Abstract
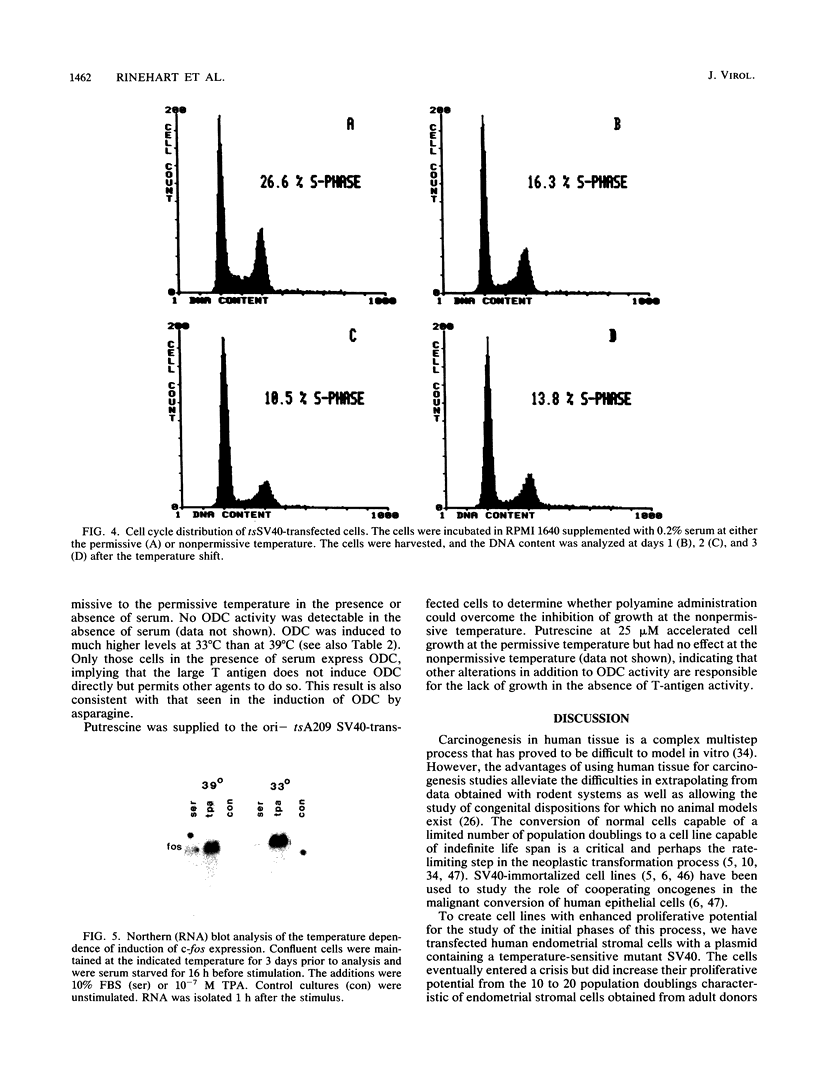
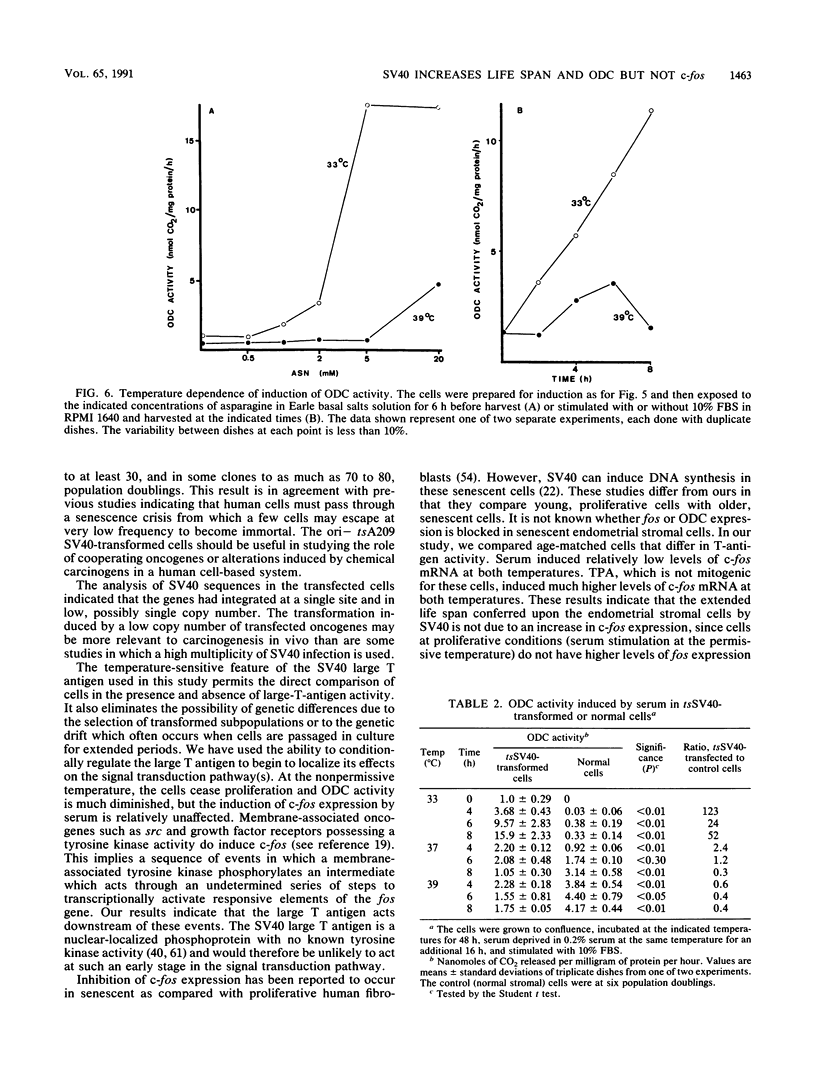
Human endometrial stromal cells transfected with an origin-defective, temperature-sensitive simian virus 40 recombinant plasmid are dependent on T-antigen function for proliferation and at the permissive temperature have an extended life span in culture. Southern blot analysis indicates that the transfected gene is present in low copy number, possibly at a single integration site. Normal stromal cells are capable of 10 to 20 population doublings in culture. Transfected cultures have been carried at the permissive temperature to 80 population doublings before crisis. In the multistep model of malignant transformation of human cells, these cells represent one of the earliest stages: extended but finite life span. We have used these cells to investigate alterations in signal transduction that may be responsible for this early stage of transformation caused by the large T antigen. Temperature shift experiments indicate that the expression of ornithine decarboxylase (ODC) but not of c-fos is altered by the large T antigen. Induction of c-fos by serum or 12-O-tetradecanoylphorbol-13-acetate is independent of temperature. However, in the transfected cells, the induction of ODC by asparagine or serum is greatly enhanced at the permissive temperature. This result indicates that the large T antigen acts downstream of c-fos but upstream of ODC expression in the signal-transducing cascade.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang S. E. In vitro transformation of human epithelial cells. Biochim Biophys Acta. 1986;823(3):161–194. doi: 10.1016/0304-419x(86)90001-6. [DOI] [PubMed] [Google Scholar]
- Chen K., Heller J., Canellakis E. S. Studies on the regulation of ornithine decarboxylase activity by the microtubules: the effect of colchicine and vinblastine. Biochem Biophys Res Commun. 1976 Jan 26;68(2):401–408. doi: 10.1016/0006-291x(76)91159-1. [DOI] [PubMed] [Google Scholar]
- Chou J. Y. Human placental cells transformed by tsA mutants of simian virus 40: a model system for the study of placental functions. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1409–1413. doi: 10.1073/pnas.75.3.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian B. J., Loretz L. J., Oberley T. D., Reznikoff C. A. Characterization of human uroepithelial cells immortalized in vitro by simian virus 40. Cancer Res. 1987 Nov 15;47(22):6066–6073. [PubMed] [Google Scholar]
- Clark R., Stampfer M. R., Milley R., O'Rourke E., Walen K. H., Kriegler M., Kopplin J., McCormick F. Transformation of human mammary epithelial cells by oncogenic retroviruses. Cancer Res. 1988 Aug 15;48(16):4689–4694. [PubMed] [Google Scholar]
- Cohen D. R., Curran T. fra-1: a serum-inducible, cellular immediate-early gene that encodes a fos-related antigen. Mol Cell Biol. 1988 May;8(5):2063–2069. doi: 10.1128/mcb.8.5.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Whyte P. RB and the cell cycle: entrance or exit? Cell. 1989 Sep 22;58(6):1009–1011. doi: 10.1016/0092-8674(89)90495-9. [DOI] [PubMed] [Google Scholar]
