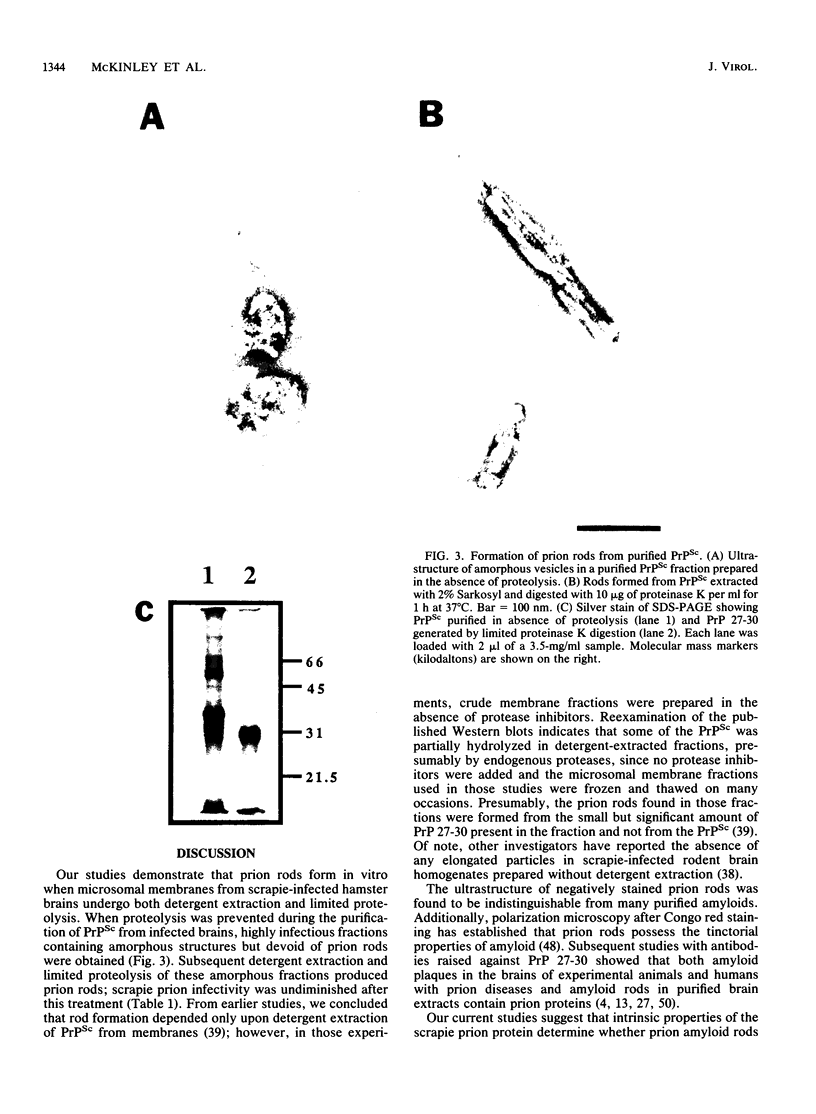
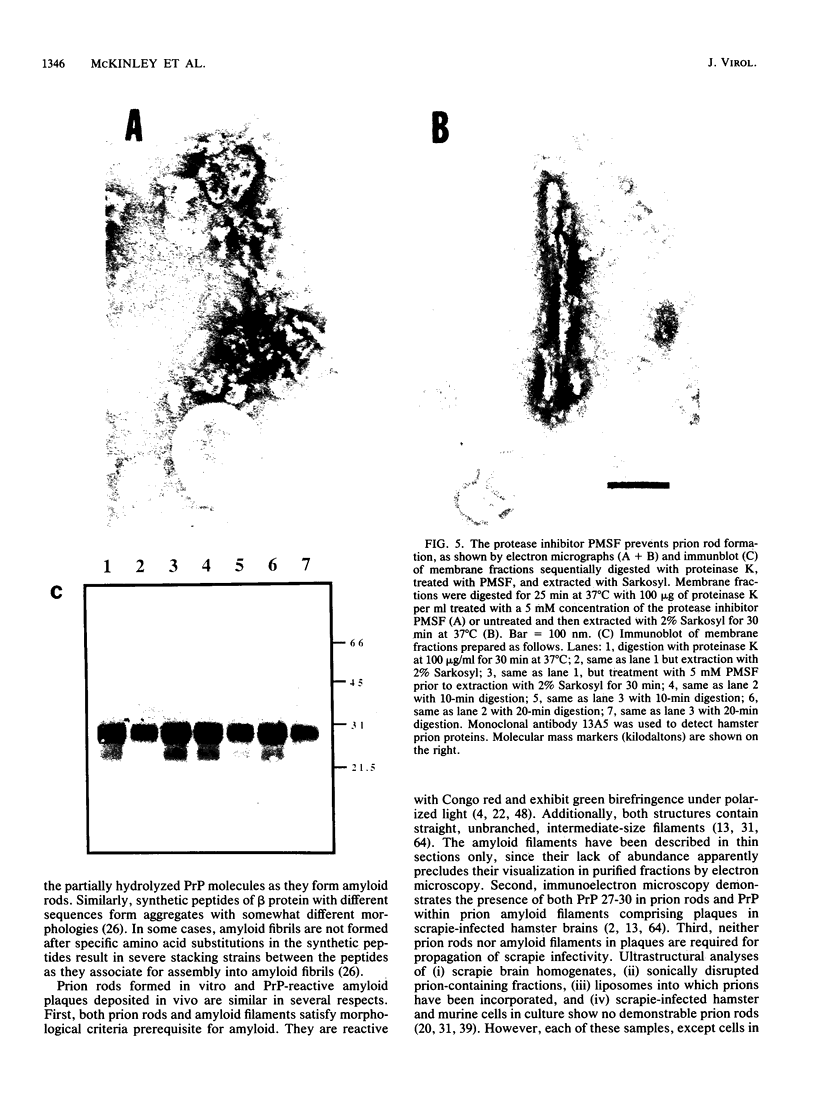
Scrapie prion infectivity can be enriched from hamster brain homogenates by using limited proteolysis and detergent extraction. Purified fractions contain both scrapie infectivity and the protein PrP 27-30, which is aggregated in the form of prion rods. During purification, PrP 27-30 is produced from a larger membrane protein, PrPSc, by limited proteolysis with proteinase K. Brain homogenates from scrapie-infected hamsters do not contain prion rods prior to exposure to detergents and proteases. To determine whether both detergent extraction and limited proteolysis are required for the formation of prion rods, microsomal membranes were prepared from infected brains in the presence of protease inhibitors. The isolated membranes were then detergent extracted as well as protease digested to evaluate the effects of these treatments on the formation of prion rods. Neither detergent (2% Sarkosyl) extraction nor limited proteinase K digestion of scrapie microsomes produced recognizable prion amyloid rods. Only after combining detergent extraction with limited proteolysis were numerous prion rods observed. Rod formation was influenced by the protease concentration, the specificity of the protease, and the duration of digestion. Rod formation also depended upon the detergent; some combinations of protease and detergent did not produce prion amyloid rods. Similar results were obtained with purified PrPSc fractions prepared by repeated detergent extractions in the presence of protease inhibitors. These fractions contained amorphous structures but not rods; however, prion rods were produced upon conversion of PrPSc to PrP 27-30 by limited proteolysis. We conclude that the formation of prion amyloid rods in vitro requires both detergent extraction and limited proteolysis. In vivo, amyloid filaments found in the brains of animals with scrapie resemble prion rods in their width and their labeling with prion protein (PrP) antisera; however, filaments are typically longer than rods. Whether limited proteolysis and some process equivalent to detergent extraction are required for amyloid filament formation in vivo remains to be established.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baringer J. R., Prusiner S. B. Experimental scrapie in mice: ultrastructural observations. Ann Neurol. 1978 Sep;4(3):205–211. doi: 10.1002/ana.410040303. [DOI] [PubMed] [Google Scholar]
- Barry R. A., McKinley M. P., Bendheim P. E., Lewis G. K., DeArmond S. J., Prusiner S. B. Antibodies to the scrapie protein decorate prion rods. J Immunol. 1985 Jul;135(1):603–613. [PubMed] [Google Scholar]
