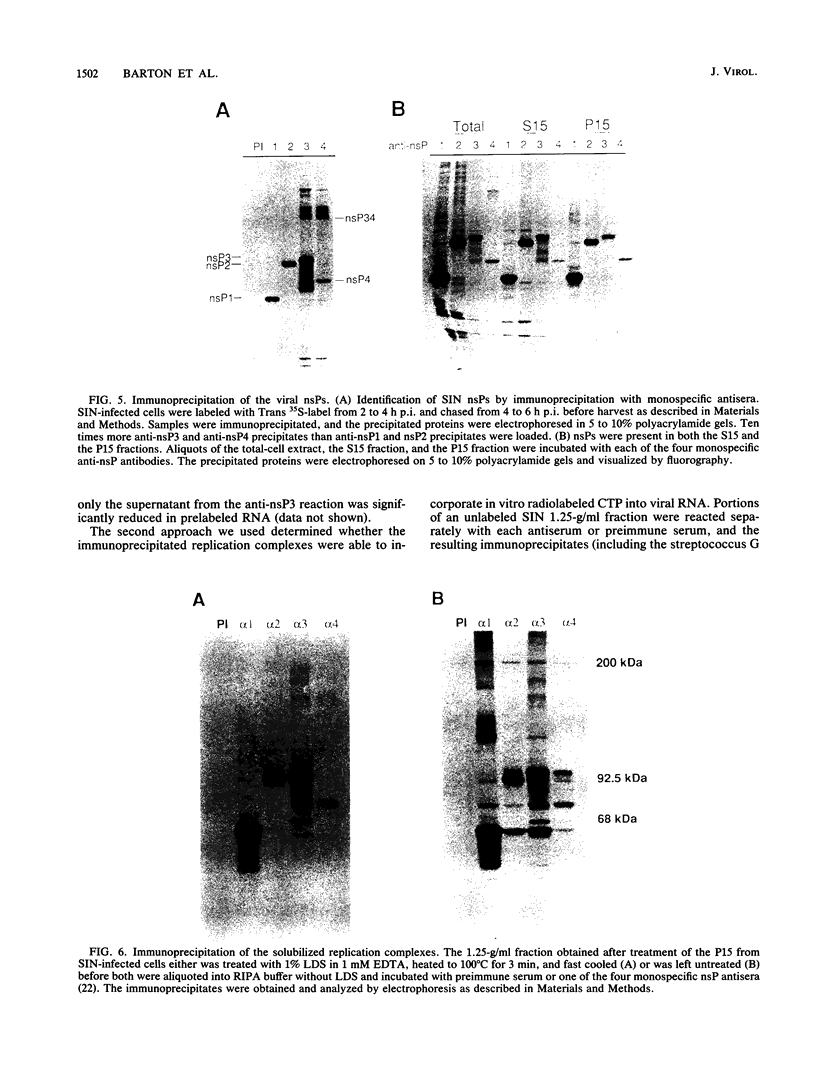
Abstract
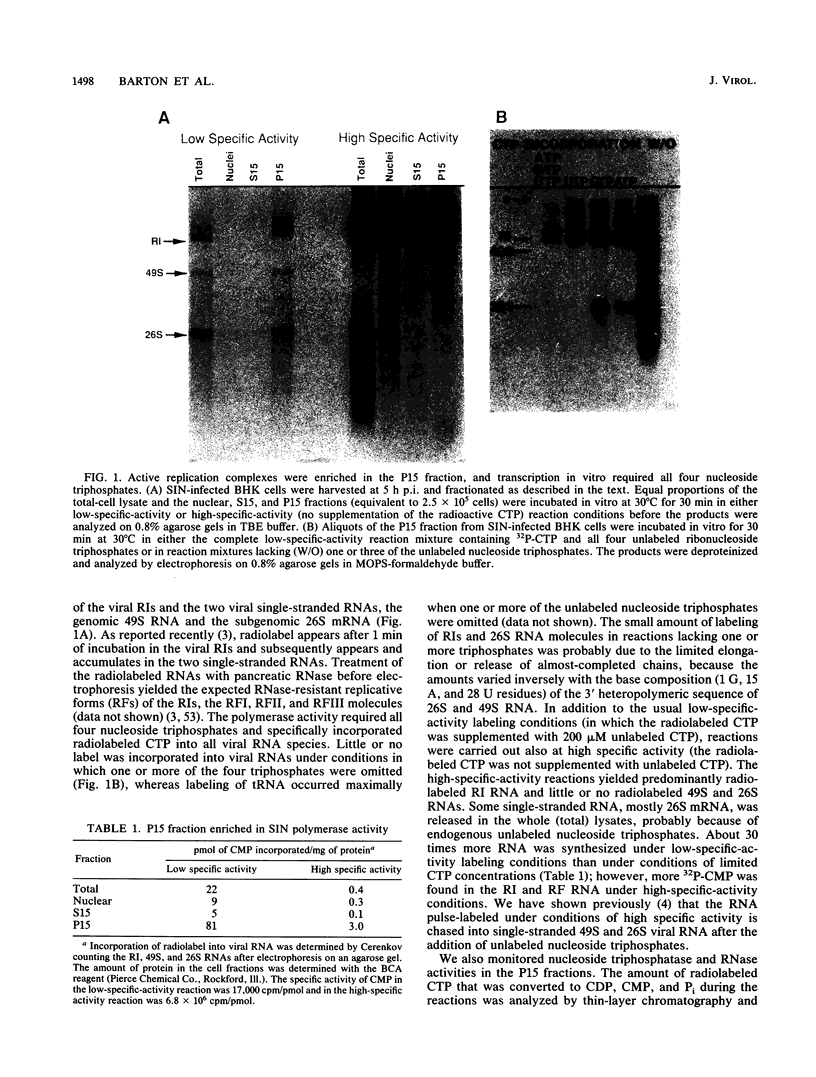
Alphavirus replication complexes that are located in the mitochondrial fraction of infected cells which pellets at 15,000 x g (P15 fraction) were used for the in vitro synthesis of viral 49S genome RNA, subgenomic 26S mRNA, and replicative intermediates (RIs). Comparison of the polymerase activity in P15 fractions from Sindbis virus (SIN)- and Semliki Forest virus (SFV)-infected cells indicated that both had similar kinetics of viral RNA synthesis in vitro but the SFV fraction was twice as active and produced more labeled RIs than SIN. When assayed in vitro under conditions of high specific activity, which limits incorporation into RIs, at least 70% of the polymerase activity was recovered after detergent treatment. Treatment with Triton X-100 or with Triton X-100 plus deoxycholate (DOC) solubilized some prelabeled SFV RIs but little if any SFV or SIN RNA polymerase activity from large structures that also contained cytoskeletal components. Treatment with concentrations of DOC greater than 0.25% or with 1% Triton X-100-0.5% DOC in the presence of 0.5 M NaCl released the polymerase activity in a soluble form, i.e., it no longer pelleted at 15,000 x g. The DOC-solubilized replication complexes, identified by their polymerase activity in vitro and by the presence of prelabeled RI RNA, had a density of 1.25 g/ml, were 20S to 100S in size, and contained viral nsP1, nsP2, phosphorylated nsP3, nsP4, and possibly nsP34 proteins. Immunoprecipitation of the solubilized structures indicated that the nonstructural proteins were complexed together and that a presumed cellular protein of approximately 120 kDa may be part of the complex. Antibodies specific for nsP3, and to a lesser extent antibodies to nsP1, precipitated native replication complexes that retained prelabeled RIs and were active in vitro in viral RNA synthesis. Thus, antibodies to nsP3 bound but did not disrupt or inhibit the polymerase activity of replication complexes in vitro.