- Curran T., Peters G., Van Beveren C., Teich N. M., Verma I. M. FBJ murine osteosarcoma virus: identification and molecular cloning of biologically active proviral DNA. J Virol. 1982 Nov;44(2):674–682. doi: 10.1128/jvi.44.2.674-682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T., Teich N. M. Candidate product of the FBJ murine osteosarcoma virus oncogene: characterization of a 55,000-dalton phosphoprotein. J Virol. 1982 Apr;42(1):114–122. doi: 10.1128/jvi.42.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- Dyson N., Buchkovich K., Whyte P., Harlow E. The cellular 107K protein that binds to adenovirus E1A also associates with the large T antigens of SV40 and JC virus. Cell. 1989 Jul 28;58(2):249–255. doi: 10.1016/0092-8674(89)90839-8. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Ludlow J. W., Marsilio E., DeCaprio J. A., Millikan R. C., Cheng S. H., Paucha E., Livingston D. M. An N-terminal transformation-governing sequence of SV40 large T antigen contributes to the binding of both p110Rb and a second cellular protein, p120. Cell. 1989 Jul 28;58(2):257–267. doi: 10.1016/0092-8674(89)90840-4. [DOI] [PubMed] [Google Scholar]
- Finkel M. P., Biskis B. O., Jinkins P. B. Virus induction of osteosarcomas in mice. Science. 1966 Feb 11;151(3711):698–701. doi: 10.1126/science.151.3711.698. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Levine A. J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989 Jun 30;57(7):1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Franza B. R., Jr, Sambucetti L. C., Cohen D. R., Curran T. Analysis of Fos protein complexes and Fos-related antigens by high-resolution two-dimensional gel electrophoresis. Oncogene. 1987 May;1(2):213–221. [PubMed] [Google Scholar]
- Fujii M., Shalloway D., Verma I. M. Gene regulation by tyrosine kinases: src protein activates various promoters, including c-fos. Mol Cell Biol. 1989 Jun;9(6):2493–2499. doi: 10.1128/mcb.9.6.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon J. V., Lane D. P. p53 and DNA polymerase alpha compete for binding to SV40 T antigen. Nature. 1987 Oct 1;329(6138):456–458. doi: 10.1038/329456a0. [DOI] [PubMed] [Google Scholar]
- Gluzman Y., Frisque R. J., Sambrook J. Origin-defective mutants of SV40. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):293–300. doi: 10.1101/sqb.1980.044.01.033. [DOI] [PubMed] [Google Scholar]
- Gorman S. D., Cristofalo V. J. Reinitiation of cellular DNA synthesis in BrdU-selected nondividing senescent WI-38 cells by simian virus 40 infection. J Cell Physiol. 1985 Oct;125(1):122–126. doi: 10.1002/jcp.1041250116. [DOI] [PubMed] [Google Scholar]
- Gotoh S., Gelb L., Schlessinger D. SV40-transformed human diploid cells that remain transformed throughout their limited lifespan. J Gen Virol. 1979 Feb;42(2):409–414. doi: 10.1099/0022-1317-42-2-409. [DOI] [PubMed] [Google Scholar]
- Green M. R. When the products of oncogenes and anti-oncogenes meet. Cell. 1989 Jan 13;56(1):1–3. doi: 10.1016/0092-8674(89)90975-6. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Harris C. C. Human tissues and cells in carcinogenesis research. Cancer Res. 1987 Jan 1;47(1):1–10. [PubMed] [Google Scholar]