- Barry R. A., Prusiner S. B. Monoclonal antibodies to the cellular and scrapie prion proteins. J Infect Dis. 1986 Sep;154(3):518–521. doi: 10.1093/infdis/154.3.518. [DOI] [PubMed] [Google Scholar]
- Bendheim P. E., Barry R. A., DeArmond S. J., Stites D. P., Prusiner S. B. Antibodies to a scrapie prion protein. Nature. 1984 Aug 2;310(5976):418–421. doi: 10.1038/310418a0. [DOI] [PubMed] [Google Scholar]
- Bignami A., Parry H. B. Aggregations of 35-nanometer particles associated with neuronal cytopathic changes in natural scrapie. Science. 1971 Jan 29;171(3969):389–390. doi: 10.1126/science.171.3969.389. [DOI] [PubMed] [Google Scholar]
- Bignami A., Parry H. B. Electron microscopic studies of the brain of sheep with natural scrapie. I. The fine structure of neuronal vacuolation. Brain. 1972;95(2):319–326. doi: 10.1093/brain/95.2.319. [DOI] [PubMed] [Google Scholar]
- Bolton D. C., Bendheim P. E., Marmorstein A. D., Potempska A. Isolation and structural studies of the intact scrapie agent protein. Arch Biochem Biophys. 1987 Nov 1;258(2):579–590. doi: 10.1016/0003-9861(87)90380-8. [DOI] [PubMed] [Google Scholar]
- Bots G. T., de Man J. C., Verjaal A. Virus-like particles in brain tissue from two patients with Creutzfeldt-Jakob disease. Acta Neuropathol. 1971;18(3):267–270. doi: 10.1007/BF00685073. [DOI] [PubMed] [Google Scholar]
- Cho H. J., Greig A. S., Corp C. R., Kimberlin R. H., Chandler R. L., Millson G. C. Virus-like particles from both control and scrapie-affected mouse brain. Nature. 1977 Jun 2;267(5610):459–460. doi: 10.1038/267459a0. [DOI] [PubMed] [Google Scholar]
- Cho H. J., Greig A. S. Isolation of 14-nm virus-like particles from mouse brain infected with scrapie agent. Nature. 1975 Oct 23;257(5528):685–686. doi: 10.1038/257685a0. [DOI] [PubMed] [Google Scholar]
- David-Ferreira J. F., David-Ferreira K. L., Gibbs C. J., Jr, Morris J. A. Scrapie in mice: ultrastructural observations in the cerebral cortex. Proc Soc Exp Biol Med. 1968 Jan;127(1):313–320. doi: 10.3181/00379727-127-32680. [DOI] [PubMed] [Google Scholar]
- DeArmond S. J., McKinley M. P., Barry R. A., Braunfeld M. B., McColloch J. R., Prusiner S. B. Identification of prion amyloid filaments in scrapie-infected brain. Cell. 1985 May;41(1):221–235. doi: 10.1016/0092-8674(85)90076-5. [DOI] [PubMed] [Google Scholar]
- DeArmond S. J., Mobley W. C., DeMott D. L., Barry R. A., Beckstead J. H., Prusiner S. B. Changes in the localization of brain prion proteins during scrapie infection. Neurology. 1987 Aug;37(8):1271–1280. doi: 10.1212/wnl.37.8.1271. [DOI] [PubMed] [Google Scholar]
- Diener T. O. PrP and the nature of the scrapie agent. Cell. 1987 Jun 19;49(6):719–721. doi: 10.1016/0092-8674(87)90607-6. [DOI] [PubMed] [Google Scholar]