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Blobel G. Immunoprecipitation of proteins from cell-free translations. Methods Enzymol. 1983;96:111–120. doi: 10.1016/s0076-6879(83)96012-3. [DOI] [PubMed] [Google Scholar]
- Baric R. S., Carlin L. J., Johnston R. E. Requirement for host transcription in the replication of Sindbis virus. J Virol. 1983 Jan;45(1):200–205. doi: 10.1128/jvi.45.1.200-205.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton D. J., Sawicki S. G., Sawicki D. L. Demonstration in vitro of temperature-sensitive elongation of RNA in Sindbis virus mutant ts6. J Virol. 1988 Oct;62(10):3597–3602. doi: 10.1128/jvi.62.10.3597-3602.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewley J. P., Kennedy S. I. Purification and polypeptide composition of Semliki Forest virus RNA polymerase. J Gen Virol. 1976 Sep;32(3):395–411. doi: 10.1099/0022-1317-32-3-395. [DOI] [PubMed] [Google Scholar]
- Condreay L. D., Adams R. H., Edwards J., Brown D. T. Effect of actinomycin D and cycloheximide on replication of Sindbis virus in Aedes albopictus (mosquito) cells. J Virol. 1988 Aug;62(8):2629–2635. doi: 10.1128/jvi.62.8.2629-2635.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross R. K., Gomatos P. J. Concomitant methylation and synthesis in vitro of Semliki Forest virus (SFV) ss RNAs by a fraction from infected cells. Virology. 1981 Oct 30;114(2):542–554. doi: 10.1016/0042-6822(81)90234-8. [DOI] [PubMed] [Google Scholar]
- Ding M. X., Schlesinger M. J. Evidence that Sindbis virus NSP2 is an autoprotease which processes the virus nonstructural polyprotein. Virology. 1989 Jul;171(1):280–284. doi: 10.1016/0042-6822(89)90539-4. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Levin J. G., Grimley P. M., Berezesky I. K. Membrane-associated replication complex in arbovirus infection. J Virol. 