- Huschtscha L. I., Holliday R. Limited and unlimited growth of SV40-transformed cells from human diploid MRC-5 fibroblasts. J Cell Sci. 1983 Sep;63:77–99. doi: 10.1242/jcs.63.1.77. [DOI] [PubMed] [Google Scholar]
- Jat P. S., Sharp P. A. Cell lines established by a temperature-sensitive simian virus 40 large-T-antigen gene are growth restricted at the nonpermissive temperature. Mol Cell Biol. 1989 Apr;9(4):1672–1681. doi: 10.1128/mcb.9.4.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- Livingston D. M., Bradley M. K. The simian virus 40 large T antigen. A lot packed into a little. Mol Biol Med. 1987 Apr;4(2):63–80. [PubMed] [Google Scholar]
- Martin R. G., Chou J. Y. Simian virus 40 functions required for the establishment and maintenance of malignant transformation. J Virol. 1975 Mar;15(3):599–612. doi: 10.1128/jvi.15.3.599-612.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura T., Zerrudo Z., Hayflick L. Senescent human diploid cells in culture: survival, DNA synthesis and morphology. J Gerontol. 1979 May;34(3):328–334. doi: 10.1093/geronj/34.3.328. [DOI] [PubMed] [Google Scholar]
- McCormick J. J., Maher V. M. Towards an understanding of the malignant transformation of diploid human fibroblasts. Mutat Res. 1988 Jun;199(2):273–291. doi: 10.1016/0027-5107(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Wang C., Tjian R. Positive and negative regulation of transcription in vitro: enhancer-binding protein AP-2 is inhibited by SV40 T antigen. Cell. 1987 Sep 11;50(6):847–861. doi: 10.1016/0092-8674(87)90512-5. [DOI] [PubMed] [Google Scholar]
- Nagata Y., Diamond B., Bloom B. R. The generation of human monocyte/macrophage cell lines. Nature. 1983 Dec 8;306(5943):597–599. doi: 10.1038/306597a0. [DOI] [PubMed] [Google Scholar]
- Neufeld D. S., Ripley S., Henderson A., Ozer H. L. Immortalization of human fibroblasts transformed by origin-defective simian virus 40. Mol Cell Biol. 1987 Aug;7(8):2794–2802. doi: 10.1128/mcb.7.8.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann E., Schaefer-Ridder M., Wang Y., Hofschneider P. H. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1(7):841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile C., Martin R. G. Stable stem-loop and cruciform DNA structures: isolation of mutants with rearrangements of the palindromic sequence at the simian virus 40 replication origin. Intervirology. 1986;25(3):158–171. doi: 10.1159/000149671. [DOI] [PubMed] [Google Scholar]
- O'Brien T. G., Simsiman R. C., Boutwell R. K. Induction of the polyamine-biosynthetic enzymes in mouse epidermis by tumor-promoting agents. Cancer Res. 1975 Jul;35(7):1662–1670. [PubMed] [Google Scholar]