- Field E. J., Narang H. K. An electron-microscopic study of scrapie in the rat: further observations on "inclusion bodies" and virus-like particles. J Neurol Sci. 1972 Nov;17(3):347–364. doi: 10.1016/0022-510x(72)90038-x. [DOI] [PubMed] [Google Scholar]
- Field E. J., Raine C. S. Observations on "dense-body" structure in nerve cells with special reference to scrapie. Res Vet Sci. 1966 Jul;7(3):292–295. [PubMed] [Google Scholar]
- Gabizon R., McKinley M. P., Groth D. F., Kenaga L., Prusiner S. B. Properties of scrapie prion protein liposomes. J Biol Chem. 1988 Apr 5;263(10):4950–4955. [PubMed] [Google Scholar]
- Gabizon R., McKinley M. P., Groth D., Prusiner S. B. Immunoaffinity purification and neutralization of scrapie prion infectivity. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6617–6621. doi: 10.1073/pnas.85.18.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabizon R., McKinley M. P., Prusiner S. B. Purified prion proteins and scrapie infectivity copartition into liposomes. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4017–4021. doi: 10.1073/pnas.84.12.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabizon R., Prusiner S. B. Prion liposomes. Biochem J. 1990 Feb 15;266(1):1–14. doi: 10.1042/bj2660001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts). N Engl J Med. 1980 Jun 5;302(23):1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Goldgaber D., Lerman M. I., McBride O. W., Saffiotti U., Gajdusek D. C. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer's disease. Science. 1987 Feb 20;235(4791):877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- Hsiao K., Baker H. F., Crow T. J., Poulter M., Owen F., Terwilliger J. D., Westaway D., Ott J., Prusiner S. B. Linkage of a prion protein missense variant to Gerstmann-Sträussler syndrome. Nature. 1989 Mar 23;338(6213):342–345. doi: 10.1038/338342a0. [DOI] [PubMed] [Google Scholar]
- Kirschner D. A., Inouye H., Duffy L. K., Sinclair A., Lind M., Selkoe D. J. Synthetic peptide homologous to beta protein from Alzheimer disease forms amyloid-like fibrils in vitro. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6953–6957. doi: 10.1073/pnas.84.19.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto T., Ogomori K., Tateishi J., Prusiner S. B. Formic acid pretreatment enhances immunostaining of cerebral and systemic amyloids. Lab Invest. 1987 Aug;57(2):230–236. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowenstein D. H., Butler D. A., Westaway D., McKinley M. P., DeArmond S. J., Prusiner S. B. Three hamster species with different scrapie incubation times and neuropathological features encode distinct prion proteins. Mol Cell Biol. 1990 Mar;10(3):1153–1163. doi: 10.1128/mcb.10.3.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley M. P., Braunfeld M. B., Bellinger C. G., Prusiner S. B. Molecular characteristics of prion rods purified from scrapie-infected hamster brains. J Infect Dis. 1986 Jul;154(1):110–120. doi: 10.1093/infdis/154.1.110. [DOI] [PubMed] [Google Scholar]