1972 Sep;10(3):504–515. doi: 10.1128/jvi.10.3.504-515.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froshauer S., Kartenbeck J., Helenius A. Alphavirus RNA replicase is located on the cytoplasmic surface of endosomes and lysosomes. J Cell Biol. 1988 Dec;107(6 Pt 1):2075–2086. doi: 10.1083/jcb.107.6.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomatos P. J., Käriäinen L., Keränen S., Ranki M., Sawicki D. L. Semliki Forest virus replication complex capable of synthesizing 42S and 26S nascent RNA chains. J Gen Virol. 1980 Jul;49(1):61–69. doi: 10.1099/0022-1317-49-1-61. [DOI] [PubMed] [Google Scholar]
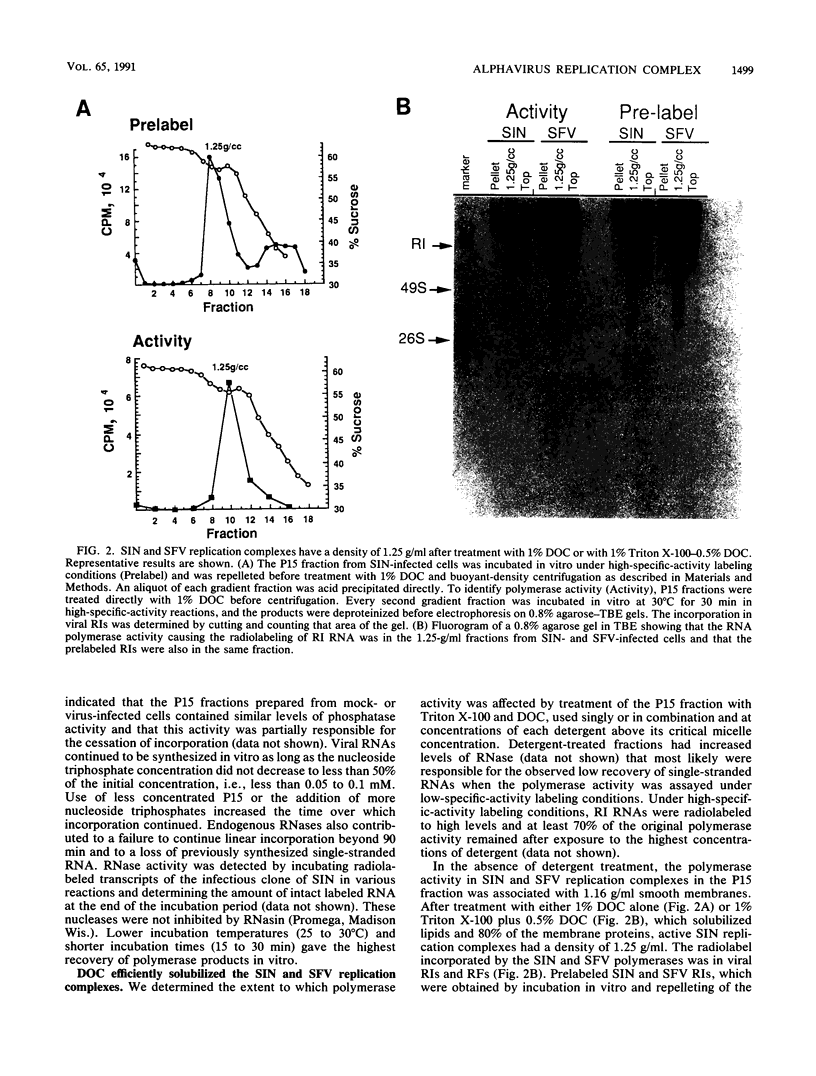
- Gorbalenya A. E., Koonin E. V. Viral proteins containing the purine NTP-binding sequence pattern. Nucleic Acids Res. 1989 Nov 11;17(21):8413–8440. doi: 10.1093/nar/17.21.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grakoui A., Levis R., Raju R., Huang H. V., Rice C. M. A cis-acting mutation in the Sindbis virus junction region which affects subgenomic RNA synthesis. J Virol. 1989 Dec;63(12):5216–5227. doi: 10.1128/jvi.63.12.5216-5227.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimley P. M., Levin J. G., Berezesky I. K., Friedman R. M. Specific membranous structures associated with the replication of group A arboviruses. J Virol. 1972 Sep;10(3):492–503. doi: 10.1128/jvi.10.3.492-503.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun J. B., Brinton M. A. Separation of functional West Nile virus replication complexes from intracellular membrane fragments. J Gen Virol. 1988 Dec;69(Pt 12):3121–3127. doi: 10.1099/0022-1317-69-12-3121. [DOI] [PubMed] [Google Scholar]
- Hahn Y. S., Grakoui A., Rice C. M., Strauss E. G., Strauss J. H. Mapping of RNA- temperature-sensitive mutants of Sindbis virus: complementation group F mutants have lesions in nsP4. J Virol. 1989 Mar;63(3):1194–1202. doi: 10.1128/jvi.63.3.1194-1202.