- Pereira-Smith O. M., Smith J. R. Functional simian virus 40 T antigen is expressed in hybrid cells having finite proliferative potential. Mol Cell Biol. 1987 Apr;7(4):1541–1544. doi: 10.1128/mcb.7.4.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit C. A., Gardes M., Feunteun J. Immortalization of rodent embryo fibroblasts by SV40 is maintained by the A gene. Virology. 1983 May;127(1):74–82. doi: 10.1016/0042-6822(83)90372-0. [DOI] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radna R. L., Caton Y., Jha K. K., Kaplan P., Li G., Traganos F., Ozer H. L. Growth of immortal simian virus 40 tsA-transformed human fibroblasts is temperature dependent. Mol Cell Biol. 1989 Jul;9(7):3093–3096. doi: 10.1128/mcb.9.7.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddel R. R., Ke Y., Gerwin B. I., McMenamin M. G., Lechner J. F., Su R. T., Brash D. E., Park J. B., Rhim J. S., Harris C. C. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer Res. 1988 Apr 1;48(7):1904–1909. [PubMed] [Google Scholar]
- Rhim J. S., Jay G., Arnstein P., Price F. M., Sanford K. K., Aaronson S. A. Neoplastic transformation of human epidermal keratinocytes by AD12-SV40 and Kirsten sarcoma viruses. Science. 1985 Mar 8;227(4691):1250–1252. doi: 10.1126/science.2579430. [DOI] [PubMed] [Google Scholar]
- Rinehart C. A., Jr, Lyn-Cook B. D., Kaufman D. G. Gland formation from human endometrial epithelial cells in vitro. In Vitro Cell Dev Biol. 1988 Oct;24(10):1037–1041. doi: 10.1007/BF02620878. [DOI] [PubMed] [Google Scholar]
- Rinehart C. A., Jr, Viceps-Madore D., Fong W. F., Ortiz J. G., Canellakis E. S. The effect of transport system A and N amino acids and of nerve and epidermal growth factors on the induction of ornithine decarboxylase activity. J Cell Physiol. 1985 Jun;123(3):435–441. doi: 10.1002/jcp.1041230321. [DOI] [PubMed] [Google Scholar]
- Rittling S. R., Brooks K. M., Cristofalo V. J., Baserga R. Expression of cell cycle-dependent genes in young and senescent WI-38 fibroblasts. Proc Natl Acad Sci U S A. 1986 May;83(10):3316–3320. doi: 10.1073/pnas.83.10.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack G. H., Jr Human cell transformation by simian virus 40--a review. In Vitro. 1981 Jan;17(1):1–19. doi: 10.1007/BF02618025. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P., Ransone L. J., Lamph W. W., Verma I. M. Direct interaction between fos and jun nuclear oncoproteins: role of the 'leucine zipper' domain. Nature. 1988 Dec 15;336(6200):692–695. doi: 10.1038/336692a0. [DOI] [PubMed] [Google Scholar]
- Seshadri T., Campisi J. Repression of c-fos transcription and an altered genetic program in senescent human fibroblasts. Science. 1990 Jan 12;247(4939):205–209. doi: 10.1126/science.2104680. [DOI] [PubMed] [Google Scholar]
- Small M. B., Gluzman Y., Ozer H. L. Enhanced transformation of human fibroblasts by origin-defective simian virus 40. Nature. 1982 Apr 15;296(5858):671–672. doi: 10.1038/296671a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stahl H., Knippers R. The simian virus 40 large tumor antigen. Biochim Biophys Acta. 1987 Oct 9;910(1):1–10. doi: 10.1016/0167-4781(87)90088-1. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright W. E., Pereira-Smith O. M., Shay J. W. Reversible cellular senescence: implications for immortalization of normal human diploid fibroblasts. Mol Cell Biol. 1989 Jul;9(7):3088–3092. doi: 10.1128/mcb.9.7.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]