- McKinley M. P., DeArmond S. J., Torchia M., Mobley W. C., Prusiner S. B. Acceleration of scrapie in neonatal Syrian hamsters. Neurology. 1989 Oct;39(10):1319–1324. doi: 10.1212/wnl.39.10.1319. [DOI] [PubMed] [Google Scholar]
- Merz P. A., Kascsak R. J., Rubenstein R., Carp R. I., Wisniewski H. M. Antisera to scrapie-associated fibril protein and prion protein decorate scrapie-associated fibrils. J Virol. 1987 Jan;61(1):42–49. doi: 10.1128/jvi.61.1.42-49.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz P. A., Rohwer R. G., Kascsak R., Wisniewski H. M., Somerville R. A., Gibbs C. J., Jr, Gajdusek D. C. Infection-specific particle from the unconventional slow virus diseases. Science. 1984 Jul 27;225(4660):437–440. doi: 10.1126/science.6377496. [DOI] [PubMed] [Google Scholar]
- Merz P. A., Somerville R. A., Wisniewski H. M., Iqbal K. Abnormal fibrils from scrapie-infected brain. Acta Neuropathol. 1981;54(1):63–74. doi: 10.1007/BF00691333. [DOI] [PubMed] [Google Scholar]
- Merz P. A., Somerville R. A., Wisniewski H. M., Manuelidis L., Manuelidis E. E. Scrapie-associated fibrils in Creutzfeldt-Jakob disease. Nature. 1983 Dec 1;306(5942):474–476. doi: 10.1038/306474a0. [DOI] [PubMed] [Google Scholar]
- Merz P. A., Wisniewski H. M., Somerville R. A., Bobin S. A., Masters C. L., Iqbal K. Ultrastructural morphology of amyloid fibrils from neuritic and amyloid plaques. Acta Neuropathol. 1983;60(1-2):113–124. doi: 10.1007/BF00685355. [DOI] [PubMed] [Google Scholar]
- Meyer R. K., McKinley M. P., Bowman K. A., Braunfeld M. B., Barry R. A., Prusiner S. B. Separation and properties of cellular and scrapie prion proteins. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2310–2314. doi: 10.1073/pnas.83.8.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narang H. K. An electron microscopic study of natural scrapie sheep brain: further observations on virus-like particles and paramyxovirus-like tubules. Acta Neuropathol. 1974;28(4):317–329. doi: 10.1007/BF00685286. [DOI] [PubMed] [Google Scholar]
- Narang H. K. An electron microscopic study of the scrapie mouse and rat: further observations on virus-like particles with ruthenium red and lanthanum nitrate as a possible trace and negative stain. Neurobiology. 1974;4(6):349–363. [PubMed] [Google Scholar]