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn Y. S., Strauss E. G., Strauss J. H. Mapping of RNA- temperature-sensitive mutants of Sindbis virus: assignment of complementation groups A, B, and G to nonstructural proteins. J Virol. 1989 Jul;63(7):3142–3150. doi: 10.1128/jvi.63.7.3142-3150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi M., Hanafusa H. Association of p60src with Triton X-100-resistant cellular structure correlates with morphological transformation. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2312–2316. doi: 10.1073/pnas.84.8.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy W. R., Hahn Y. S., de Groot R. J., Strauss E. G., Strauss J. H. Synthesis and processing of the nonstructural polyproteins of several temperature-sensitive mutants of Sindbis virus. Virology. 1990 Jul;177(1):199–208. doi: 10.1016/0042-6822(90)90473-5. [DOI] [PubMed] [Google Scholar]
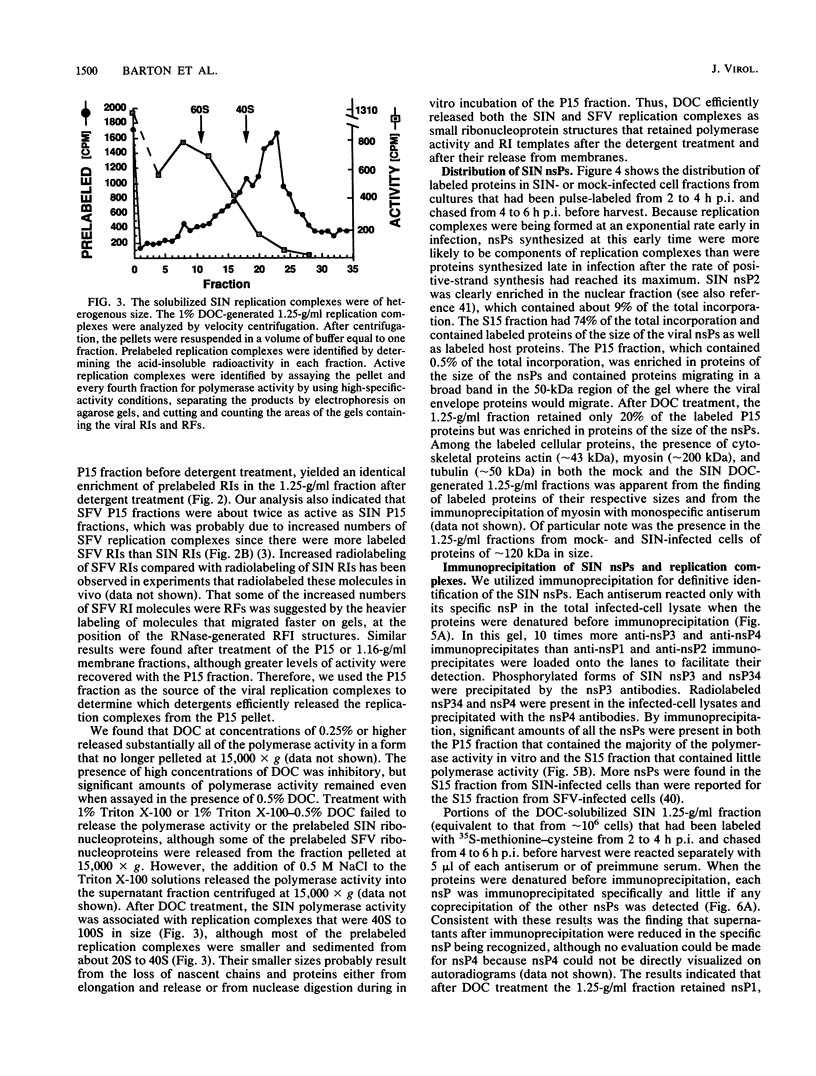
- Hardy W. R., Strauss J. H. Processing the nonstructural polyproteins of Sindbis virus: study of the kinetics in vivo by using monospecific antibodies. J Virol. 1988 Mar;62(3):998–1007. doi: 10.1128/jvi.62.3.998-1007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy W. R., Strauss J. H. Processing the nonstructural polyproteins of sindbis virus: nonstructural proteinase is in the C-terminal half of nsP2 and functions both in cis and in trans. J Virol. 1989 Nov;63(11):4653–4664. doi: 10.1128/jvi.63.11.4653-4664.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes R. J., Buck K. W. Complete replication of a eukaryotic virus RNA in vitro by a purified RNA-dependent RNA polymerase. Cell. 1990 Oct 19;63(2):363–368. doi: 10.1016/0092-8674(90)90169-f. [DOI] [PubMed] [Google Scholar]
- Hill V. M., Summers D. F. A minor microtubule-associated protein is responsible for the stimulation of vesicular stomatitis virus transcription in vitro. J Gen Virol. 1990 Feb;71(Pt 2):289–298. doi: 10.1099/0022-1317-71-2-289. [DOI] [PubMed] [Google Scholar]
- Kamer G., Argos P. Primary structural comparison of RNA-dependent polymerases from plant, animal and bacterial viruses. Nucleic Acids Res. 1984 Sep 25;12(18):7269–7282. doi: 10.1093/nar/12.18.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keränen S., Käriäinen L. Functional defects of RNA-negative temperature-sensitive mutants of Sindbis and Semliki Forest viruses. J Virol. 1979 Oct;32(1):19–29. doi: 10.1128/jvi.32.1.19-29.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
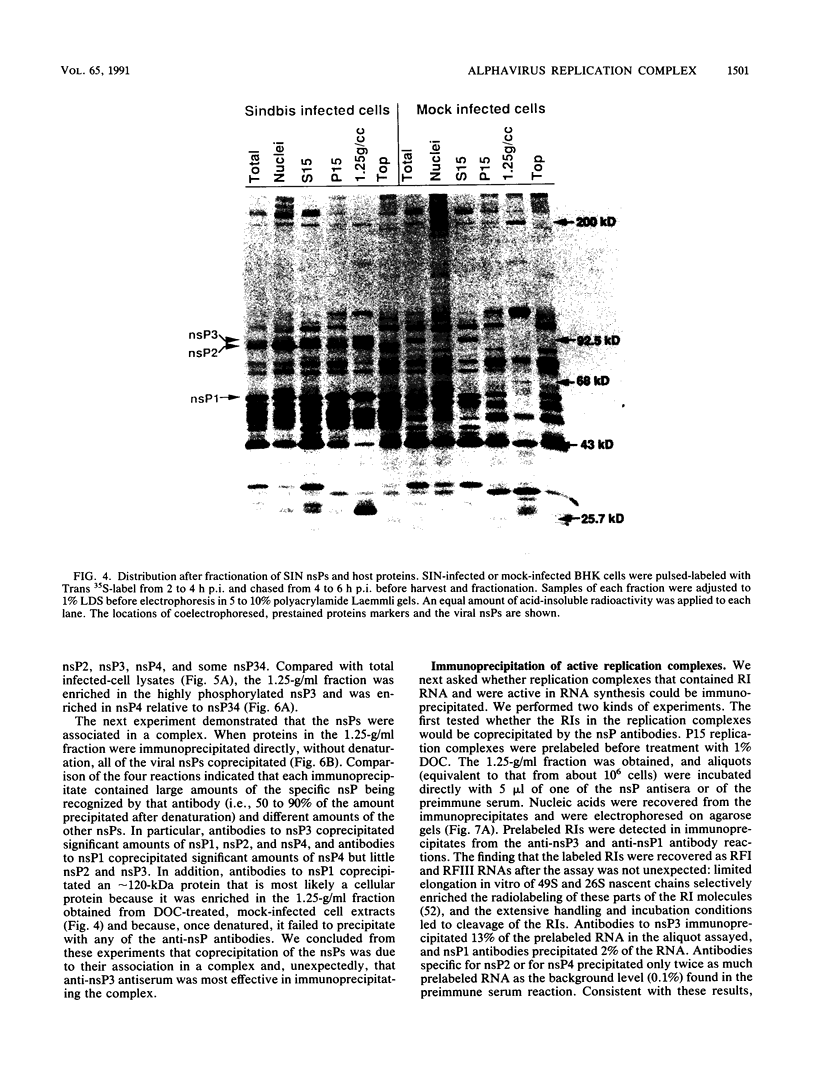
- Keränen S., Ruohonen L. Nonstructural proteins of Semliki Forest virus: synthesis, processing, and stability in infected cells. J Virol. 1983 Sep;47(3):505–515. doi: 10.1128/jvi.47.3.505-515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal K. J., Stollar V. Temperature-sensitive host-dependent mutants of Sindbis virus. Virology. 1981 Oct 15;114(1):140–148. doi: 10.1016/0042-6822(81)90260-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemm J. A., Durbin R. K., Stollar V., Rice C. M. Mutations which alter the level or structure of nsP4 can affect the efficiency of Sindbis virus replication in a host-dependent manner. J Virol. 1990 Jun;64(6):3001–3011. doi: 10.1128/jvi.64.6.3001-3011.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. P., La Starza M. W., Hardy W. R., Strauss J. H., Rice C. M. Phosphorylation of Sindbis virus nsP3 in vivo and in vitro. Virology. 1990 Nov;179(1):416–427. doi: 10.1016/0042-6822(90)90310-n. [DOI] [PubMed] [Google Scholar]
- Li G. P., Rice C. M. Mutagenesis of the in-frame opal termination codon preceding nsP4 of Sindbis virus: studies of translational readthrough and its effect on virus replication. J Virol. 1989 Mar;63(3):1326–1337. doi: 10.1128/jvi.63.3.1326-1337.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. B. Does the cytoskeleton play a significant role in animal virus replication? J Theor Biol. 1982 Nov 7;99(1):173–191. doi: 10.1016/0022-5193(82)90397-6. [DOI] [PubMed] [Google Scholar]