- Narang H. K. Virus-like particles in natural scrapie of the sheep. Res Vet Sci. 1973 Jan;14(1):108–110. [PubMed] [Google Scholar]
- Oesch B., Westaway D., Wälchli M., McKinley M. P., Kent S. B., Aebersold R., Barry R. A., Tempst P., Teplow D. B., Hood L. E. A cellular gene encodes scrapie PrP 27-30 protein. Cell. 1985 Apr;40(4):735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Bolton D. C., Groth D. F., Bowman K. A., Cochran S. P., McKinley M. P. Further purification and characterization of scrapie prions. Biochemistry. 1982 Dec 21;21(26):6942–6950. doi: 10.1021/bi00269a050. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Cochran S. P., Groth D. F., Downey D. E., Bowman K. A., Martinez H. M. Measurement of the scrapie agent using an incubation time interval assay. Ann Neurol. 1982 Apr;11(4):353–358. doi: 10.1002/ana.410110406. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D. F., Bolton D. C., Kent S. B., Hood L. E. Purification and structural studies of a major scrapie prion protein. Cell. 1984 Aug;38(1):127–134. doi: 10.1016/0092-8674(84)90533-6. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., McKinley M. P., Bowman K. A., Bolton D. C., Bendheim P. E., Groth D. F., Glenner G. G. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983 Dec;35(2 Pt 1):349–358. doi: 10.1016/0092-8674(83)90168-x. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Scott M., Foster D., Pan K. M., Groth D., Mirenda C., Torchia M., Yang S. L., Serban D., Carlson G. A. Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell. 1990 Nov 16;63(4):673–686. doi: 10.1016/0092-8674(90)90134-z. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Scrapie prions. Annu Rev Microbiol. 1989;43:345–374. doi: 10.1146/annurev.mi.43.100189.002021. [DOI] [PubMed] [Google Scholar]
- Roberts G. W., Lofthouse R., Allsop D., Landon M., Kidd M., Prusiner S. B., Crow T. J. CNS amyloid proteins in neurodegenerative diseases. Neurology. 1988 Oct;38(10):1534–1540. doi: 10.1212/wnl.38.10.1534. [DOI] [PubMed] [Google Scholar]
- Roberts G. W., Lofthouse R., Brown R., Crow T. J., Barry R. A., Prusiner S. B. Prion-protein immunoreactivity in human transmissible dementias. N Engl J Med. 1986 Nov 6;315(19):1231–1233. doi: 10.1056/NEJM198611063151919. [DOI] [PubMed] [Google Scholar]
- Rohwer R. G. Scrapie-associated fibrils. Lancet. 1984 Jul 7;2(8393):36–36. doi: 10.1016/s0140-6736(84)92013-0. [DOI] [PubMed] [Google Scholar]
- Scott M., Foster D., Mirenda C., Serban D., Coufal F., Wälchli M., Torchia M., Groth D., Carlson G., DeArmond S. J. Transgenic mice expressing hamster prion protein produce species-specific scrapie infectivity and amyloid plaques. Cell. 1989 Dec 1;59(5):847–857. doi: 10.1016/0092-8674(89)90608-9. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Abraham C. R., Podlisny M. B., Duffy L. K. Isolation of low-molecular-weight proteins from amyloid plaque fibers in Alzheimer's disease. J Neurochem. 1986 Jun;46(6):1820–1834. doi: 10.1111/j.1471-4159.1986.tb08501.x. [DOI] [PubMed] [Google Scholar]