- Martin E. M., Sonnabend J. A. Ribonucleic acid polymerase catalyzing synthesis of double-stranded arbovirus ribonucleic acid. J Virol. 1967 Feb;1(1):97–109. doi: 10.1128/jvi.1.1.97-109.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E. M. Studies on the RNA polymrase of some temperature-sensitive mutants of Semliki Forest virus. Virology. 1969 Sep;39(1):107–117. doi: 10.1016/0042-6822(69)90352-3. [DOI] [PubMed] [Google Scholar]
- Mi S., Durbin R., Huang H. V., Rice C. M., Stollar V. Association of the Sindbis virus RNA methyltransferase activity with the nonstructural protein nsP1. Virology. 1989 Jun;170(2):385–391. doi: 10.1016/0042-6822(89)90429-7. [DOI] [PubMed] [Google Scholar]
- Michel M. R., Gomatos P. J. Semliki forest virus-specific RNAs synthesized in vitro by enzyme from infected BHK cells. J Virol. 1973 Jun;11(6):900–914. doi: 10.1128/jvi.11.6.900-914.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer S. A., Baker S. C., Horikami S. M. Host cell proteins required for measles virus reproduction. J Gen Virol. 1990 Apr;71(Pt 4):775–783. doi: 10.1099/0022-1317-71-4-775. [DOI] [PubMed] [Google Scholar]
- Nakhasi H. L., Rouault T. A., Haile D. J., Liu T. Y., Klausner R. D. Specific high-affinity binding of host cell proteins to the 3' region of rubella virus RNA. New Biol. 1990 Mar;2(3):255–264. [PubMed] [Google Scholar]
- Peränen J., Rikkonen M., Liljeström P., Käriäinen L. Nuclear localization of Semliki Forest virus-specific nonstructural protein nsP2. J Virol. 1990 May;64(5):1888–1896. doi: 10.1128/jvi.64.5.1888-1896.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peränen J., Takkinen K., Kalkkinen N., Käriäinen L. Semliki Forest virus-specific non-structural protein nsP3 is a phosphoprotein. J Gen Virol. 1988 Sep;69(Pt 9):2165–2178. doi: 10.1099/0022-1317-69-9-2165. [DOI] [PubMed] [Google Scholar]
- Poch O., Sauvaget I., Delarue M., Tordo N. Identification of four conserved motifs among the RNA-dependent polymerase encoding elements. EMBO J. 1989 Dec 1;8(12):3867–3874. doi: 10.1002/j.1460-2075.1989.tb08565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter K. R. The cytomatrix: a short history of its study. J Cell Biol. 1984 Jul;99(1 Pt 2):3s–12s. doi: 10.1083/jcb.99.1.3s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadt R., Jaspars E. M. Purification and characterization of brome mosaic virus RNA-dependent RNA polymerase. Virology. 1990 Sep;178(1):189–194. doi: 10.1016/0042-6822(90)90393-6. [DOI] [PubMed] [Google Scholar]
- Ranki M., Käriäinen L. Solubilized RNA replication complex from Semliki Forest virus-infected cells. Virology. 1979 Oct 30;98(2):298–307. doi: 10.1016/0042-6822(79)90553-1. [DOI] [PubMed] [Google Scholar]
- Sawicki D. L., Sawicki S. G. Functional analysis of the A complementation group mutants of Sindbis HR virus. Virology. 1985 Jul 15;144(1):20–34. doi: 10.1016/0042-6822(85)90301-0. [DOI] [PubMed] [Google Scholar]
- Sawicki D. L., Sawicki S. G., Keränen S., Käriäinen L. Specific Sindbis virus-coded function for minus-strand RNA synthesis. J Virol. 1981 Aug;39(2):348–358. doi: 10.1128/jvi.39.2.348-358.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki D., Barkhimer D. B., Sawicki S. G., Rice C. M., Schlesinger S. Temperature sensitive shut-off of alphavirus minus strand RNA synthesis maps to a nonstructural protein, nsP4. Virology. 1990 Jan;174(1):43–52. doi: 10.1016/0042-6822(90)90052-s. [DOI] [PubMed] [Google Scholar]
- Sawicki S. G., Sawicki D. L. The effect of overproduction of nonstructural proteins on alphavirus plus-strand and minus-strand RNA synthesis. Virology. 1986 Jul 30;152(2):507–512. doi: 10.1016/0042-6822(86)90157-1. [DOI] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Replication of Sindbis virus. II. Multiple forms of double-stranded RNA isolated from infected cells. J Mol Biol. 1972 Nov 28;71(3):615–631. doi: 10.1016/s0022-2836(72)80027-5. [DOI] [PubMed] [Google Scholar]