- Serban D., Taraboulos A., DeArmond S. J., Prusiner S. B. Rapid detection of Creutzfeldt-Jakob disease and scrapie prion proteins. Neurology. 1990 Jan;40(1):110–117. doi: 10.1212/wnl.40.1.110. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Kisilevsky R., Willmer J., Prusiner S. B., DeArmond S. J. Sulfated glycosaminoglycans in amyloid plaques of prion diseases. Acta Neuropathol. 1989;77(4):337–342. doi: 10.1007/BF00687367. [DOI] [PubMed] [Google Scholar]
- Somerville R. A., Ritchie L. A., Gibson P. H. Structural and biochemical evidence that scrapie-associated fibrils assemble in vivo. J Gen Virol. 1989 Jan;70(Pt 1):25–35. doi: 10.1099/0022-1317-70-1-25. [DOI] [PubMed] [Google Scholar]
- Somerville R. A. Ultrastructural links between scrapie and Alzheimer's disease. Lancet. 1985 Mar 2;1(8427):504–506. doi: 10.1016/s0140-6736(85)92097-5. [DOI] [PubMed] [Google Scholar]
- Stahl N., Borchelt D. R., Hsiao K., Prusiner S. B. Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell. 1987 Oct 23;51(2):229–240. doi: 10.1016/0092-8674(87)90150-4. [DOI] [PubMed] [Google Scholar]
- Taraboulos A., Serban D., Prusiner S. B. Scrapie prion proteins accumulate in the cytoplasm of persistently infected cultured cells. J Cell Biol. 1990 Jun;110(6):2117–2132. doi: 10.1083/jcb.110.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk E., Teplow D. B., Hood L. E., Prusiner S. B. Purification and properties of the cellular and scrapie hamster prion proteins. Eur J Biochem. 1988 Sep 1;176(1):21–30. doi: 10.1111/j.1432-1033.1988.tb14246.x. [DOI] [PubMed] [Google Scholar]
- Vernon M. L., Horta-Barbosa L., Fuccillo D. A., Sever J. L., Baringer J. R., Birnbaum G. Virus-like particles and nucleoprotein-type filaments in brain tissue from two patients with Creutzfeldt-Jakob disease. Lancet. 1970 May 9;1(7654):964–966. doi: 10.1016/s0140-6736(70)91095-0. [DOI] [PubMed] [Google Scholar]
- Westaway D., Goodman P. A., Mirenda C. A., McKinley M. P., Carlson G. A., Prusiner S. B. Distinct prion proteins in short and long scrapie incubation period mice. Cell. 1987 Nov 20;51(4):651–662. doi: 10.1016/0092-8674(87)90134-6. [DOI] [PubMed] [Google Scholar]
- Wiley C. A., Burrola P. G., Buchmeier M. J., Wooddell M. K., Barry R. A., Prusiner S. B., Lampert P. W. Immuno-gold localization of prion filaments in scrapie-infected hamster brains. Lab Invest. 1987 Dec;57(6):646–656. [PubMed] [Google Scholar]