- Sreevalsan T., Yin F. H. Sindbis virus-induced viral ribonucleic acid polymerase. J Virol. 1969 Jun;3(6):599–604. doi: 10.1128/jvi.3.6.599-604.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E. G., Lenches E. M., Strauss J. H. Mutants of sindbis virus. I. Isolation and partial characterization of 89 new temperature-sensitive mutants. Virology. 1976 Oct 1;74(1):154–168. doi: 10.1016/0042-6822(76)90137-9. [DOI] [PubMed] [Google Scholar]
- Strauss E. G., Levinson R., Rice C. M., Dalrymple J., Strauss J. H. Nonstructural proteins nsP3 and nsP4 of Ross River and O'Nyong-nyong viruses: sequence and comparison with those of other alphaviruses. Virology. 1988 May;164(1):265–274. doi: 10.1016/0042-6822(88)90644-7. [DOI] [PubMed] [Google Scholar]
- Strauss E. G., Rice C. M., Strauss J. H. Sequence coding for the alphavirus nonstructural proteins is interrupted by an opal termination codon. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5271–5275. doi: 10.1073/pnas.80.17.5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takkinen K., Peränen J., Keränen S., Söderlund H., Käriäinen L. The Semliki-Forest-virus-specific nonstructural protein nsP4 is an autoproteinase. Eur J Biochem. 1990 Apr 20;189(1):33–38. doi: 10.1111/j.1432-1033.1990.tb15456.x. [DOI] [PubMed] [Google Scholar]
- Wang Y. F., Sawicki S. G., Sawicki D. L. Sindbis virus nsP1 functions in negative-strand RNA synthesis. J Virol. 1991 Feb;65(2):985–988. doi: 10.1128/jvi.65.2.985-988.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot R. J., Hardy W. R., Shirako Y., Strauss J. H. Cleavage-site preferences of Sindbis virus polyproteins containing the non-structural proteinase. Evidence for temporal regulation of polyprotein processing in vivo. EMBO J. 1990 Aug;9(8):2631–2638. doi: 10.1002/j.1460-2075.1990.tb07445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]









